Seminars in Cancer Biology xxx (xxxx) xxx
Available online 11 August 2019
1044-579X/© 2019 Elsevier Ltd. All rights reserved.
Ginger and its active compounds in cancer therapy: From folk uses to
nano-therapeutic applications
M.F. Mahomoodally
a, M.Z. Aumeeruddy
a, Kannan R.R. Rengasamy
b,c,*, S. Roshan
d,
S. Hammad
e,f, J. Pandohee
a,g, Xuebo Hu
h, G. Zengin
iaDepartment of Health Sciences, Faculty of Medicine and Health Sciences, University of Mauritius, R´eduit, Mauritius bBionanotechnology Research Group, Ton Duc Thang University, Ho Chi Minh City, Viet Nam
cFaculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, Viet Nam
dDeccan School of Pharmacy, Darussalam, Aghapura, Hyderabad, 500001, Telangana, India
eSchool of Pharmacy, Monash University, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia fInstitute of Pharmaceutical Sciences (IPS), University of Veterinary & Animal Sciences (UVAS), Lahore, Pakistan
gCentre for Integrative Metabolomics and Computational Biology, School of Science, Edith Cowan University, Joondalup, WA 6027, Australia hCollege of Plant Sciences and Technology, Huazhong Agricultural University, Wuhan, China
iDepartment of Biology, Faculty of Science, Selcuk University, Turkey
A R T I C L E I N F O Keywords: Zingiber officinale Microbiome Gingerol Shogaol Enzyme inhibition Combination therapy Nanoformulation A B S T R A C T
Ginger is a spice that is renowned for its characteristic aromatic fragrance and pungent taste, with documented healing properties. Field studies conducted in several Asian and African countries revealed that ginger is used traditionally in the management of cancer. The scientific community has probed into the biological validation of its extracts and isolated compounds including the gingerols, shogaols, zingiberene, and zingerone, through in- vitro and in-vivo studies. Nonetheless, an updated compilation of these data together with a deep mechanistic approach is yet to be provided. Accordingly, this review highlights the mechanisms and therapeutics of ginger and its bioactive compounds focused on a cancer context and these evidence are based on the (i) cytotoxic effect against cancer cell lines, (ii) enzyme inhibitory action, (iii) combination therapy with chemotherapeutic and phenolic compounds, (iv) possible links to the microbiome and (v) the use of nano-formulations of ginger bioactive compounds as a more effective drug delivery strategy in cancer therapy.
1. Introduction
The ginger plant is a herbaceous flowering plant that belongs to the Zingiberaceae family. Scientifically named as Zingiber officinale Roscoe, the perennial plant consists of a pseudo-stem, yellow flowers and tu-berous rhizomes, which are also known as ginger root or commonly called ginger. It is the rhizomes of ginger plants that are the most sought- after portion due to its aromatic odour and pungent taste. Ginger is therefore an important ingredient for culinary purposes. Originally from Asia, the most common custom usage of ginger was as a flavouring agent in its various form and this could include fresh, dried, pickled, powdered and preserved and, more interestingly, as a tonic root to treat many ailments for its medicinal benefits. Nowadays, the plant is grown on a large scale across the world in tropical regions, such as the West Indies, Africa and India, for its consumption as a spice, dietary supplement [1].
As a natural product, ginger is a complex spice composed of carbo-hydrates, water, protein, lipids, fibres and volatile essential oils [2,3]. Using advanced mass spectrometry-based identifications, chemical analysis of the essential oil reveals that the root contains hundreds of different compounds [4,5]. The majority of compounds identified in ginger are from the chemical classes gingerols, shogaols, zigiberenes and zingerone (chemical structures shown in Fig. 1). To a lesser extent, terpene components such as bisabolene, farnesene, sesquiphellandrene, curcumene and phytosterols, vitamins and minerals can also be found in specific varieties of ginger. Thus, the nutraceutical value of ginger is attributed to its complexity of bioactive compounds especially the major phenolic classes such as the gingerols, shogaols, zingiberene, paradol, and zingerone [6].
Gingerols (23–25%) are part of the phenolic compound class and these volatile organic compounds are known to account for the strong * Corresponding author at: Ton Duc Thang University, Ho Chi Minh City, Viet Nam.
E-mail address: rengasamy.kannan@tdtu.edu.vn (K.R.R. Rengasamy).
Contents lists available at ScienceDirect
Seminars in Cancer Biology
journal homepage: www.elsevier.com/locate/semcancerhttps://doi.org/10.1016/j.semcancer.2019.08.009
taste of fresh Zingiber officinale. 6-gingerol is the main compound accountable for the pungency of the rhizome, while the other gingerols (4-, 8-, 10- and 12-gingerol) are present in lower amounts (1–10%). However, being thermally labile, these compounds are converted to shogaols at high temperatures, for example while cooking, and gives ginger its spicy-sweet aroma. The biological properties of gingerols and shogaols are recognized to possess antimicrobial, anticancer, antioxi-dant, anti-inflammatory, and anti-allergic propensities [7,8]. Shogaols are known to have anti-coughing effects, gingerol on the other hand accounts for the analgesic properties of ginger. In addition to the phe-nolics, diarylheptanoids and zingerone have also been identified in ginger and are believed to be bioactive compounds contributing to health benefits [5,6,9].
Using ginger in the treatment of cancer is not a new concept, it has been a customary practice in the folk medicine throughout the world and has been showed to be effective by several field studies. In India, the juice and decoction of the rhizome is taken orally [10,11]. In Singapore, the cooked rhizome is used as a mean for cancer prevention [12]. An infusion of the rhizome is used against breast cancer by the Palestinians [13]. In addition, as a general cancer treatment, a decoction of the root is prepared from a mix of ginger root, turmeric, and honey, and two doses are ingested on a daily basis. A concoction can also be made from a mix of ginger root, nigella, and camel milk, and one glass is taken daily before breakfast [14]. Another recipe used by the Palestinians to manage stomach and liver cancer uses 100 g of the ground dried rhizomes boiled in water and given twice daily after meals [13]. In Morocco, ginger root is ground with honey and taken orally [15]. Another traditional use of ginger is as a paste; in Ghana the root/rhizome is ground and the resulting mixture is either orally consumed or used as a massage remedy for stomach and brain cancer [16].
Recently, pharmacological studies have probed into the validation of such claims, and now, ginger together with its associated bioactive compounds have been proven to have potential anticancer properties. In this context, this review aims at highlighting the cytotoxic effect of ginger and its compounds against various types of cancer, focusing on its enzyme inhibitory mechanism, combination therapy with other agents and the potential of nano-formulations of ginger bioactive compounds as a novel beneficial in cancer treatment.
2. Overview of anti-cancer properties of ginger
There has been an increasing amount of studies showing promising results on the cytotoxic effect of ginger extracts and its bioactive com-pounds against several cancer cells including breast, cervical, colorectal, leukemia, liver, lung, nasopharyngeal, ovarian, prostate, and retino-blastoma (see detailed findings and mechanisms in Table 1 and Fig. 2). 2.1. Breast cancer
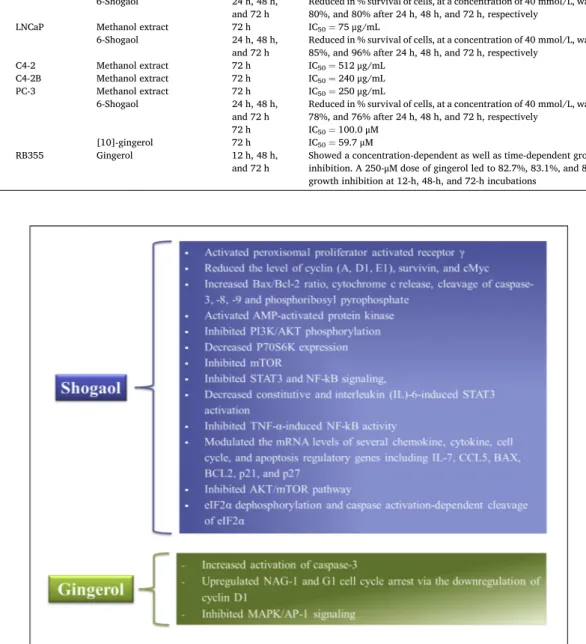
Ansari et al [17] showed that the methanolic solution of ginger displayed a time-dependent cytotoxic effect on the breast cancer cell line (MDA-MB-231), with IC50 of 86.7 and 57.5μg/mL after 24 and 48 h incubation, respectively. In addition, the two derivatives of 6-oxo-sho-gaol isolated from ginger, namely Z-6-oxo-6-sho6-oxo-sho-gaol and Z-6-oxo-8-sh-ogaol, showed potent cytotoxic action on MCF-7 cell, with IC50 of 6.27 and 47.22 μM, respectively, after 48 h exposure [5]. 6-shogaol was found to hinder the growth of cancer cell (breast & colon) by activating the peroxisomal proliferator activated receptor γ (PPARγ) [18]. It has been observed [19] that 10-gingerol caused a considerable upsurge in the initiation of caspase-3 and inhibited orthotopic tumour growth of spontaneous breast cancer metastasis. More pertinently, the authors reported that 10-gingerol inhibit metastasis to multiple organs (including lung, bone, and brain).
