ContentslistsavailableatScienceDirect
Particuology
j ou rn a l h o m ep a g e :w w w . e l s e v i e r . c o m / l o c a t e / p a r t i c
Novel
one-step
synthesis
of
silica
nanoparticles
from
sugarbeet
bagasse
by
laser
ablation
and
their
effects
on
the
growth
of
freshwater
algae
culture
Nalan
Oya
San
a,b,c,
Canan
Kurs¸
ungöz
c,
Yasin
Tümtas¸
c,
Öncay
Yas¸
a
c,
Bülend
Ortac¸
c,∗,
Turgay
Tekinay
a,b,∗∗aPolatlıScienceandLiteratureFaculty,BiologyDepartment,GaziUniversity,Ankara06900,Turkey bLifeSciencesApplicationandResearchCenter,GaziUniversity,Ankara06830,Turkey
cUNAMInstituteofMaterialsScienceandNanotechnology,BilkentUniversity,Ankara06800,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received21September2013
Receivedinrevisedform6November2013 Accepted25November2013 Keywords: Laserablation One-stepsynthesis Raman Silicananoparticle Microalgae
a
b
s
t
r
a
c
t
Scientificresearchinvolvingnanotechnologyhasgrownexponentiallyandhasledtothedevelopment ofengineerednanoparticles(NPs).SilicaNPshavebeenusedinnumerousscientificandtechnological applicationsoverthepastdecade,necessitatingthedevelopmentofefficientmethodsfortheirsynthesis. Recentstudieshaveexploredthepotentialoflaserablationasaconvenientwaytopreparemetaland oxideNPs.Duetoitshighsilicacontent,lowcost,andwidespreadavailability,sugarbeetbagasseishighly suitableasarawmaterialforproducingsilicaNPsvialaserablation.Inthisstudy,twodifferentNP pro-ductionmethodswereinvestigated:laserablationandNaOHtreatment.Wedevelopedanovel,one-step methodtoproducesilicaNPsfromsugarbeetbagasseusinglaserablation,andwecharacterizedthesilica NPsusingenvironmentalscanningelectronmicroscopy(ESEM),energydispersivespectrometry(EDS), dynamiclightscattering(DLS),transmissionelectronmicroscopy(TEM),attenuatedtotal reflectance-Fouriertransforminfraredspectroscopy(ATR–FTIR),X-rayphotoelectronspectroscopy(XPS)andRaman spectroscopy.EDSanalysisandXPSconfirmedthepresenceofsilicaNPs.TheNPsproducedbylaser ablationweresmaller(38–190nm)thanthoseproducedbyNaOHtreatment(531–825nm).Finally,we demonstratedpositiveeffectsofsilicaNPsproducedfromlaserablationonthegrowthofmicroalgae,and thus,ournovelmethodmaybebeneficialasanenvironmentallyfriendlyproceduretoproduceNPs.
©2014PublishedbyElsevierB.V.onbehalfofChineseSocietyofParticuologyandInstituteofProcess Engineering,ChineseAcademyofSciences.
1. Introduction
Silica is beneficial to many plants (Ding, Ma, Shui, Wan, &
Li,2005).Itiswellknownthatcertainplants,includinggrasses
(Poaceae),rice(Oryzasativa),sugarbeet(Betavulgaris),and
horse-tail(Equisetum),containhighlevelsofbiogenicsilica(Sun&Gong,
2001).Inparticular,sugarbeetisanattractivesourceofbiogenic
sil-icabecausethesilicacontentofthisplantismainlyconcentrated
inbagasse.Sugarbeetbagasseisproduced inlargequantitiesas
anagro-industrialbyproductandisoftenusedasboilerfuelfor
generatingsteamduringtheprocessingofsugar.
∗ Correspondingauthor.Tel.:+903122903526;fax:+903122664365. ∗∗ Correspondingauthorat:PolatlıScienceandLiteratureFaculty,Biology Depart-ment,GaziUniversity,Ankara06900,Turkey.Tel.:+903124846270;
fax:+903124846271.
