Contents lists available atScienceDirect
Microchemical Journal
journal homepage:www.elsevier.com/locate/microc
A colorimetric and fluorometric probe for hydrazine through subsequent
ring-opening and closing reactions: Its environmental applications
Serkan Erdemir
a,⁎, Sait Malkondu
baDepartment of Chemistry, Science Faculty, Selcuk University, Konya 42031, Turkey
bDepartment of Environmental Engineering, Faculty of Engineering, Giresun University, Giresun 28200, Turkey
A R T I C L E I N F O Keywords: Optical sensing Fluorescence Hydrazine Detection ICT A B S T R A C T
Hydrazine (N2H4), as one of the essential industrial chemicals, is main water contaminant and highly toxic for human health. Therefore, the rapid, selective and sensitive detection strategies for hydrazine are needed. The present study describes the first example of a receptor based on pyrazolone and hydrazide formation by sub-sequent ring-opening and closing reactions displayed distinct naked eye colorimetric and visual fluorescence sensing towards hydrazine with rapid reactivity and very good selectivity over various anions, cations and amines. The probe BC was obtained in good yield by three stages. The sensing mechanism was confirmed by1H NMR and13C NMR spectra. Upon interaction with hydrazine, coumarin ring was easily opened and pink color followed by purple was formed. The more hydrazine addition triggered pyrazolone ring formation with yellow colored solution in company with the remarkable cyan blue turn-on fluorescence output. An easy-to-produce TLC test strip enables to determine hydrazine in the solution. The results were successfully shown to be con-venient and efficient for hydrazine detection. Therefore, the present strategy is supposed to be widely applied to fabricate fluorescent sensors for detection of hydrazine.
1. Introduction
Hydrazine is one of the major industrial inorganic chemicals and has been widely utilized in the agricultural, pharmaceutical, cosmetic, and food industries[1–3]. Due to its high hydrogen content, the ab-sence of contamination, easy storage and transportation, hydrazine instead of hydrogen is promising a main hydrogen storage material for well-established fuel cells [4,5]. Since the 1960s, hydrazine has been also well applied in space propulsion systems as a liquid propellant[6]. Hydrazine in chemical industries act as catalyst, emulsifiers, inhibitor, reducing agent, dye stuff and explosives[7–12]. In spite of these many applications, hydrazine is regarded as extremely poisonous to human and animals. It is listed as a major category of environmental con-taminants. It is readily absorbed by dermal, inhalation and oral uptake and a model toxin, which induces adverse impacts on diverse organs and system damage[13–15], especially for nervous system[16]. Be-cause of hydrazine's high degree of toxicity, U.S. Environmental Pro-tection Agency (EPA) classifies it to be possible human carcinogen with a threshold limit value (TLV) of 10 ppb [17,18]. Hence, considerable effort has been paid recently to develop powerful means for the tracking and detection of hydrazine due to its detrimental impacts on environment and human health.
Up to now, several analytical techniques for the determination of hydrazine have been developed focused on the application of chemi-luminescence [19], GC–MS [20], HPLC [21], coulometry [22], ti-trimetry [23], amperometry [24] and ion-selective electrode [25]. These methods should be ideally reproducible, specified, simple, rapid and sensitive. Additionally, they require complicated processing pro-cedures and large stationary instruments. However, colorimetry and fluorescence methods are considered to be promising candidate for sensitive and selective detection of hydrazine due to the low cost, short response time and on-line monitoring. In the last few decades, design and development of selective and sensitive chemosensors for the monitoring biologically, chemically and environmentally important compounds have become a highly significant for technological appli-cations and academic value[26–29].
A number of chemodosimeters for the detection of hydrazine have been developed very recently, and most of their sensing strategies were based on deprotection of the leaving group by a specific deprotecting agent[30–35]. Among the deprotection groups for hydrazine are 4-bromo butyrate, acetyl, phthalimides, malonitriles, aldehyde, benzoic acids. One approach is substitution of dicyano- or monocyanovinyl groups with hydrazine [36,37]. Electron-withdrawing cyanovinyl group is attached to the fluorophore to form a charge transfer from the
https://doi.org/10.1016/j.microc.2019.104375
Received 6 August 2019; Received in revised form 18 October 2019; Accepted 25 October 2019 ⁎Corresponding author.