2.2. Cervical cancer
A study by Liu et al [20] found that 6-shogaol exhibited high inhi-bition (IC50=14.75 μM) against HeLa cells, which was more effective compared to α-zingiberene (IC50=60.6μg/mL), 6-gingerol (IC50=96.32μM), and [10]-gingerol (IC50=52.4μM) [21–23]. The essential oil was also found to be cytotoxic to SiHa cells, showing IC50 value of 38.6μg/mL, more effective than α-zingiberene (IC50=46.2μg/mL) [21]. 6-gingerol blocked the cell cycle in G0/G1-phase in HeLa cell and eventually caused cells death. The com-pound reduced cyclin (A, D1, E1) expression, slightly decreased CDK-1, p21, and p27 while cyclin B1 and E1 protein were unaffected. Western blot analysis also indicated the pathway of apoptotic activation, with an increased Bax/Bcl-2 ratio, cytochrome c release, cleavage of caspase-3, -8, -9 and phosphoribosyl pyrophosphate (PRPP) in challenged cells. Consequently, 6-gingerol triggered AMP-activated protein kinase
Table 1
Cytotoxicity of ginger and bioactive compounds against cancer cell lines.
Cancer type Cell line Extract type/compound Incubation
time Findings References
Breast MDA-MB-231 Methanol extract 24 h and 48 h 24 h: IC50=86.7μg/mL
48 h: IC50=57.5μg/mL
[17] Ethyl acetate fraction
obtained from crude methanolic extract
24 h At 100μg/mL, reduced the cell viability by 44% [43]
MCF-7 Ethyl acetate fraction obtained from crude methanolic extract
24 h At 100μg/mL, reduced the cell viability by 36% [43]
Essential oil 24 h IC50=82.6μg/mL [21] (Z)-6-oxo- [6]-shogaol 48 h IC50=6.27 μM [5] (Z)-6-oxo- [8]-shogaol 48 h IC50=47.22 μM [5]
α-zingiberene 24 h IC50=172.0μg/mL [21]
Cervical HeLa Chloroform extract 48 h IC50=74.32μg/mL [44]
Ethanol extract 48 h IC50=33.78μg/mL [44]
Essential oil 24 h MTT assay; IC50=141.4μg/mL
NRU assay; IC50=129.9μg/mL [29] 24 h IC50=46.0μg/mL [21] Methanol extract 24 h and 48 h 24 h: IC50=46.5μg/mL
48 h: IC50=37.5μg/mL [17] 6-shogaol 24 h IC50=14.75 μM [20] 72 h IC50=62.5μM [22] α-zingiberene 24 h IC50=60.6μg/mL [21] 6-gingerol 48 h IC50=96.32μM [23] [10]-gingerol 72 h IC50=52.4μM [22] SiHa α-zingiberene 24 h IC50=46.2μg/mL [21] Essential oil 24 h IC50=38.6μg/mL Colorectal HCT15 6-Gingerol 24 h IC50=100μM [45] HCT-116 6-Gingerol 24 h IC50=160.42μM [26]
Ethyl acetate fraction obtained from crude methanolic extract
24 h and 48 h Reduced the viability by 79 % and 88% at 200μg/mL at 24 h and 48 h,
respectively [43]
Ethanol extract 24 h IC50=496μg/mL [46] [6]-Gingerol 48 h,72 h or
96 h Dose- and and time-dependent cytotoxic effect, with an ICμM 50 value of 283
[32] SW480 Ethyl acetate fraction
obtained from crude methanolic extract
24 h and 48 h Reduced the viability by 55 % and 76% at 200μg/mL at 24 h and 48 h,
respectively [43]
[10]-Gingerol 24 h With 50-100μM, [10]-gingerol reduced the cell viability in a
concentration-dependent manner [47] [6]-Gingerol 48 h,72 h or
96 h Dose- and and time-dependent cytotoxic effect, with an ICμM 50 value of 205
[32] LoVo Ethyl acetate fraction
obtained from crude methanolic extract
24 h and 48 h Reduced the viability by 59 % and 80% at 200μg/mL at 24 h and 48 h,
respectively [43]
HT 29 Ethanol extract 24 h IC50=455μg/mL [46] Mucus-
secreting HT29 Essential oil 24 h IC50=40μL/mL [48] Non-mucus-
secreting HT- 29
Essential oil 24 h IC50=60μL/mL [48]
Leukemia Raw 264.7 cell 6-Gingerol 24 h IC50=102μM [45]
HL-60 α-zingiberene 24 h IC50=80.3μg/mL [21] Essential oil 24 h IC50=39.1μg/mL [21] 6-shogaol 72 h IC50=7.9μM [22] [10]-gingerol 72 h IC50=75.4μM [22] K562 6-shogaol 72 h IC50=24.2μM [22] [10]-gingerol 72 h IC50=112.5μM [22]
Liver HepG2 Essential oil 24 h IC50=635.1μg/mL [29]
Ethyl acetate fraction obtained from crude methanolic extract
24 h At 100μg/mL, reduced the cell viability by 30% [43]
(Z)-6-oxo- [6]-shogaol 48 h IC50=8.92 μM [5] (Z)-6-oxo- [8]-shogaol 48 h IC50=45.14 μM [5] (E)- [4]-isoshogaol 48 h IC50=14.87 μM [5] BEL-7404 6-shogaol 72 h IC50=11.8μM [22] [10]-gingerol 72 h IC50=95.2μM [22] Lung H-1299 6-gingerol 24 h IC50=136.73μM [26] A549 6-shogaol 72 h IC50=22.9μM [22] [10]-gingerol 72 h IC50=85.4μM [22] Murine
fibrosarcoma L929 Essential oil 3 h IC50
=41μg/mL [49]
Chloroform extract 48 h IC50=87.28μg/mL [44] Ethanol extract 48 h IC50=101.0μg/mL [44]
(AMPK), and inhibited PI3K/AKT phosphorylation, thus leading to a decrease in P70S6K expression and ultimately inhibited mTOR [23]. 2.3. Prostate cancer
Ginger and its active compounds were also found to reduce the cell viability of a number of prostate cancer cells including DU145, LNCaP, C4-2, C4-2B, and PC-3. 10-gingerol (IC50=59.7μM) was more cyto-toxic than 6-shogaol (IC50=100.0μM) against PC-3 cell [22]. In addi-tion, at a concentration of 40μmol/L, 6-shogaol abridged the survival of DU145, LNCaP, and PC-3 by 80%, 96%, and 76%, respectively, after 72 h exposure [24]. The authors also found that 6-shogaol inhibited
STAT3 and NF-kB signaling, decreased interleukin (IL)-6-induced STAT3 initiation and also inhibited TNF-α-induced NF-kB activity. Moreover, 6-shogaol decreased the level of numerous STAT3 and NF-kB-regulated target genes at the proteinomic stage, such as cyclin D1, survivin, and cMyc. 6-shogaol also modulated the mRNA levels of several chemokine, cytokine, cell cycle, and apoptosis regulatory genes including IL-7, CCL5, BAX, BCL2, p21, and p27. 6-gingerol also induced dose- and time-dependent apoptosis in LNCaP human prostate cancer cells mainly via caspase-3 expression with the resulting degradation of PARP [25].