E-mailaddresses:ortac@unam.bilkent.edu.tr(B.Ortac¸),ttekinay@gazi.edu.tr
(T.Tekinay).
In recent years, there hasbeen an increasing trend toward
themoreefficientuseofagro-industrialby-productsfor animal
nutrition,fuel,andfermentativeproducts.Severalprocessesand
productsusingsugarbeetbagasseastherawmaterialhavebeen
reported,particularlyinpulpandpaperproduction;itisalsoused
asafeedstockinfermentationprocesses(Alves,Felipe,Silva,Silva,
&Prata,1998;Pandey,Soccol,Nigam,&Soccol,2000).However,
sugarbeetbagassecanalsobeprocessedtoproducehigh-purity
sil-ica,exceeding99%purityandprimarilybearingK2O,andMgOas
impurities(Affandi,Setyawan,Winardi,Purwanto,&Balgis,2009).
Assuch,bagasseisaneconomicallyviablerawmaterialforsilica
nanoparticle(NP)production.
Nanoparticlesarefrequentlyusedinseveral
nanotechnologi-calapplications.Inparticular,silicaNPsarewidelyusedindrugs,
cosmetics,printertoners,varnishes,andfoodpreservatives(Baek
&An,2011;Bagwe,Hilliard,&Tan,2006;Huaetal.,2009; Lin, Huang,Zhou,&Ma,2006).Inaddition,theuseofsilicaNPshas
recently beenextended tothe biomedicaland biotechnological
1674-2001/$–seefrontmatter©2014PublishedbyElsevierB.V.onbehalfofChineseSocietyofParticuologyandInstituteofProcessEngineering,ChineseAcademyofSciences.
silicaNPsynthesismaybeunsustainableandcostprohibitiveinthe
nearfuture.Therefore,itishighlydesirabletoidentifyalternative
approachestoreduceproductioncosts.
MostNPsynthesistechniques,suchasphysicalvapor
deposi-tion(Yousefi&Muhamad,2010),precipitation(Yang&Hu,2010),
solvothermal/hydrothermalmethods(Wang,Shi,Qi,&Liu,2010),
andsol–gelmethods(e.g.,sol–gelcombustion),areexpensiveand
complex and offer only limited control over particle size and
sizeuniformity.Inrecentyears,pulsed-laserablationofsolidsin
solutionhasattractedinterestduetoitsversatilityandlowcost
(Alkis,Oruc¸,Ortac¸,Kos¸ger,&Okyay,2012;Amendola&Meneghett, 2009;Wu,Dickinson,&Lele,2012).Sajti,Sattari, Chichkov,and Barcikowski(2010)demonstratedrecentlythebulksynthesis of
NPsbylaserablation,yieldingceramicNPsonascaleofseveral
grams.Theirstudyindicatesthepotentialfeasibilityoflaser
abla-tionforlarge-scalesynthesisapplications.
Based on the available literature (Affandi et al., 2009), we
hypothesizedthatsugarbeetbagasse,whichisinherentlyrichin
silica,canbeusedtosynthesizesilicaNPs.Inthisstudy,wedescribe
forthefirsttimetheuseoflaserablationforthesynthesisof
sil-ica NPsfrom agro-industrialbyproducts.It is alsoimportantto
investigatetheeffects ofnanomaterialexposureontheaquatic
environment.Greenalgaeareknowntobesensitivetomany
chem-icals.Theyhavebeenconsideredindicatorsofthebioactivityof
industrial wastes,and theyvaryin theirresponsestoa variety
oftoxicants.Theirecologicalpositionatthebaseofmostaquatic
foodwebsandtheiressentialrolesinnutrientcyclingandoxygen
productionarecriticaltomanyecosystems.Therefore,we
exam-inedhowsilicaNPsimpactthegrowthofafreshwatergreenalgae
speciesthatisamongthemostwidespreadofallalgae:Chlorella
vulgaris.Ourresultsmayaidthedevelopmentofenvironmentally
friendlyandeconomicallyattractivealternativestocurrentNP
pro-ductionmethods.