E-mail addresses:serdemir82@gmail.com,serdemir82@selcuk.edu.tr(S. Erdemir).
Available online 28 October 2019
0026-265X/ © 2019 Elsevier B.V. All rights reserved.
electron donor groups upon excitation. Simply, a fluorophore including ortho-hydroxy aromatic aldehyde functionality can undergo con-densation by hydrazine to give an Schiff base [38,39]. Hydrazone transformation has remarkable effect on the photophysical properties of a fluorophore. Another strategy utilize the cleavage of linker C]C bond in hemicyanine dyes, and resulting in a considerable change in the fluorescence output [40,41]. However, just a few study were reported through the cyclization reaction [42,43]. Therefore, it is very essential to develop novel sensing pathways for the determination of hydrazine. Here, a new type of reaction-based chromogenic and fluorescent sensor benzothiazole coumarin derivative (BC) for hydrazine detection through pyrazolone formation is reported first. The present probe has also ability to detect hydrazine via a chromogen carbohydrazide in-termediate.
2. Experimental
2.1. General
All chemicals used in this study were purchased from Sigma-Aldrich, Across and used without any further purification. NMR spectra were recorded on a Varian 400 MHz instrument. For NMR spectra, DMSO‑d6 and CDCl3 were used as solvents. Chemical shifts are ex-pressed in δ units and1H–1H coupling constants in Hz. UV–vis spectra were measured on a UV1280 spectrophotometer (Shimadzu). TOF-MS analysis was realized on an Agilent 6230 equipment. Fluorescence spectra were recorded at room temperature by Perkin Elmer LS 55 with the excitation and emission slit widths at 5 nm.
2.2. Synthesis of probe BC
As depicted inScheme 1, the synthesis of 1 was realized in two steps from the condensation reaction of benzothiazole and 5‑bromo‑2‑hy-droxy benzaldehyde followed by conversion of it to corresponding al-dehyde derivative (1) via Duff reaction[44]. Then, the probe BC was synthesized by reaction of 1 with methyl acetoacetate in presence of piperidine via knoevenegal condensation reaction. For this, a solution of 1 (0.2 g, 0.60 mmol), methyl acetoacetate (0.077 g, 0.66 mmol) and piperidine (65 µL) in ethanol (40 mL) was refluxed for 12 h. After this
period, the produced yellow precipitate was filtered, and recrystallized from ethanol to obtain BC. Yield: 70%; FTIR (ATR); 1747 cm−1(C]O), 1712 cm−1(C]O);1H NMR (400 MHz, 25 °C, DMSO‑d6) δ 8.62 (s, 1 H, ArH), 8.57 (s, 1 H, -CeCH), 8.28 (s, 1 H, ArH), 8.20 (d, 1 H,
J == 7.59 Hz, ArH), 8.02 (d, 1 H, J == 7.59 Hz, ArH), 7.55 (t, 1 H, J == 7.59 Hz, ArH), 7.47 (t, 1 H, J == 7.59 Hz, ArH), 2.57 (s, 3 H,
-CH3);13C NMR (100 MHz, 25 °C, DMSO‑d6) δ 195.01, 158.81, 157.22, 151.82, 151.19, 146.11, 136.27, 135.32, 134.74, 127.39, 126.47, 126.00, 123.61, 122.90, 122.61, 121.65, 116.85, 30.45; Anal. Calcd for C18H10BrNO3S (398.96): C, 54.02; H, 2.52; N, 3.50. Found: C, 54.07; H, 2.60; N, 3.62. TOF-MS: calcd. for C18H10BrNO3S+(M+H)+: 401.9600 found:4,019,678
2.3. Analytical procedure
For the fluorescence and UV–vis measurements, the stock solutions of probe BC were prepared (5.0 µM for fluorescence and 50.0 µM for UV–vis) in DMSO/H2O (v/v, 8/2). The stock solution of hydrazine (0.01 M) was prepared by using hydrazine hydrate (80% water solu-tion). In addition, the guest anions (as tetrabutylammonium) and ca-tions (as perchlorate) in H2O, semicarbazide, thiosemicarbazide, hy-droxylamine (as its chloride) and phenyl hydrazine in DMSO/H2O (8/2, v/v) were used for the sensing behavior experiment. Titrations ex-periments were realized directly in 3 mL of cuvette by successive ad-dition of hydrazine and other species using a proper micropipette.