Table 1 (continued)
Cancer type Cell line Extract type/compound Incubation
time Findings References
6-Gingerol 24 h IC50=102μM [45]
Nasopharyngeal CNE 6-shogaol 72 h IC50=43.8μM [22]
[10]-gingerol 72 h IC50=88.1 μM [22]
Ovarian SKOV-3 Extract (Sigma-Aldrich:
W252108) 24 h, 48 h and 72 h ICrespectively 50 was 97μg/mL, 60μg/mL and 40μg/mL at 24 h, 48 h and 72 h, [50]
Prostate DU145 Methanolic fraction 24 h IC50=45.3μg/mL [51]
Methanol extract 72 h IC50=95μg/mL [52] 6-Shogaol 24 h, 48 h,
and 72 h Reduced in % survival of cells, at a concentration of 40 mmol/L, was 64%, 80%, and 80% after 24 h, 48 h, and 72 h, respectively [24] LNCaP Methanol extract 72 h IC50=75μg/mL [52]
6-Shogaol 24 h, 48 h,
and 72 h Reduced in % survival of cells, at a concentration of 40 mmol/L, was 67%, 85%, and 96% after 24 h, 48 h, and 72 h, respectively [24] C4-2 Methanol extract 72 h IC50=512μg/mL [52]
C4-2B Methanol extract 72 h IC50=240μg/mL [52] PC-3 Methanol extract 72 h IC50=250μg/mL [52]
6-Shogaol 24 h, 48 h,
and 72 h Reduced in % survival of cells, at a concentration of 40 mmol/L, was 66%, 78%, and 76% after 24 h, 48 h, and 72 h, respectively [24] 72 h IC50=100.0μM [22]
[10]-gingerol 72 h IC50=59.7μM [22]
Retinoblastoma RB355 Gingerol 12 h, 48 h,
and 72 h Showed a concentration-dependent as well as time-dependent growth inhibition. A 250-μM dose of gingerol led to 82.7%, 83.1%, and 85.2% growth inhibition at 12-h, 48-h, and 72-h incubations
[53]
2.4. Lung cancer
The cytotoxic effect of 6-gingerol (IC50=136.73μM after 24 h exposure) on H-1299 lung cancer cell was proved by Lv et al [26]. Peng et al [22] found that 6-shogaol (IC50=22.9μM) was more effective than 10-gingerol (IC50=85.4μM) against A549 lung cancer cell. When evaluated against human lung cancer cells, 6-shogaol was also observed to initiate autophagy via the inhibition of AKT/mTOR pathway [27]. 2.5. Liver cancer
Ginger extract was observed to diminish the raised activity of NFκB and TNF-α in rats with liver cancer [28]. In addition, other ginger de-rivatives such as Z-6-oxo-6-shogaol, Z-6-oxo-8-shogaol and E-4-iso-shogaol, exhibited cytotoxic effect against HepG2 cell, with IC50 scores of 8.92 μM, 45.14 μM, 14.87 μM, respectively [5], which was more effective than the essential oil (IC50=635.1μg/mL) [29]. 72 h exposure of 6-shogaol also showed higher cytotoxic effect on BEL-7404 compared to 10-gingerol [22]⋅
2.6. Blood cancer
6-shogaol was a more effective cytotoxic agent against HL-60 and K562 leukemic cell (supported by IC50 values of 7.9μM and 24.2μM, respectively), which was more effective compared to 10-gingerol [22]. Also, Liu et al [30] observed that 6-shogaol specifically stimulated apoptosis in transformed and primary cellular leukemia. Data from immunoblotting studies revealed that 6-shogaol caused apoptosis by a process which involves eIF2α dephosphorylation and caspase activation-dependent breakdown of eIF2α.
2.7. Colorectal cancer
A plethora of supporting information that ginger and its bioactive derivatives are cytotoxic to several colon cancer cell lines with HCT15, HCT116, SW480, LoVo, and HT29 cells (see Table 1). It has been [31] observed that 6-gingerol caused apoptosis in colorectal cancer cells by upregulating NAG-1 and G1 cell cycle arrest via the down-regulation of cyclin D1. Several mechanisms seem to be responsible for the action of 6-gingerol, including protein degradation along with β-catenin, PKCε, and GSK-3β pathways. 6-gingerol also stimulated caspase-dependent apoptosis and prevented phorbol myristate acetate-induced prolifera-tion in colon cancer cells through the inhibiprolifera-tion of MAPK/AP-1 signaling. On top of that, using p53(-/-) and p53(+/+) HCT-116 colo-rectal cancer cells, the researchers confirmed that p53/p21 was the principal pathway contributing to the G2/M cell cycle arrest induced by 6-shogaol [32]. It was [33] found that 6-shogaol induced-apoptosis primarily via the pathway involving the mitochondria, and the Bcl-2 family might play as a central regulator.
3. Links to the microbiome
With the increasing evidence that ginger has beneficial effects on colorectal and intestinal cancer, the search to demystify the links and relationship between the bioactive compounds present in ginger and the microbiome is becoming more and more critical. The microbiome is the set of microorganisms that live inside a system; the human body itself is home to 10–100 trillion symbiotic microbial cells, most of which live in the human gut alone [34]. It estimated that the total amount of bacterial cells living in a human significantly outnumber human cells and that microbes in the human intestines play a vital role in maintaining a healthy gut function. Some of these functional contributions include harvesting essential nutrients from non-digestible food such as fibres [35], vitamin synthesis [36] and the immune system [37]. The core human microbiome is in constant interaction with its host and is sus-ceptible to changes in various factors such as the host lifestyle, diet,
genotype, physiology, environment and disease status. In other words, the microbiome and its human host are in a symbiotic relationship where microbes have access to food components and habitable gut environment while simultaneously providing humans beneficial by-product metabolites. A disbalance in this relationship has been shown to lead to inflammatory bowel disease and colorectal cancer. Diet therefore plays an essential role in establishing overall health.
The various ways ginger can be employed to prevent/manage/ and/ or treat gastrointestinal cancer has been reviewed in many occasions [36–40]. Most recently, Ganaie et al [41] investigated the chemo-preventive efficacy of zingerone on colon carcinogenesis in Wistar rats. The four groups of rodents were given normal saline (con-trol), 1,2-dimethylhydrazine (dose rate used was 20 mg/kg), zingerone (dose rate 20 mg/kg) and zingerone again (dose rate 100 mg/kg). Key findings were the decreased activity of cytochrome P4502E1 and decreased ROS level with the ingestion of zingerone meaning that sup-plementation of zingerone has the capability to be used as a chemo-preventive agent. Ganaie et al [41] also discussed the potential of zingerone to regulate action of xenobiotic enzymes of Phase I cyto-chrome P4502E1 and its ability to suppressed NF-kB-p65, COX-2, iNOS and PCNA, Ki-67.
Similarly, Wang et al [42] found that the gut microbiome has an essential function in the metabolic pathway of 6-shogaol by reducing the double bond and ketone group. They showed that 6-shogaol and its metabolites interacts with the human gut microbiota and in mice microbiota through various chemical reactions in the intestines. As a result, the products of the chemicals processes adds to the anti-inflammation characteristics of ginger which are key in its anti-cancerous activities with many studies linking ginger to inhibition of free radical damage, which is the cause of many cancers, and espe-cially in its potential to control signaling molecules such as NF-κB, STAT3, MAPK, PI3K, ERK1/2, Akt, TNF-α, COX-2, cyclin D1, cdk, MMP-9, surviving, cIAP-1, XIAP and Bcl-2.
4. Enzyme inhibition
A number of enzymes are presently targeted as biomarkers in the diagnosis or treatment of cancer. Targeting cellular metabolism associ-ated enzymes linked to cancer progression is considered to offer much promise but remains a largely unexplored topic [54]⋅
4.1. Cytochrome P450
Cytochrome 450, shortened to CYP450, comprises a collection of enzymes whose main function is the oxidative catalysis of numerous endogenous and exogenous constituents. Such enzymes are involved in the phase I metabolism of 80% of commonly used drugs, including anticancer molecules, and therefore, CYP450 inhibitors are presently being investigated to improve the pharmacokinetics of several anti-cancer drugs [55].
Langhammer and Nilsen [56] found that ginger extracts obtained from commercially available ginger capsule inhibited CYP activities such as CYP1A2, CYP2D6, CYP3A4, with IC50 scores of 320, 445, and 565μg/mL, respectively. In addition, it was [57] observed that 6-, 8-, and 10-gingerol blocked the action of CYP450 activity. The three gin-gerols exhibited potent inhibition on CYP2C9 activity, modest inhibition on CYP2C19 and CYP3A4, and weakly inhibited CYP2D6. Among them, 8-gingerol showed the highest inhibition of P450 enzymes displaying IC50 values of 6.8, 12.5, 8.7, and 42.7μmol/L against CYP2C9, CYP2C19, CYP3A4, and CYP2D6, respectively. Another study by Muk-kavilli et al [58] also found that 6-, 8-, and 10-gingerol, and 6-shogaol repressed the main membrane-bound players of the CYP450 enzyme system, including CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A enzymes.
4.2. Cyclooxygenase (COX)
Cyclooxygenase enzymes (specifically COX-2) are key molecular targets for cancer intervention at both early and late stages. COX-2 is over-expressed during carcinogenesis, and seems to be involved in the start and progression of tumor [59]. 10- gingerol, 8-shogaol, and 10-sho-gaol were found to hinder COX-2 with IC50 scores of 32, 17.5 and 7.5μM, respectively [60]. In another study by Tjendraputra et al [61], they observed that 10-gingerol, 6-shogaol, and 8-paradol exhibited potent COX-2 inhibition with IC50 values of 3.7, 2.1, 3.4μM, respec-tively. In addition, 8-gingerdiol (IC50=12.5μM), 8- gingerol (IC50=10μM), and its positional isomer (IC50=15.8μM) displayed comparatively strong COX-2 inhibitory activity.
4.3. Matrix metalloproteinases
Matrix metalloproteinases (MMPs) is a family of structurally related zinc dependent endopeptidases which are key players for extracellular matrix degradation, hence, are associated with the invasion and metastasis of tumor. So far, more than 20 human MMPs have been acknowledged, among which, MMP-2 and -9 are thought to be most significant in tumor invasion due to their role in the degradation of type IV collagen. Currently, agents reducing the expression of either MMP-2 or MMP-9 have been found to effectively inhibit cancer cell invasion [62]. Interestingly, 6-shogaol was found to dose-dependently decrease MMP-9 gene activation, protein expression and secretion by blocking nuclear factor-kB activation [62]. Zingerone also displayed strong anti-angiogenic activity by inhibiting MMP-2 and MMP-9 during tumor progression [63].