2. Materialsandmethods
2.1. Nanoparticleproduction
Sugarbeetbagasse wasobtainedfromtheAnkaraSugar
Fac-tory,Etimesgut,Ankara,Turkey.Twoseparatetreatments were
adopted toextractsilica fromthebagasse samples. Inthe first
approach,bagasseashwasobtainedbycalciningsugarbeetbagasse
at500◦Cfor12h.Onegramofbagasseashwasthentreatedwith
concentratedHCl:HNO3=1:3(v/v)at35◦Cfor2handoven-dried
at 60◦C. Then, 50mL of water was added to the residue, and
thesolutionwasalkalized toa pHof13–14withconcentrated
NaOH.Followingovernightincubation,thealkalinesolutionwas
sampleswerefilteredwitha0.22mfiltertoremovethefibers
fromtheNPsolution.
2.2. Nanoparticlecharacterization
Themorphologyandelementalcompositionofrawbagassewas
measuredbyanenvironmentalscanningelectronmicroscopewith
EDS(ESEM,Quanta200FEG,FEIInstruments,USA).Theparticlesize
anddistributionofparticlesdispersedindistilledwaterwere
mea-suredusingdynamiclightscattering(DLS)(MalvernInstruments
Ltd.,Malvern,UK).ThestabilityofthesilicaNPswasmeasuredfrom
thezetapotentialofthesolution(NanoZS,MalvernInstruments
Ltd.,Malvern,UK).ThemorphologyofsilicaNPswasalsoanalyzed
usingaFEITecnaiG2F30transmissionelectronmicroscope(TEM)
connectedtoahighresolutionimagingsystem.NPsampleswere
preparedbydryingatotalof2Lofthelaserablatedmixtureon
carboncoatedcoppergridsatroomtemperature.
Fourier transform infrared spectroscopy (ATR/FTIR) analysis
wasperformed using a Nicolet 6700(Thermo Fisher Scientific,
USA)ATR–FTIRspectrometer.Spectrawereobtainedwithinthe
4000–500cm−1rangewitharesolutionof4cm−1(Bruker,Vertex
70withHyperionScanningMicroscope,Germany).Thesamples
(100L)wereplacedintheattenuatedtotalreflectance(ATR,ZnSe)
analyzerandanalyzed.
X-rayphotoelectronspectroscopy(XPS)(K␣-Monochromated
highperformance)(Thermo,USA)measurementswereperformed
inanultra-highvacuum(UHV)withaconventionalX-raysource
(Mg-K␣).
Ramanspectrawereacquiredatroomtemperaturebyusinga
WitecAlpha300S+RamanModule(Witec,Germany).Asolid-state
532nmwavelengthlaserwasusedforexcitation.Raman
measure-mentsofsinglespectraweretakenat50×magnificationandwith
2.03sintegrationtimes.
2.3. EffectsofsilicananoparticlesonC.vulgarisgrowth
ThealgaC.vulgariswasobtainedfromaculturecollectionat
GaziUniversityLifeSciencesApplicationandResearchCenterand
sub-culturedinthelaboratory.C.vulgariswascultivatedin
steril-izedTapmedium(Tris–acetate–phosphate)underanillumination
intensity of 4000lux. The temperature in the air-conditioned
growthchamberwasmaintainedat25◦C.WeexposedsilicaNPs
producedfromtwodifferenttreatmentstoalgalcultures.Thealgal
densityofthreereplicateswasthencalculatedbymeasuringthe
opticaldensity (OD) at a wavelengthof 750nmwith a UV–vis
spectrophotometer(Shimadzu,UV-1201V,Japan).Medium
Fig.1.Flowdiagramoftheprocedureusedtoproducesilicananoparticlesfromsugarbeetbagasse.
Fig.2.(a)ESEMimagesand(b)EDSspectrumofrawsugarbeetbagasse.
cultures werevisuallyinspectedforcontaminationusinga light
microscope.