3. Results and discussion
3.1. Colorimetric sensing properties of BC for hydrazine
The UV–vis spectral change of BC was examined after addition of 100.0 µM of various cations, anions and amines to the solution of BC (50 µM) in DMSO/H2O (v/v, 8/2). The free probe BC exhibited two maximal absorption bands at around 306 and 369 nm. As seen inFig.1a, the unique absorption change at 520 nm with appearance of the pink in solution was observed by the addition of only hydrazine among the tested amines, which can be ascribed to the chromenyl ring-opening followed by the hydrazide formation (BHT). Interestingly, the pink color of BHT was converted to purple within ~10 min due to the for-mation of pyrazolone ring (BPN) via intramolecular cyclization reac-tions, and a new band was observed at 595 nm (Fig. 1b), indicating that BC can be used as a visual probe for rapid detection of hydrazine. Subsequently, the more addition of hydrazine led to instant color change from purple to yellow (Fig.1c). This is probably owing to basic character of hydrazine which induces the deprotonation of -OH in BPN and its solvation effect. As well as other amines, the anions and cations could not cause any significant absorption changes in this period as shown in Figure S6.
Scheme 1. Synthetic route for probe BC.
Scheme 2. The possible reaction mechanism of probe BC with hydrazine.
To investigate the minute details of hydrazine induced ring-opening and closing of BC, UV–vis titration experiments were also realized. As depicted inFig. 2a, by the addition of hydrazine (0–2.0 equiv.) to a solution of BC (50.0 µM), the absorption band at 369 nm was decreased
and a new absorbance band was observed at 520 nm for BHT (possibly due to the chromenyl ring-opening in BC). Also, the gradually addition of hydrazine induced to formation of two weak absorbance bands at Fig. 1. (a) UV–vis spectra of BC (50.0 µM) in presence of various amines (2.0
equiv.); (b) after 10 min.; (c) the more addition of amines (10.0 equiv.) to the existing solutions.
Fig. 2. (a) Absorption titration of BC (50.0 μM) with the addition of increasing amounts of hydrazine (0.0–2.0 equiv.); (b) UV–vis spectral changes of the pink solution within 10 min.; (c) UV–vis titration of the purple solution after the more addition of hydrazine (0–10.0 equiv.) in DMSO/H2O (8/2, v/v).
426 and 595 nm belong to BPN and its deprotonation form (BPN−), which formed during the reaction, respectively. Consecutively, the time dependent UV–vis absorption spectra of the pink-color solution (BHT) was recorded during 0–10 min, and the results revealed the transfor-mation of BHT to BPN by ring-closing. With increasing time, the ab-sorbance band at 520 nm was gradually decreased, and a new band was appeared at 595 nm along with ~75 nm red shift. The absorbance in-tensity at 595 nm reached a stable level within about 10 min (Fig. 2b). In other words, the intramolecular ring-closing of BHT to form BPN was completed in this time. UV–vis titration experiments continued with the addition of hydrazine (0–10.0 equiv.) to the resulting solution of BPN and the changes were displayed inFig. 2c. As it can be seen that the addition of hydrazine induced the decrease in absorbance intensity at 595 nm and the emergence of a new band at 426 nm, which indicated formation of BPN's deprotonation form (BPN−) and solvation by hy-drazine, and the color of solution changed from purple to yellow. Also, the absorbance intensity at 520 nm indicated an excellent linear re-lationship (R2= 0.991) with the concentration of hydrazine in the
range of 0–100.0 μM. The detection limit for hydrazine was found to be 9.40 µM based on 3σ/k (Fig. S7).