Moreover, Weng et al [64] observed that treatment of HepG2 and Hep3B cells with 6-shogaol (1, 2.5, 5, and 10μM) or 6-gingerol (5, 10, 25, and 50μM), respectively, suppressed MMP-9 activity in a dose-dependent manner while the activity of MMP-2 was not signifi-cantly changed. Upon treatment of HepG2 cells for 24 h with 0–10μM 6-shogaol or 0–50μM 6-gingerol, the mRNA expression of MMP9 was nearly unchanged, except for 1 μM 6-shogaol treatment which caused a dramatic reduction in the levels of MMP-9 mRNA. On the other hand, 24 h treatment of 6-shogaol or 6-gingerol in Hep3B cells reduced the mRNA expression of MMP-9. Nonetheless, a decrease in MMP-9 mRNA level was only observed after treatment with 6-gingerol.
Additionally, it was found that 6-gingerol treatment reduced MMP-2 and MMP-9 activities in MDA-MB-231 cells [65]. Also, 6-gingerol, at levels above 5μM, was able to reduce the quantity of MMP-2 protein in the culture supernatant, but the level of MMP-9 protein remained un-changed. However, 6-gingerol reduced the mRNA expression of both MMP-2 and MMP-9.
4.4. Telemerase
Telomerase is another key target in cancer therapy. Without telo-merase, the length of telomeres of typical somatic cells are reduced at every cell division. When a telomere is shortened to a critical length at the cellular level, that cell is prompted to cellular senescence. Therefore, cellular senescence induced by telomere reduction is regarded as a tumor suppressor mechanism. However, more than 85% of all cancers are able of maintaining the length of their telomeres by reactivating telomerase to prevent cancer cells from the replicative senescence [66, 67].
Navakoon et al [66] showed that long-term incubation with sub-cytotoxic concentration of ginger extract (ethyl acetate fraction), which allowed A549 cells to multiply normally, stimulated telomere reduction which subsequently induced cellular senescence in these cells, demonstrated by an upsurge in the senescence associated β‑galactosidase positive cells and a decrease in clonogenicity.
The transcriptional regulation of telomerase reverse transcriptase in human (hTERT) also tend to be a major mechanism towards controlling
the activity of telomerase. c-Myc is one transcription factor, which, collectively with Max, binds to the E-box on the hTERT promoter, thereby activating hTERT expression [67]. Tuntiwechapikul et al [67] observed that ginger extract (ethyl acetate) can time- and concentration-dependently inhibit the activity of the two main molec-ular targets of cancer, hTERT and c-Myc, in A549 lung cancer cells. The cancer cells displayed reduced telomerase activity due to decreased protein manufacture rather than direct down regulation of telomerase. The decrease of hTERT expression tends to concur with the decrease of c-Myc activity.
4.5. Leukotriene A4 hydrolase
The proinflammatory enzyme, leukotriene A4 hydrolase (LTA4H), is involved in the biosynthesis of leukotriene B4 (LTB4), a chemo- attractant which causes an intense inflammatory reaction associated with cancer development. LTA4H is reported to be highly expressed in colorectal carcinoma.
El-Naggar et al [68] found that methyl shogaol and 4′-O-prenyl- [6]-gingerol displayed high LTA4H aminopeptidase (IC50=4.92 and 3.01μM, respectively) and epoxide hydrolase inhibitory activities (IC50=11.27 & 7.25μM, respectively). Among the tested gingerols, 10-gingerol was the most efficient inhibitor of LTA4H aminopeptidase (IC50=21.59μM) and epoxide hydrolase (IC50=15.24μM). These re-sults corroborates with the observed in vitro cytotoxic effect of ginger and isolated bioactive phytochemicals against colorectal cancer cells, as mentioned above, which indicates their potential as natural inhibitors of aminopeptidase and epoxide hydrolase for colorectal cancer.
5. Combinational therapy
The combination of two or more types of treatment to specifically target cancer-inducing or cell sustaining pathways has become a fundamental of cancer therapy. This approach can potentially act in a synergistic and/or additive way and decreases drug resistance and toxicity [69].
In the study of Brahmbhatt et al [70], numerous binary blends of ginger biophenolics were tested at several concentrations against the human prostate cancer PC-3 cells. They found that cell proliferation could be hindered by binary combinations of ginger bioactives. 83% of gingerol combinations (6-gingerol+8-gingerol, 8-gingerol+10-gingerol, 6-gingerol+10-gingerol) inhibited PC-3 proliferation in a synergistic manner. Another example is the grouping gingerol (90%) and 6-shogaol (6-shogaol+6-gingerol, 6-shogaol+8-gingerol, and 6-sho-gaol+10-gingerol) displayed synergism. Very strong synergism was exhibited by 80% of the 6-gingerol+6-shogaol combinations. Due to the ginger matrix complexity and minute amount of biophenolics, the re-searchers further investigated if increasing their individual amounts in the ginger extract could cause an enhanced antiproliferative effect. They observed that, specifically, ginger extract+6-gingerol and ginger extract+10-gingerol in combination displayed remarkable synergistic antiproliferative activity.
Furthermore, Hakim et al [71] showed that ginger extract (aqueous) causes a greater inhibitory effect on the division of HCT 116 colorectal cancer cells (IC50=3 mg/mL) as opposed to Gelam honey (IC50=75 mg/mL). Interestingly, the collective action of the two nat-ural agents (3 mg/mL ginger+75 mg/mL Gelam honey) synergistically reduced the IC50 of Gelam honey (22 mg/mL). Extract of ginger was also found to enhance the apoptosis effect of 5-fluorouracil.
Additionally, combination of γ-Tocotrienol and 6-gingerol synergis-tically induced cytotoxicity and apoptosis in HT-29 and SW837 colo-rectal cancer cells, corresponding to IC50 values of 105 and 70μg/mL, respectively. Additional benefits were a rise in apoptosis after 24 h of treatment (21.2% and 55.4% in HT-29 and SW837, respectively) with unaffected normal hepatic cells and morphological changes such as cell shrinkage and pyknosis [72]. Combination of 6-gingerol (45μM) with
paclitaxel (0.36μM) was able to reduce 83.2% growth of HeLa cells, which was more effective compared to 6- gingerol (45μM) with 5-FU (22.5μM) (52% inhibition) or 6-gingerol treatment alone (10.75% in-hibition) [23]. In addition, combined treatment of zingerone and its novel derivative synergistically suppressed hepatocellular carcinoma metastasis by the inhibition of the TGF-β1 induced epithelial-mesenchymal transition and suppressed migration and inva-sion of hepatocellular [73].
Rahman et al [74] studied the anticancer properties of a number of compounds including 6-gingerol, epigallocatechin gallate (EGCG), asi-aticoside (AS) and vitamin E (tocotrienol-rich fraction (TRF)). EGCG + 6-gingerol synergistically induced apoptosis and inhibited the growth of 1321N1 and LN18 glioma cancer cells. On the other hand, all tested combinations (TRF + 6-gingerol, TRF + EGCG and EGCG + 6-gingerol) were reported to be antagonistic on SW1783. Moreover, Kotowski et al [75] found that although 6-shogaol combined with cisplatin displayed an antagonistic effect, when coupled with irradiation exhibited a syn-ergistic decrease of clonogenic survival in head and neck squamous cell carcinoma cell lines.
Moreover, gingerol synergised cytotoxicity of doxorubicin on liver cancer cells without influencing the cellular pharmacokinetics. Also, gingerol decreased the IC50 of doxorubicin against HepG2 and Huh7 liver by 10- and 4-fold, respectively. On top of that, treatment with only doxorubicin induced cell growth at S-phase and G2/M-phase, while its blending with gingerol arrested cell cycle at the G2/M-phase. In addi-tion, co-incubation with 6-gingerol (30 μM) caused a complete blockage of the exaggerated vasoconstriction and impaired vascular relaxation caused by doxorubicin [76]. In addition, ElAshmawy et al [77] reported that co-treatment of ginger extract (100 mg/kg orally day after day) alongside doxorubicin (4 mg/kg i.p. for 4 cycles every 5 days), admin-istered to mice starting on the 12th day of inoculation of Ehrlich ascites carcinoma cells, markedly amplified survival rate, reduced tumor vol-ume, raised the level of phosphorylated AMPK (PAMPK), and improved related pathways in mice compared to doxorubicin group. Moreover, histopathological findings showed improved apoptosis and absence of multinucleated cells in tumor tissue of the ginger extract + doxorubicin group.