3. Resultsanddiscussion
Inthepresentstudy,thesilicaNPswerepreparedusingtwo
differentmethods:(1)calcinationofsugarbeetbagasseand
sub-sequenttreatmentofsugarbeetbagasse ashwithNaOHand (2)
synthesisofsugarbeetbagasseusinglaserablation(Fig.1).
Fig.2(a)and(b)shows theESEM imagesandEDS spectrum,
respectively, ofrawbagasse. TheESEMmicrographofraw
sug-arbeet bagasse (Fig. 2(a)) clearly revealsits fibrous texture. In
Fig.3.TheparticlenumberdistributionsofsilicaNPsobtainedby(a)NaOH treat-mentand(b)laserablation.
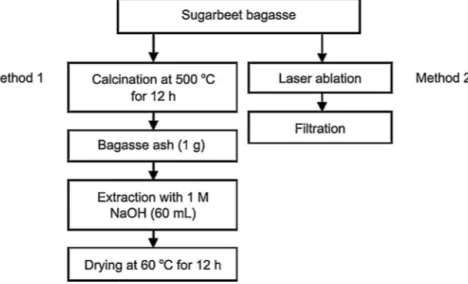
Fig.4. (a)TEMimageofsilicaNPs,inset:sizedistribution,(b)XPSanalysisrecordedfromsilicaNPs,(c)amorphousstructureofsilicaNPs,and(d)nanocrystal(NC)structure ofsilicaNPs.
addition,EDS analysis showedthat rawsugarbeet bagasse was
primarilycomposedofmetaloxides,suchasMg,K,andSi(Fig.2(b)).
Fig.3showstheintensityandparticlenumberdistributionsof
silicaNPsofvarioussizesafter(a)NaOHtreatmentand(b)laser
ablation.Fig.3(a)demonstratesthatthesilicaparticlesproduced
bycalcinationandNaOHtreatmentweresubstantiallylargerthan
thoseformedvialaserablation,withasizerangeof531–825nm.
Incontrast,silicaNPsformedvialaserablationwereintherange
of38–190nm,withanaveragesizeof∼74nm(Fig.3(b)).
AdetailedTEManalysiswasperformedtoidentifythestructural
propertiesofthecolloidalnanoparticlesolution(CNS).Fig.4shows
thattheCNSwassuccessfullyproducedbypulsedlaserablation
methodindeionizedwater.TheCNSconsistsofspheroid-shaped
anduniformlydispersednanoparticles,withoutanyaggregation.
Inaddition,zetapotentialmeasurementswerecarriedout,andthe
zetapotentialwas−21.0mV,indicatingthatthesilicaNPswere
sta-ble.ThedataindicatetheCNSindeionizedwaterwasstableand
welldispersedafterthelaserablationprocess.Toobtainaccurate
particlesizeinformation,wemeasuredthesizesof150particles
observedintheTEMimages.AsapparentfromtheinsetofFig.4(a),
nanoparticlesrangedinsizebetween40and160nm,withan
aver-ageparticlesizeof100nm.Toverifythechemicalcomposition
oftheCNS, XPSanalysiswasperformed.Thepeak at102.43eV
correspondstotheSi2pspectra,indicatingtheexistenceofSi O
bondsofSiO2nanoparticles(Yang,Kuperman,Coombs,
Mamiche-Afara,&Ozin,1996).ThesedatashowthatthecolloidalNPsolution
consistsofSiO2 NPs(Fig.4(b)).Inaddition,Fig.4(a)showsthat
theone-stepproductionmethodyieldedNPswithamorphousand
crystalstructure.Fig.4(c)showsanisolatedamorphousNP,and
Fig.4(d) shows an isolated nanocrystal(NC). The lattice-fringe
structureapparentintheHR-TEMimageofthesingleisolatedNC
evidencesthegenerationofnanoparticlesintheformofa
crys-tallinestructurebylaserablationtechnique.