3.2. Fluorometric sensing properties of BC for hydrazine
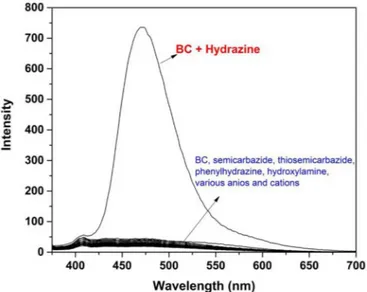
The fluorescence sensing ability of BC for hydrazine was in-vestigated in DMSO/H2O (v/v, 8/2). As shown inFig. 3, upon addition of various analytes under the same conditions with some amines (semicarbazide, thiosemicarbazide, hydroxylamine, phenyl hydrazine), anions (F−, Cl−, Br−, I−, ClO
4−, NO3−, NO2−, HSO4−, SO42−, HPO42−, H2PO4−) and cations (Na+, Li+, Ca2+, Ba2+, Mg2+, Ni2+, Ag+, Fe2+, Fe3+, Al3+, Co2+, Zn2+, Cd2+, Pb2+, Hg2+, Cu2+, Cr3+, Mn2+) indicated almost no changes to the fluorescent spectra of BC, in sharp contrast to hydrazine (Fig. 3). The reason for selecting these tested competing analytes is that they possibly coexist in the water samples. To check the sensitivity of BC, the fluorescence titration stu-dies of BC (5.0 µM) was also realized in the presence of hydrazine (0–50.0 µM) in DMSO/H2O (v/v, 8/2) at 25 °C. In the fluorescence spectra (Fig. 4a), one can see that BC showed non-fluorescence when it was excited at 365 nm due to the strong ICT (intramolecular charge transfer) effect. However, the addition of hydrazine resulted in the progressive appearance of a new strong emission band at 472 nm. Significantly, the specific reaction between β-ketoester moiety in BC and hydrazine yielded the products of pyrazolone and then its depro-tonation form, which affected the intramolecular electron density dis-tribution, resulting in the fluorescence response to hydrazine in aqu-eous solution. In addition, the stochiometric ratio between BC and hydrazine was determined by Job plot and found to be 1:2 (Fig.S8). On the basis of 1:2 of stoichiometry, the logarithmic association constants (logKa) were calculated to be 10.14 M−2for BC towards hydrazine from the Benesi–Hildebrand equation (Fig. S9). According to fluorescence titrations data, the detection limit of BC for hydrazine was determined to be 2.76 × 10−7M (8.85 ppb) (Fig. S10), which is sufficiently low for monitoring the hydrazine. On the other hand, the time-dependent fluorescence changes of BC (5.0 μM) in the presence of 50.0 μM hy-drazine in DMSO/H2O solution (8:2, v/v) were measured for kinetic studies (Fig. 4b). After the addition of hydrazine (50.0 μM), the fluor-escence intensity at 472 nm gradually enhanced depending on reaction time, and the fluorescence intensity at 472 nm of BC reached a stable value within about 30 min. The reaction rate constant of BC with hy-drazine was found to be 0.147 min−1 assuming pseudo-first-order Fig. 3. Fluorescence spectra of BC (5.0 µM) in presence of various amines,
anions and cations in DMSO/H2O (v/v, 8/2).
Fig. 4. (a) Fluorescence titrations profile of BC (5.0 µM) in the presence of increasing concentration of (0–50 µM) of hydrazine; (b) Time-dependent fluorescence changes of probe BC (5.0 µM) in DMSO/H2O (8:2, v/v) in the presence of 50 µM hydrazine.
kinetics (Fig.S11).
Photostability is one of the most important properties for devel-oping fluorescent sensors. Emission intensity of BC displayed the neg-ligible decrease upon the probe solution was irritated for 10 h through with a 400 W iodine–tungsten lamp at room temperature. As seen from in Fig. S12, about 94% of the initial emission intensity of BCeN2H4was remained. Therefore, the results suggest that BCeN2H4has the good photostability property.
In view of practical applications, fluorogenic performance of BC in the absence or presence of hydrazine was tested at 472 nm over a wide range of pH from 2.0 to 12.0 (Fig.S13). The emissions intensities of free probe BC was quite low and not varied remarkably at the tested pH ranges, showing that BC was stable. However, after treatment of BC with hydrazine, emission intensities were greatly influenced along with the tested pH scale. With the decline of pH from 6.0 to 2.0, a decrease trend was monitored N2H4for BCeN2H4due to protonation of N2H4in acidic solution. On the contrary, the response of BC for N2H4was pH-dependent and demonstrated a positive and almost linear trend in neutral and basic solutions. Therefore, the results showed that BC had high potential to detect N2H4in natural water samples and especially at physiological pH.