A number of clinical trials also proved that supplementation of ginger helps to decrease the severity of acute chemotherapy-induced nausea in cancer patients [78–81]. Nausea and vomiting are among the most common and troubling adverse effects associated with chemotherapy [79]. Nevertheless, one of the likely side effect of radio-therapy to the neck and head area is xerostomia, which is a subjective feeling of dry mouth accompanied by decreased salivation. Shooriabi et al [82] observed that ginger can improve the xerostomia symptoms by increasing the rate of salivary secretion in patients undergoing radio-therapy. Nevertheless, Chamani et al [83], reported that ginger decreased the severity of dry mouth in patients with post-radiation xerostomia but did not improve dry mouth symptoms or the patients’ quality of life.
6. Nanotherapeutic application
The emerging field of nanomedicine has the ability to change the way cancer therapy is being provided [84,85]. By targeting the vascular tissue and cellular characteristics exclusive to cancer cells [86], nano-medicine ensures that the drugs are delivered at the right sites, therefore making drug delivery and absorption more successful [87,88]. Even though the potential of nanotechnology in the treatment/management of cancer is a recent and novel development [89,90], significant research has been done in the area in order to optimize its mechanism [91], pharmacokinetics [92] and delivery [93] as well as the searches for new nano-composites such as graphene [94,95] or hybrid polymer-metal composites for drug delivery [96]. Nanomedicine reduces the chances of developing cancer cells that are resistant [97], and more importantly, it may allow for more than one type of cancer to be targeted during one
treatment [98].
The benefits and therapeutic applications of liposomal based drug delivery have been reviewed [99,100] and while a vast number of ap-plications are in colon cancer, its significance in oral cancer therapy is also discussed [101]. In particular, the work carried out by Khalili et al [102] found that PEGylated nanoliposomal formulation of gingerol increased its cytotoxic effect against breast cancer MCF-7 cell at a reduced concentration, allowing a slower drug release, when compared with liposomal and standard gingerol. PEGylation allowed a controlled drug release and protect the liposome against the immune system and lipase enzymes which cause lipid solvation, thereby enabling a longer blood circulation system and enough time to reach the target tumor cell. Similarly, Behroozeh et al [103] observed that PEGylated nanoniosomal gingerol showed higher cytotoxicity on T47D breast cancer cell than the standard drug. More than 76% of the gingerol drug were enclosed in the PEGylated nanoniosomal formulation and the gingerol release from the nanoparticles consisted of an instant and fast release phase at the start and then a slower release phase. Applications have also been carried out in the treatment of lung cancer [104].
6-shogaol enriched liposomes in a DMPG-Na carrier also showed enhanced in vitro and in vivo anticancer action. The release of 6-shogaol from the liposomes was slower than that from ginger oleoresin at all the tested pH ranges, which showed reduced outcome on the dissolution of 6-shogaol from the inner core compartment of the liposomes. In addi-tion, the 6-shogaol rich ginger oleoresin loaded liposomes showed better cytotoxic effect than the 6-shogaol rich ginger oleoresin and the blank liposomes, which can be justified to a greater cellular uptake of lipo-somes through phagocytosis or the fusion process of lipid lipolipo-somes. Moreover, the 6-shogaol rich ginger oleoresin loaded liposomes, (100, 200 and 400 mg/kg body weight) caused an increase in life span in mice with Dalton’s Ascitic Lymphoma up to 91.5%, 92.5% and 93.5%, respectively, as opposed to the batch challenged with only the 6-shogaol rich ginger oleoresin (78%, 81% and 84%, respectively) [105].
Additionally, Zhang et al [106] showed the specificity of nano-particles, functionalized with ginger bioactives, to colon cancer cells and the stability and non-toxicity of the ingested nanoparticles in solutions similar to the stomach and intestine matrices and conditions. Mouse colitis models showed that the nanoparticles were mainly absorbed in the intestinal epithelial cells and macrophages and were effective in preventing colitis associated cancer. Both tumor numbers per mouse and tumor loads were significantly decreased by the ginger derived nano-particles treatment of azoxymethane/dextran sodium sulfate colitis-associated cancer-induced mice. In addition, the level of pro-inflammatory cytokine and cyclin D1 mRNA were also decreased, suggesting that a reduction in cell proliferation during colitis-associated cancer development.
Zhang et al [107] further reconstructed the lipid groups of ginger functionalized nanoparticles to ginger-derived nanovectors (GDNVs) to behave as a delivery platform for doxorubicin in the treatment of colon cancer. Comparable to the previously functionalized ginger nano-particles, the GDNVs were proven to be non-toxic and absorbed by cancer cells in the large intestines and more importantly the GDNVs have the capacity to encapsulate doxorubicin (95.9% at a ginger lipid concentration of 100μmol/L), and displayed a better pH-dependent drug-release profile compared to commercially available liposomal-doxorubicin. On top of that, the doxorubicin-GDNVs were very stable at 4◦C (up to 25 days), retained their ability to load doxo-rubicin, and exhibited a time-dependent release profile which decreased the release of doxorubicin from GDNVs and resulted to a sustained cytotoxic effect against colon cancer cells.
The researchers then generated nanoparticles with a high affinity to Colon-26 tumors in vivo by modifying the ginger-derived nanoparticles to incorporate folic acid, a molecule showing high affinity for folate receptors. These receptors are highly expressed on many types of tu-mors, while being almost undetectable on healthy cells. The authors demonstrated that the folic acid nanovectors efficiently loaded and
delivered doxorubicin into cancer cells and inhibited tumor growth. 7. Conclusion
The current review showcases the cytotoxicity propensities of ginger and its main bioactive compounds to a number of cancer types with particular attention on breast, cervical, colorectal, leukemia, liver, lung, nasopharyngeal, ovarian, prostate, and retinoblastoma types. Further evidence is presented to show that apoptotic mechanisms are dependent on cancer type. Ginger and its active compounds also exerted inhibitory effect against key enzymes linked to cancer progression including cy-tochrome 450, cyclooxygenase-2, matrix metalloproteinase-2 and -9, telomerase, and leukotriene A4 hydrolase. In addition, an enhancement in the efficacy of chemotherapeutic drugs and reduction of the side ef-fects of radiotherapy was exhibited by ginger. More interestingly how-ever, future work remains to be done to provide a comprehensive understanding of the interactions of the bioactive phytochemicals in ginger in the human body system through in-vivo preclinical trials. Also, the application of nanotechnology seems to be a better delivery system for ginger compounds. Nonetheless, further pharmacodynamic and pharmacokinetic studies to determine the molecular pathway exhibited by these nano-formulations and their bioavailability are needed. Last but not least, since ginger is traditionally used against cancer, there is a need for more human clinical trials to validate the exact dose and mode of administration as practiced traditionally to establish its efficacy and observe any possible adverse reactions.
Funding support
No specific funding was disclosed. Declaration of Competing Interest
The authors made no disclosures. References
[1] L.-Q. Peng, J. Cao, L.-J. Du, Q.-D. Zhang, J.-J. Xu, Y.-B. Chen, Y.-T. Shi, R.-R. Li, Rapid ultrasonic and microwave-assisted micellar extraction of zingiberone, shogaol and gingerols from gingers using biosurfactants, J. Chromatogr. A 1515 (2017) 37–44.
[2] J. Prakash, Chemical composition and antioxidant properties of ginger root (Zingiber officinale), J. Med. Plants Res. 4 (24) (2010) 2674–2679.
[3] D. Pan, C. Zeng, W. Zhang, T. Li, Z. Qin, X. Yao, Y. Dai, Z. Yao, Y. Yu, X. Yao, Non- volatile pungent compounds isolated from Zingiber officinale and their mechanisms of action, Food Funct. 10 (2) (2019) 1203–1211. [4] B.H. Ali, G. Blunden, M.O. Tanira, A. Nemmar, Some phytochemical,
pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research, Food Chem. Toxicol. 46 (2) (2008) 409–420.
[5] Z. Li, Y. Wang, M. Gao, W. Cui, M. Zeng, Y. Cheng, J. Li, Nine new gingerols from the rhizoma of Zingiber officinale and their cytotoxic activities, Molecules 23 (2) (2018) 315.
[6] Y. Liu, R.J. Whelan, B.R. Pattnaik, K. Ludwig, E. Subudhi, H. Rowland, N. Claussen, N. Zucker, S. Uppal, D.M. Kushner, Terpenoids from Zingiber officinale (Ginger) induce apoptosis in endometrial cancer cells through the activation of p53, PLoS One 7 (12) (2012), e53178.
[7] M.-J. Ko, H.-H. Nam, M.-S. Chung, Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction, Food Chem. 270 (2019) 149–155.
[8] R.B. Semwal, D.K. Semwal, S. Combrinck, A.M. Viljoen, Gingerols and shogaols: important nutraceutical principles from ginger, Phytochemistry 117 (2015) 554–568.
[9] J.-P. Ma, X.-L. Jin, L. Yang, Z.-L. Liu, Two new diarylheptanoids from the rhizomes of Zingiber officinale, Chin. Chem. Lett. 15 (11) (2004) 1306–1308. [10] R. Silambarasan, M. Ayyanar, An ethnobotanical study of medicinal plants in
Palamalai region of Eastern Ghats, India, J. Ethnopharmacol. 172 (2015) 162–178.