ProductionofSiNPshaspreviouslybeenreportedfrom
silica-containingagro-industrialwaste,suchasricehuskashandcoffee
(Estevez,Vargas,Casta ˜no,&Rodriguez,2009;Li&Wang,2008).
Inthesestudies,Si NPswereobtainedbyashingfollowed bya
chemicaltreatmentof6horlonger.Ourresultsdemonstratethat
laserablationcanbeusedasaone-step,uncomplicatedmethod
toproducesilicaNPsthataresignificantlysmallerthanthose
pro-ducedbychemicalmethods,suchascalcinationfollowedbyNaOH
treatment.
InfraredspectraofthesilicaNPssynthesizedusingtwodifferent
methodswererecordedbyFTIRspectroscopyandarepresentedin
Fig.5.AsseeninFig.5,silicapeaksareclearlyvisible.The
Fig.5. FTIRspectraofNPsobtainedfromlaserablationofbagasseandNaOH-treated bagasseash.
region.Thebandsat1200–1000cm−1and807areduetothe
asym-metricandsymmetricstretchingmodesofSi O Si(Beganskien ˙e,
Sirutkaitis,Kurtinaitien ˙e,Juˇsk ˙enas,&Kareiva,2004).Thebandat
955cm−1wasascribedtotheSiO Hasymmetrybendingvibration.
Thebroad peak atapproximately3200–3600cm−1 corresponds
to the stretching vibrations of hydroxyl groups, whereas the
band at 1630–1640cm−1 is due to the deformation of water
moleculesabsorbedthroughthesilicaparticlesurface(Martinez,
Ruiz,Vorobiev,Perez-Robles,&Gonzalez-Hernandez,1998).The
peaks centered at approximately 1100cm−1 show an obvious
broadeningandashoulderinthe1160–1290cm−1range.This
pat-terncanbeattributedtotheasymmetricstretchingvibrationsof
thetetrahedralSiO4coordinationunits(Pol,Gedanken,&
Calderon-Moreno,2003;Wangetal.,2011).Similaritiesbetweenthespectra
ofthetwosilicasamplesindicatethatthepretreatmentdoesnot
affectthechemicalstructure ofthesynthesizedsilica,although
NaOHtreatmentproducedmoreresiduesthanthelaserablation
method.
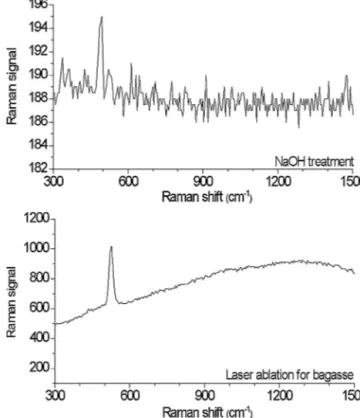
Throughitssensitivitytovibrationalproperties,Raman
scatter-ingoffersavaluabletoolforunderstandingstructuralaspectsof
materials(Dreschera&Kneipp,2012;Woods&Bain,2012).Inthis
work,theWitecAlpha300S+RamanModulewith532nm
excita-tionwasusedtocharacterizethebondpropertiesofsilicaNPs.The
resultsofRamanscatteringinthespectralrangeof300–3600cm−1
areshowninFig.6.Severaldifferencesareapparentamongthe
Ramanspectraofthethreesamples.ThenoiselevelintheNaOH
treatmentspectraismuchhigherthanthatofthelaserablation
treatment,althoughbothspectrawererecordedunderthesame
conditions.The probablesourceofthis highnoise is thelarger
amountofporewaterincorporatedinNPsduetoNaOHtreatment.
Wefoundanotablepeakat522cm−1 inbothtreatments,which
isclosetothevalueobservedforbulksilicon(Inada,Nakagawana,
Umezu,&Sugimura,2002).
Fig.7 shows theoptical density (OD)of C. vulgarisexposed
to silica NPs produced by laser ablation and NaOH treatment.