[11] R. Silambarasan, J. Sureshkumar, J. Krupa, S. Amalraj, M. Ayyanar, Traditional herbal medicines practiced by the ethnic people in Sathyamangalam forests of Western Ghats, India, Eur. J. Integr. Med. 16 (2017) 61–72.
[12] Y.-Y. Siew, S. Zareisedehizadeh, W.-G. Seetoh, S.-Y. Neo, C.-H. Tan, H.-L. Koh, Ethnobotanical survey of usage of fresh medicinal plants in Singapore, J. Ethnopharmacol. 155 (3) (2014) 1450–1466.
[13] N.A. Jaradat, R. Shawahna, A.M. Eid, R. Al-Ramahi, M.K. Asma, A.N. Zaid, Herbal remedies use by breast cancer patients in the West Bank of Palestine, J. Ethnopharmacol. 178 (2016) 1–8.
[14] M.S. Ali-Shtayeh, R.M. Jamous, N.M. Salameh, R.M. Jamous, A.M. Hamadeh, Complementary and alternative medicine use among cancer patients in Palestine with special reference to safety-related concerns, J. Ethnopharmacol. 187 (2016) 104–122.
[15] F. Kabbaj, B. Meddah, Y. Cherrah, E. Faouzi, Ethnopharmacological profile of traditional plants used in Morocco by cancer patients as herbal therapeutics, Phytopharmacology 2 (2) (2012) 243–256.
[16] C. Agyare, V. Spiegler, A. Asase, M. Scholz, G. Hempel, A. Hensel, An ethnopharmacological survey of medicinal plants traditionally used for cancer treatment in the Ashanti region, ghana, J. Ethnopharmacol. 212 (2018) 137–152. [17] J.A. Ansari, M.K. Ahmad, A.R. Khan, N. Fatima, H.J. Khan, N. Rastogi, D.
P. Mishra, A.A. Mahdi, Anticancer and antioxidant activity of Zingiber officinale Roscoe rhizome, Indian J. Exp. Biol. 54 (2016) 767–773.
[18] B.S. Tan, O. Kang, C.W. Mai, K.H. Tiong, A.S.-B. Khoo, M.R. Pichika, T. D. Bradshaw, C.-O. Leong, 6-Shogaol inhibits breast and colon cancer cell proliferation through activation of peroxisomal proliferator activated receptor γ (PPARγ), Cancer Lett. 336 (1) (2013) 127–139.
[19] A.C.B. Martin, A.M. Fuzer, A.B. Becceneri, J.A. da Silva, R. Tomasin, D. Denoyer, S.-H. Kim, K.A. McIntyre, H.B. Pearson, B. Yeo, [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo, Oncotarget 8 (42) (2017) 72260.
[20] Q. Liu, Y.-B. Peng, L.-W. Qi, X.-L. Cheng, X.-J. Xu, L.-L. Liu, E.-H. Liu, P. Li, The cytotoxicity mechanism of 6-Shogaol-treated HeLa human cervical cancer cells revealed by label-free shotgun proteomics and bioinformatics analysis, Evid. Based Complement. Altern. Med. 2012 (2012).
[21] Y. Lee, Cytotoxicity evaluation of essential oil and its component from Zingiber officinale Roscoe, Toxicol. Res. 32 (3) (2016) 225–230.
[22] F. Peng, Q. Tao, X. Wu, H. Dou, S. Spencer, C. Mang, L. Xu, L. Sun, Y. Zhao, H. Li, Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger, Fitoterapia 83 (3) (2012) 568–585.
[23] F. Zhang, J.-G. Zhang, J. Qu, Q. Zhang, C. Prasad, Z.-J. Wei, Assessment of anti- cancerous potential of 6-gingerol (Tongling White Ginger) and its synergy with drugs on human cervical adenocarcinoma cells, Food Chem. Toxicol. 109 (2017) 910–922.
[24] A. Saha, J. Blando, E. Silver, L. Beltran, J. Sessler, J. DiGiovanni, 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling, Cancer Prev. Res. 7 (6) (2014) 627–638.
[25] H.-W. Kim, D.-H. Oh, C. Jung, D.-D. Kwon, Y.-C. Lim, Apoptotic effects of 6- gingerol in LNCaP human prostate cancer cells, Soonchunhyang Med. Sci. 17 (2) (2011) 75–79.
[26] L. Lv, H. Chen, D. Soroka, X. Chen, T. Leung, S. Sang, 6-Gingerdiols as the major metabolites of 6-gingerol in cancer cells and in mice and their cytotoxic effects on human cancer cells, J. Agric. Food Chem. 60 (45) (2012) 11372–11377. [27] J.-Y. Hung, Y.-L. Hsu, C.-T. Li, Y.-C. Ko, W.-C. Ni, M.-S. Huang, P.-L. Kuo, 6-
Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells, J. Agric. Food Chem. 57 (20) (2009) 9809–9816.
[28] S.H.M. Habib, S. Makpol, N.A.A. Hamid, S. Das, W.Z.W. Ngah, Y.A.M. Yusof, Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats, Clinics 63 (6) (2008) 807–813.
[29] P. Santos, G. Avanço, S. Nerilo, R. Marcelino, V. Janeiro, M. Valadares, M. Machinski, Assessment of cytotoxic activity of rosemary (Rosmarinus officinalis L.), turmeric (Curcuma longa L.), and ginger (Zingiber officinale R.) essential oils in cervical cancer cells (HeLa), Sci. World J. 2016 (2016). [30] Q. Liu, Y.-B. Peng, P. Zhou, L.-W. Qi, M. Zhang, N. Gao, E.-H. Liu, P. Li, 6-Shogaol
induces apoptosis in human leukemia cells through a process involving caspase- mediated cleavage of eIF2α, Mol. Cancer 12 (1) (2013) 135.
[31] S.H. Lee, M. Cekanova, S.J. Baek, Multiple mechanisms are involved in 6- gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells, Mol. Carcinog. 47 (3) (2008) 197–208.
[32] E. Radhakrishnan, S.V. Bava, S.S. Narayanan, L.R. Nath, A.K.T. Thulasidasan, E. V. Soniya, R.J. Anto, [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP- 1 signaling, PLoS One 9 (8) (2014), e104401.
[33] L.-W. Qi, Z. Zhang, C.-F. Zhang, S. Anderson, Q. Liu, C.-S. Yuan, C.-Z. Wang, Anti- colon cancer effects of 6-shogaol through G2/M cell cycle arrest by p53/p21- cdc2/cdc25A crosstalk, Am. J. Chin. Med. 43 (04) (2015) 743–756. [34] Y. Zhu, R.F. Warin, D.N. Soroka, H. Chen, S. Sang, Metabolites of ginger
component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: chemical synthesis and biological evaluation, PLoS One 8 (1) (2013), e54677.
[35] L. Gentschew, L.R. Ferguson, Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases, Mol. Nutr. Food Res. 56 (4) (2012) 524–535. [36] M. Levy, E. Blacher, E. Elinav, Microbiome, metabolites and host immunity, Curr.
Opin. Microbiol. 35 (2017) 8–15.
[37] Y.K. Lee, S.K. Mazmanian, Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 330 (6012) (2010) 1768–1773.
[38] K. Singletary, Ginger: an overview of health benefits, Nutr. Today 45 (4) (2010) 171–183.
[39] P.J. Turnbaugh, R.E. Ley, M. Hamady, C.M. Fraser-Liggett, R. Knight, J.I. Gordon, The human microbiome project, Nature 449 (7164) (2007) 804.
[40] S. Prasad, A.K. Tyagi, Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer, Gastroenterol. Res. Pract. 2015 (2015) 11. [41] M.A. Ganaie, A. Al Saeedan, H. Madhkali, B.L. Jan, T. Khatlani, I.A. Sheikh, M.
U. Rehman, K. Wani, Chemopreventive efficacy zingerone (4-[4-hydroxy-3- methylphenyl] butan-2-one) in experimental colon carcinogenesis in Wistar rats, Environ. Toxicol. 34 (2019) 610–625.
[42] C.-Z. Wang, L.-W. Qi, C.-S. Yuan, Cancer chemoprevention effects of ginger and its active constituents: potential for new drug discovery, Am. J. Chin. Med. 43 (07) (2015) 1351–1363.
[43] G.H. Park, J.H. Park, H.M. Song, H.J. Eo, M.K. Kim, J.W. Lee, M.H. Lee, K.- H. Cho, J.R. Lee, H.J. Cho, Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells, BMC Complement. Altern. Med. 14 (1) (2014) 408.
[44] I. Karaboz, Antimicrobial and cytotoxic activities of Zingiber officinalis extracts, FABAD J. Pharm. Sci 33 (2010) 76–85.
[45] M. Kumara, M. Shylajab, P. Nazeemc, T. Babu, 6-Gingerol is the most potent anticancerous compound in ginger (Zingiber officinale Rosc.), J. Dev. Drugs 6 (2017) 6–11.
[46] S. Abdullah, S.A.Z. Abidin, N.A. Murad, S. Makpol, W.Z.W. Ngah, Y.A.M. Yusof, Ginger extract (Zingiber officinale) triggers apoptosis and G0/G1 cells arrest in HCT 116 and HT 29 colon cancer cell lines, Afr. J. Biochem. Res. 4 (5) (2010) 134–142.
[47] C.-Y. Chen, Y.-W. Li, S.-Y. Kuo, Effect of [10]-gingerol on [ca2+] i and cell death in human colorectal cancer cells, Molecules 14 (3) (2009) 959–969.
[48] M.A. Al-Tamimi, B. Rastall, I.M. Abu-Reidah, Chemical composition, cytotoxic, apoptotic and antioxidant activities of main commercial essential oils in Palestine: a comparative study, Medicines 3 (4) (2016) 27.
[49] K. Jeena, V.B. Liju, R. Kuttan, Antitumor and cytotoxic activity of ginger essential oil (Zingiber officinale Roscoe), Int. J. Pharm. Pharm. Sci. 7 (8) (2015) 341–344. [50] R. Pashaei-Asl, F. Pashaei-Asl, P.M. Gharabaghi, K. Khodadadi, M. Ebrahimi,
E. Ebrahimie, M. Pashaiasl, The inhibitory effect of ginger extract on Ovarian cancer cell line; application of systems biology, Adv. Pharm. Bull. 7 (2) (2017) 241.
[51] H. Rashid, G. Umamaheswari, Evaluation of the cytotoxic effect of ginger extract against prostate Cancer model using in vitro, World J. Pharm. Pharm. Sci. 6 (2017) 1044–1053.
[52] P. Karna, S. Chagani, S.R. Gundala, P.C. Rida, G. Asif, V. Sharma, M.V. Gupta, R. Aneja, Benefits of whole ginger extract in prostate cancer, Br. J. Nutr. 107 (4) (2012) 473–484.
[53] B. Meng, H. Ii, W. Qu, H. Yuan, Anticancer effects of gingerol in retinoblastoma Cancer cells (RB355 cell line) are mediated via apoptosis induction, cell cycle arrest and upregulation of PI3K/Akt signaling pathway, Med. Sci. Monit. 24 (2018) 1980.
[54] M.H. Baig, M. Adil, R. Khan, S. Dhadi, K. Ahmad, G. Rabbani, T. Bashir, M. A. Imran, F.M. Husain, E.J. Lee, Enzyme Targeting Strategies for Prevention and Treatment of Cancer: Implications for Cancer Therapy, Seminars in Cancer Biology, Elsevier, 2017.
[55] B. Mittal, S. Tulsyan, S. Kumar, R.D. Mittal, G. Agarwal, Cytochrome P450 in Cancer Susceptibility and Treatment, Advances in Clinical Chemistry, Elsevier, 2015, pp. 77–139.
[56] A.J. Langhammer, O.G. Nilsen, In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy, Phytother. Res. 28 (4) (2014) 603–610.
[57] M. Li, P.-z. Chen, Q.-x. Yue, J.-q. Li, R.-a. Chu, W. Zhang, H. Wang, Pungent ginger components modulates human cytochrome P450 enzymes in vitro, Acta Pharmacol. Sin. 34 (9) (2013) 1237.
[58] R. Mukkavilli, S.R. Gundala, C. Yang, S. Donthamsetty, G. Cantuaria, G. R. Jadhav, S. Vangala, M.D. Reid, R. Aneja, Modulation of cytochrome P450 metabolism and transport across intestinal epithelial barrier by ginger biophenolics, PLoS One 9 (9) (2014), e108386.
[59] W.F. Anderson, A. Umar, E.T. Hawk, Cyclooxygenase inhibition in cancer prevention and treatment, Expert Opin. Pharmacother. 4 (12) (2003) 2193–2204. [60] R.B. van Breemen, Y. Tao, W. Li, Cyclooxygenase-2 inhibitors in ginger (Zingiber
officinale), Fitoterapia 82 (1) (2011) 38–43.
[61] E. Tjendraputra, V.H. Tran, D. Liu-Brennan, B.D. Roufogalis, C.C. Duke, Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells, Bioorg. Chem. 29 (3) (2001) 156–163.
[62] H. Ling, H. Yang, S.H. Tan, W.K. Chui, E.H. Chew, 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-kappaB activation, Br. J. Pharmacol. 161 (8) (2010) 1763–1777.
[63] W.-Y. Bae, J.-S. Choi, J.-E. Kim, C. Park, J.-W. Jeong, Zingerone suppresses angiogenesis via inhibition of matrix metalloproteinases during tumor development, Oncotarget 7 (30) (2016) 47232–47241.
[64] C.J. Weng, C.F. Wu, H.W. Huang, C.T. Ho, G.C. Yen, Anti-invasion effects of 6- shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells, Mol. Nutr. Food Res. 54 (11) (2010) 1618–1627. [65] H.S. Lee, E.Y. Seo, N.E. Kang, W.K. Kim, [6]-Gingerol inhibits metastasis of MDA-
MB-231 human breast cancer cells, J. Nutr. Biochem. 19 (5) (2008) 313–319. [66] N. Kaewtunjai, W. Pompimon, W. Tuntiwechapikul, Ginger (Zingiber officinale)
extract promotes telomere shortening and induces cellular senescence in A549 lung Cancer cells, Planta Med. Int. Open 4 (S 01) (2017). Mo-PO-12. [67] W. Tuntiwechapikul, T. Taka, C. Songsomboon, N. Kaewtunjai, A. Imsumran,
L. Makonkawkeyoon, W. Pompimon, T.R. Lee, Ginger extract inhibits human telomerase reverse transcriptase and c-Myc expression in A549 lung cancer cells, J. Med. Food 13 (6) (2010) 1347–1354.
[68] M.H. El-Naggar, A. Mira, F.M.A. Bar, K. Shimizu, M.M. Amer, F.A. Badria, Synthesis, docking, cytotoxicity, and LTA4H inhibitory activity of new gingerol derivatives as potential colorectal cancer therapy, Bioorg. Med. Chem. 25 (3) (2017) 1277–1285.
[69] R.B. Mokhtari, T.S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das, H. Yeger, Combination therapy in combating cancer, Oncotarget 8 (23) (2017) 38022.
[70] M. Brahmbhatt, S.R. Gundala, G. Asif, S.A. Shamsi, R. Aneja, Ginger
phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation, Nutr. Cancer 65 (2) (2013) 263–272.
[71] L. Hakim, E. Alias, S. Makpol, W.Z.W. Ngah, N.A. Morad, Y. Yusof, Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT 116 colorectal cancer cells, Asian Pac. J. Cancer Prev. 15 (11) (2014) 4651–4657.
[72] M. Yusof, S. Makpol, R. Jamal, R. Harun, N. Mokhtar, W. Wan Ngah, γ-Tocotrienol and 6-Gingerol in combination synergistically induce cytotoxicity and apoptosis in HT-29 and SW837 human colorectal cancer cells, Molecules 20 (6) (2015) 10280–10297.
[73] Y.-J. Kim, Y. Jeon, T. Kim, W.-C. Lim, J. Ham, Y.N. Park, T.-J. Kim, H. Ko, Combined treatment with zingerone and its novel derivative synergistically inhibits TGF-β1 induced epithelial-mesenchymal transition, migration and invasion of human hepatocellular carcinoma cells, Bioorg. Med. Chem. Lett. 27 (4) (2017) 1081–1088.
[74] A. Rahman, S. Makpol, R. Jamal, R. Harun, N. Mokhtar, W. Ngah, Tocotrienol- rich fraction,[6]-gingerol and epigallocatechin gallate inhibit proliferation and induce apoptosis of glioma cancer cells, Molecules 19 (9) (2014) 14528–14541. [75] U. Kotowski, L. Kadletz, S. Schneider, E. Foki, R. Schmid, R. Seemann,
D. Thurnher, G. Heiduschka, 6-shogaol induces apoptosis and enhances radiosensitivity in head and neck squamous cell carcinoma cell lines, Phytother. Res. 32 (2) (2018) 340–347.
[76] F. Al-Abbasi, E. Alghamdi, M. Baghdadi, A. Alamoudi, A. El-Halawany, H. El- Bassossy, A. Aseeri, A. Al-Abd, Gingerol synergizes the cytotoxic effects of doxorubicin against liver cancer cells and protects from its vascular toxicity, Molecules 21 (7) (2016) 886.
[77] N.E. El-Ashmawy, N.F. Khedr, H.A. El-Bahrawy, H.E.A. Mansour, Ginger extract adjuvant to doxorubicin in mammary carcinoma: study of some molecular mechanisms, Eur. J. Nutr. 57 (3) (2018) 981–989.
[78] W. Marx, A.L. McCarthy, K. Ried, L. Vitetta, D. McKavanagh, D. Thomson, A. Sali, L. Isenring, Can ginger ameliorate chemotherapy-induced nausea? Protocol of a randomized double blind, placebo-controlled trial, BMC Complement. Altern. Med. 14 (1) (2014) 134.