Understandingtheeffectsofnanomaterialexposureontheaquatic
environmentisextremelyimportant.Becauseoftheirwidespread
use,NPswilllikelymoveintoaquatic,terrestrial,andatmospheric
environments. Therefore, concerns have been raised about the
potentialenvironmentalrisksposedbyNPs.Greenalgaeareknown
tobesensitivetomanychemicals,andtheyareconsidered
indica-torsofthebioactivityofindustrialwastes.Theirecologicalposition
atthebaseofmostaquaticfoodwebsandtheiressentialrolesin
Fig.6. RamanscatteringspectraofNPsresultingfromlaserablationofbagasseand NaOHtreatedbagasseash.
nutrientcyclingandoxygenproductionarecriticaltomany
ecosys-tems.However,fewstudieshaveinvestigatedNPtoxicitytoalgae.
Zhu,Zhu,Tian,Lang,andLi(2008)studiedthetoxicityofZnO,
CuO,andTiO2NPstothegreenalgaeScenedesmusobliquus.They
foundthatZnONPswerethemosttoxic,followedbyCuONPsand
TiO2NPs.Inthepresentstudy,SiNPsdidnotinhibitalgalgrowth
butinsteadincreasedthegrowthrate.
Weietal.(2010)showedthatSiO2NPsof10–20nmindiameter
weretoxictoS.obliquus.Theyfoundthatthesmallertheparticle,
thegreateritstoxicity.ThetoxicityofSiNPsismostlikelydueto
theirsorptiontothealgalcellsurface.Inthepresentstudy,the
sizeofNPsproducedbylaserablationwas38–190nm.Therefore,
wespeculatethatthelaser-ablatedSiNPscannotsorbtothecell
surface.Inaddition,themorphologyofthealgalcellsdidnotchange
whenobservedunderanopticalmicroscope.
Majornutrients,includingcarbon,nitrogen,phosphorus,and
silica, aredefined assuchbecausetheyare essentialfor life in
Fig.7.EffectsofsilicaNPsproducedfromlaserablationandNaOHtreatmenton growthofChlorellavulgaris.
bagasse notonlytakes fulladvantageofthehighsilicacontent
ofsugarbeetbagassebutalsominimizestheenvironmentalissues
associatedwithcurrentapplicationsanddisposalmethodsof
sug-arbeetbagasse.Assuch,themethodpresentedhereinmayaidthe
developmentofneweconomicalapproaches involvingthe
syn-thesisofvaluablenanomaterialsfromagro-industrialresiduesas
alternativestotheenergy-intensiveandpotentiallyhazardous
pro-cessescurrentlyadoptedbyindustry.
Here,wereportthesuccessfulpreparationofenvironmentally
friendlysilicaNPsusinglaserablation,asconfirmedbySEM,TEM,
andDLS.OurresultsshowthattheNPsobtainedbylaserablation
aresignificantlysmaller(38–190nm)thanthosepreparedusing
chemicaltreatment.
Last,thisstudydemonstratestheeffectofsilicaNPsproduced
usingtwodifferentnanoparticlemethodsonalgalgrowth.From
ourresults,weconcludethatsilicaNPsproducedfromchemical
treatmenthaveanegativeeffectonaquaticalgae,asmanifestedby
thedecreasedgrowthofalgalcells.However,silicaNPsproduced
bylaserablationincreasedthegrowthofC.vulgaris.Ourresults
suggestthatsilicaNPsproducedbylaserablationmayposenoharm
totheaquaticenvironment.
Acknowledgments
TheauthorsthankZeynepErgülÜlgerforprocuringthe
sugar-beetbagassesamples,ÖmerFarukSarıo˘gluforhelpinanalyzing
theATR/FTIRdata,AlperDevrimÖzkanforobtainingtheRaman
spectraandAhmetEminTopalforperformingtheXPSanalysis.
References
Affandi,S.,Setyawan,H.,Winardi,S.,Purwanto,A.,&Balgis,R.(2009).Afacilemethod forproductionofhigh-puritysilicaxerogelsfrombagasseash.AdvancedPowder Technology,20,468–472.
Alkis,S.,Oruc¸,F.B.,Ortac¸,B.,Kos¸ger,A.C.,&Okyay,A.K.(2012).Aplasmonic enhancedphotodetectorbasedonsiliconnanocrystalsobtainedthroughlaser ablation.JournalofOptics,14,125001.
Alves,L.A.,Felipe,M.G.A.,Silva,J.B.A.E.,Silva,S.S.,&Prata,A.M.R.(1998). Pretreat-mentofsugarcanebagassehemicellulosehydrolysateforxylitolproductionby Candidaguilliermondii.AppliedBiochemistryandBiotechnology,70–72,89–98.
Amendola,W.V.,&Meneghett,M.(2009).Laserablationsynthesisinsolutionand sizemanipulationofnoblemetalnanoparticles.PhysicalChemistryChemical Physics,11,3805–3821.
Baek,Y.W.,&An,Y.-J.(2011).Microbialtoxicityofmetaloxidenanoparticles(CuO, NiO,ZnO,andSb2O3)toEscherichiacoli,Bacillussubtilis,andStreptococcusaureus.
ScienceoftheTotalEnvironment,409,1603–1608.
Bagwe,R.P.,Hilliard,L.R.,&Tan,W.H.(2006).Surfacemodificationofsilica nanopar-ticlestoreduceaggregationandnon-specificbinding.Langmuir,22,4357– 4362.
Bansal,V.,Ahmad,A.,&Sastry,M.(2006).Fungus-mediatedbiotransformationof amorphoussilicainricehusktonanocrystallinesilica.JournaloftheAmerican ChemicalSociety,128(43),14059–14066.
nanoparticles.GreenChemistry,12,1995–2002.
Hua,D.,Tang,J.,Jiang,J.L.,Gu,Z.Q.,Dai,L.L.,&Zhui,X.L.(2009).Controlledgrafting modificationofsilicagelviaRAFTpolymerizationunderultrasonicirradiation. MaterialsChemistryandPhysics,114,402–406.
Inada,M.,Nakagawana,H.,Umezu,I.,&Sugimura,A.(2002).EffectsofhydrogenonSi nanoparticlesformedbypulsedlaserablation.AppliedSurfaceScience,197–198, 666–669.
Lee,J.,Park,J.,Singha,K.,&Kim,W.J.(2013).Mesoporoussilicananoparticle facilitateddrugreleasethroughcascadephotosensitizeractivationand cleav-ageof singletoxygen sensitivelinker. ChemicalCommunication,49,1545– 1547.
Li,T., &Wang, T.(2008).Preparationof silicaaerogelfrom rice hullashby dryingatatmosphericpressure.MaterialsChemistryandPhysics,112,398– 401.
Lin,W.,Huang,Y.W.,Zhou,X.D.,&Ma,Y.(2006).Invitrotoxicityofsilica nanopar-ticlesinhumanlungcancercells.ToxicologyandAppliedPharmacology,217, 252–259.
Martinez,J.R.,Ruiz,F.,Vorobiev,Y.V.,Perez-Robles,F.,&Gonzalez-Hernandez, J. (1998). Infraredspectroscopy analysis of the localatomic structure in silicapreparedbysol–gel.TheJournalofChemicalPhysics, 109(17),7511– 7514.
Molenkamp,W.C.,Watanabe,M.,Miyata,H.,&Tolbert,S.H.(2004).Highlypolarized luminescencefromopticalqualityfilmsofasemiconductingpolymeraligned withinorientedmesoporoussilica.JournaloftheAmericanChemicalSociety, 126(14),4476–4477.
Pandey,A.,Soccol,C.R.,Nigam,P.,&Soccol,V.T.(2000).Biotechnologyfor agro-industrialresiduesutilisation.I:Sugarcanebagasse.BioresourceTechnology,74, 69–80.