[79] Y. Panahi, A. Saadat, A. Sahebkar, F. Hashemian, M. Taghikhani, E. Abolhasani, Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: a pilot, randomized, open-label clinical trial, Integr. Cancer Ther. 11 (3) (2012) 204–211.
[80] J.L. Ryan, C.E. Heckler, J.A. Roscoe, S.R. Dakhil, J. Kirshner, P.J. Flynn, J. T. Hickok, G.R. Morrow, Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients, Support. Care Cancer 20 (7) (2012) 1479–1489.
[81] F. Sanaati, S. Najafi, Z. Kashaninia, M. Sadeghi, Effect of ginger and chamomile on nausea and vomiting caused by chemotherapy in iranian women with breast cancer, Asian Pac. J. Cancer Prev. 17 (8) (2016) 4125–4129.
[82] M. Shooriabi, D. Ardakani, B. Mansoori, S. Satvati, R. Sharifi, The effect of ginger extract on radiotherapy-oriented salivation in patients with xerostomia: a double- blind controlled study, Pharm. Lett. 8 (15) (2016) 37–45.
[83] G. Chamani, M.R. Zarei, M. Mehrabani, N. Nakhaee, B. Kalaghchi, M. Aghili, A. Alaee, Assessment of systemic effects of ginger on salivation in patients with post-radiotherapy xerostomia, J. Oral Health Oral Epidemiol. 6 (3) (2017) 130–137.
[84] V. Kumar, P.C. Bhatt, M. Rahman, G. Kaithwas, H. Choudhry, F.A. Al-Abbasi, F. Anwar, A. Verma, Fabrication, optimization, and characterization of umbelliferone β-D-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: in vitro and in vivo studies, Int. J. Nanomed. 12 (2017) 6747–6758.
[85] M. Rahman, M.Z. Ahmad, I. Kazmi, S. Akhter, Y. Kumar, F.J. Ahmad, F. Anwar, Novel approach for the treatment of Cancer: theranostic nanomedicine, Pharmacologia 3 (2012) 371–376.
[86] A. Sohail, A. Mohammad Zaki, S. Anjali, A. Iqbal, R. Mahfoozur, A. Mohammad, J. Gaurav Kumar, A. Farhan Jalees, K. Roop Krishen, Cancer targeted metallic nanoparticle: targeting overview, recent advancement and toxicity concern, Curr. Pharm. Des. 17 (18) (2011) 1834–1850.
[87] M. Rahman, S. Beg, A. Ahmed, S. Swain, Emergence of functionalized nanomedicines in Cancer chemotherapy: recent advancements, current challenges and toxicity considerations, Recent Pat. Nanomed. 3 (2) (2013) 128–139.
[88] A. Preeti, R. Mahfoozur, B. Sarwar, A. Shivali, D. Vishal, C. Rupali, Cancer targeted magic bullets for effective treatment of Cancer, Recent Pat. Antiinfect. Drug Discov. 9 (2) (2014) 121–135.
[89] M.Z. Ahmad, S. Akhter, G.K. Jain, M. Rahman, S.A. Pathan, F.J. Ahmad, R. K. Khar, Metallic nanoparticles: technology overview & drug delivery applications in oncology, Expert Opin. Drug Deliv. 7 (8) (2010) 927–942. [90] M. Rahman, M.Z. Ahmad, I. Kazmi, S. Akhter, M. Afzal, G. Gupta, F. Jalees
Ahmed, F. Anwar, Advancement in multifunctional nanoparticles for the effective treatment of cancer, Expert Opin. Drug Deliv. 9 (4) (2012) 367–381. [91] M.Z. Ahmad, S. Akhter, M. Anwar, A. Kumar, M. Rahman, A.H. Talasaz, F.
microspheres: a mechanistic, pharmacokinetic and biochemical investigation, Drug Dev. Ind. Pharm. 39 (12) (2013) 1936–1943.
[92] P. Pandey, M. Rahman, P.C. Bhatt, S. Beg, B. Paul, A. Hafeez, F.A. Al-Abbasi, M. S. Nadeem, O. Baothman, F. Anwar, V. Kumar, Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin, Nanomed. Lond. (Lond) 13 (8) (2018) 849–870.
[93] A. Mohammad Zaki, A. Sohail, A. Iqbal, R. Mahfoozur, A. Mohammad, K. J. Gourav, J.A. Farhan, K. Roop Krishen, Development of polysaccharide based Colon Targeted drug delivery system: design and evaluation of Assam Bora rice starch based matrix tablet, Curr. Drug Deliv. 8 (5) (2011) 575–581.
[94] R. Mahfoozur, A. Mohammad Zaki, A. Javed, F. Jamia, A. Farhan Jalees, M. Gohar, A.K. Mohammad, A. Sohail, Role of graphene nano-composites in Cancer therapy: theranostic applications, metabolic fate and toxicity issues, Curr. Drug Metab. 16 (5) (2015) 397–409.
[95] M. Rahman, S. Akhter, M.Z. Ahmad, J. Ahmad, R.T. Addo, F.J. Ahmad, C. Pichon, Emerging advances in cancer nanotheranostics with graphene nanocomposites: opportunities and challenges, Nanomedicine (Lond) 10 (15) (2015) 2405–2422. [96] S. Beg, M. Rahman, A. Jain, S. Saini, P. Midoux, C. Pichon, F.J. Ahmad, S. Akhter, Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications, Drug Discov. Today 22 (4) (2017) 625–637.
[97] R. Mahfoozur, A. Mohammad Zaki, K. Imran, A. Sohail, A. Muhammad, G. Gaurav, S. Vivek Ranjan, Emergence of nanomedicine as Cancer Targeted magic bullets: recent development and need to address the toxicity apprehension, Curr. Drug Discov. Technol. 9 (4) (2012) 319–329.
[98] J. Kydd, R. Jadia, P. Velpurisiva, A. Gad, S. Paliwal, P. Rai, Targeting strategies for the combination treatment of cancer using drug delivery systems, Pharmaceutics 9 (4) (2017) 46.
[99] Mahfoozur Rahman, B. Sarwar, V. Amita, K. Imran, P. Dinesh Kumar, A. Firoz, A. A.A. Fahad, Vikas Kumar, Therapeutic applications of liposomal based drug
delivery and drug targeting for immune linked inflammatory maladies: a contemporary view Point, Curr. Drug Targets 18 (13) (2017) 1558–1571. [100] M. Rahman, V. Kumar, S. Beg, G. Sharma, O.P. Katare, F. Anwar, Emergence of
liposome as targeted magic bullet for inflammatory disorders: current state of the art, Artificial Cells, Nanomed. Biotechnol. 44 (7) (2016) 1597–1608. [101] A. Javed, A. Saima, R. Mahfoozur, R. Rehan Abdur, S. Madhur, A. Mohammad
Zaki, R. Ziyaur, T.A. Richard, A. Farhan Jalees, M. Gohar, K. Mohammad Amjad, A. Sohail, Solid matrix based lipidic nanoparticles in oral Cancer chemotherapy: applications and pharmacokinetics, Curr. Drug Metab. 16 (8) (2015) 633–644. [102] M. Khalili, A. Akbarzadeh, M. Chiani, S. Torabi, The effect of nanoliposomal and
PE gylated nanoliposomal forms of 6-gingerol on breast cancer cells, Res. J. Recent Sci. 2 (2013) 29–33.
[103] A. Behroozeh, M.M. Tabrizi, S.M. Kazemi, E. Choupani, N. Kabiri, D. Ilbeigi, A. H. Nasab, A.A. Khiyavi, A.S. Kurdi, Evaluation the anti-cancer effect of PEGylated nano-niosomal gingerol, on breast cancer cell lines (T47D), in-Vitro, Asian Pac. J. Cancer Prevent. APJCP 19 (3) (2018) 645.
[104] J. Ahmad, S. Akhter, M. Rizwanullah, S. Amin, M. Rahman, M.Z. Ahmad, M. A. Rizvi, M.A. Kamal, F.J. Ahmad, Nanotechnology-based inhalation treatments for lung cancer: state of the art, Nanotechnol. Sci. Appl. 8 (2015) 55–66. [105] K. Kemkar, S. Lohidasan, A. Sathiyanarayanan, K. Mahadik, 6-shogaol from
ginger oleoresin loaded liposomes using DMPG-Na as a carrier enhances the in- vitro and in-vivo anticancer activity, J. Appl. Pharm. Sci. 8 (2018) 1–10. [106] M. Zhang, E. Viennois, M. Prasad, Y. Zhang, L. Wang, Z. Zhang, M.K. Han, B. Xiao,
C. Xu, S. Srinivasan, Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer, Biomaterials 101 (2016) 321–340.
[107] M. Zhang, B. Xiao, H. Wang, M.K. Han, Z. Zhang, E. Viennois, C. Xu, D. Merlin, Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug- delivery approach for colon cancer therapy, Mol. Ther. 24 (10) (2016) 1783–1796.