Pol,V.G.,Gedanken,A.,&Calderon-Moreno,J.(2003).Depositionofgold nanopar-ticlesonsilicaspheres:Asonochemicalapproach.ChemistryofMaterials,15(5), 1111–1118.
Sun,L.,&Gong,K.(2001).Review:Silicon-basedmaterialsfromricehusksand theirapplications.Industrial&EngineeringChemistryResearch,40(25),5861– 5877.
Tolaymat,T.M.,ElBadawy,A.M.,Genaidy,A.,Scheckel,K.G.,Luxton,T.P.,&Suidan, M.(2010).Anevidence-basedenvironmentalperspectiveofmanufactured sil-vernanoparticleinsynthesesandapplications:Asystematicreviewandcritical appraisalofpeer-reviewedscientificpapers.ScienceoftheTotalEnvironment, 408,999–1006.
Trewyn,B.G.,Giri,S.,Slowing,I.I.,&Lin,V.S.Y.(2007).Mesoporoussilica nanopar-ticlebasedcontrolledrelease,drugdelivery,andbiosensorsystems.Chemical Communications,31,3236–3245.
Sajti,C.L.,Sattari,R.,Chichkov,B.N.,&Barcikowski,S.(2010).Gramscalesynthesis ofpureceramicnanoparticlesbylaserablationinliquid.TheJournalofPhysical ChemistryC,114,2421–2427.
Suzuki,N.,Kiba,S.,&Yamauchi,Y.(2011).Fabricationofmesoporoussilica/polymer compositesthroughsolventevaporationprocessandinvestigationoftheir excellentlowthermalexpansionproperty.PhysicalChemistryChemicalPhysics, 13(11),4957–4962.
Wang,J.,Shi,N.,Qi,Y.,&Liu,M.(2010).Reversemicellestemplateassisted fab-ricationofZnOhollownanospheresandhexagonalmicrotubesbyanovelfast microemulsion-basedhydrothermalmethod.JournalofSol–GelScienceand Tech-nology,53,101–106.
Wang,W.,Martin,J.C.,Zhang,N.,Ma,C.,Han,A.,&Sun,L.(2011).Harvesting sil-icananoparticlesfromricehusks.JournalofNanoparticleResearch,13,6981– 6990.
Wei,C.,Zhang,Y.,Guo,J.,Han,B.,Yang,X.,&Yuan,J.(2010).Effectsofsilica nanopar-ticlesongrowthandphotosyntheticpigmentcontentsofScenedesmusobliquus. JournalofEnvironmentalSciences,22,155–160.
Woods,A.D.,&Bain,C.D.(2012).TotalinternalreflectionRamanspectroscopy. Analyst,137,35–48.
Wu,J.,Dickinson,R.B.,&Lele,T.P.(2012).Investigationofinvivomicrotubuleand stressfibermechanicswithlaserablation.IntegrativeBiology,4,471–479.
Yousefi,R.,&Muhamad,M.R.(2010).Effectsofgoldcatalystsandthermal evapo-rationmethodmodificationsonthegrowthprocessofZn1−xMgxOnanowires.
JournalofSolidStateChemistry,183,1733–1739.
Yang,H.,Kuperman,A.,Coombs,N.,Mamiche-Afara,S.,&Ozin,G.(1996).Synthesis oforientedmesoporoussilicafilmsonmica.Nature,379,703–705.
Yang,Q.,&Hu,W.(2010).Anovelmercury-mediaroutetosynthesizeZnOhollow microspheres.CeramicsInternational,36,989–993.
Zhao,B.,&Zhu,L.(2009).Mixedpolymerbrush-graftedparticles:Anewclass ofenvironmentallyresponsivenanostructuredmaterials.Macromolecules,42, 9369–9383.
Zhu,X.S.,Zhu,L.,Tian,S.Y.,Lang,Y.P.,&Li,Y.(2008).Aquaticecotoxicitiesof nanoscaleTiO2,ZnOandAl2O3watersuspensions.ActaEcologicaSinica,28(8),