3.3. Sensing mechanism and environmental applications of BC for hydrazine
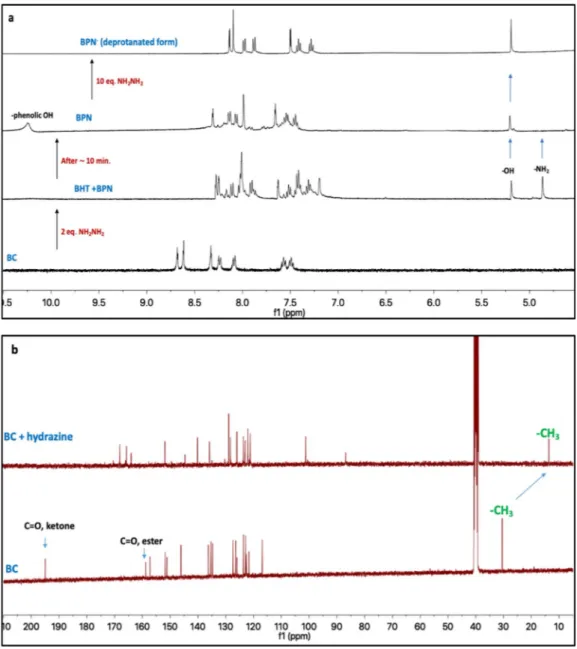
The subsequent ring-opening and closing mechanism of BC by hy-drazine was investigated by 1H NMR and 13C NMR techniques in DMSO‑d6. As seen in Fig. 5a, upon the addition of hydrazine (2.0 equiv.) to BC solution, the hydrazide-NH2 in BHT and the enol-OH signals in BPN appeared at δ 4.86 and δ 5.19 ppm, respectively, in-dicating simultaneous ring opening and closing reaction. After 10 min, 1H NMR spectra of the resulting solution was re-recorded, and the hy-drazide-NH2signal at δ 4.86 was disappeared, which shows completely the conversion of BHT to BPN. When hydrazine (10.0 equiv.) was added to this solution, the phenolic −OH signal at δ 10.24 ppm disappeared, and the aromatic protons of BPN displayed apparent upfield shifts due to the deprotonation. The opening and closing of the ring of BC in the presence of hydrazine was also supported by disappearance of ketone and ester carbonyl signals at δ 195.01 and 158.81 in13C NMR spectra, respectively. In addition, the methyl carbon shifted upfield from δ 30.45 to 13.29 ppm owing to the formation of pyrazolone (enol-form) (Fig. 5b). These data clearly show that hydrazine induced to the for-mation of pyrazolone (BPN) by subsequent ring-opening and closing reactions in BC (Scheme 2).
Fig. 5.1H NMR (a) and13C NMR (b) spectral changes of probe BC in presence of hydrazine in d 6-DMSO.
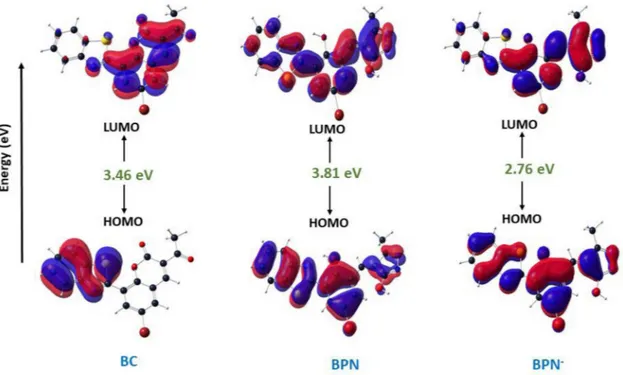
To better insight to the electronic structure of BC, BPN and BPN−, theoretical calculations using the Gaussian 16 software with the PM6 semiempirical method were carried out and differences in the spectral profiles of BC, BPN and BPN−were compared. The orbital shapes of HOMO and LUMO are given inFig. 6. In BC, the HOMO is mainly lo-calized over the benzothiazole moiety while the LUMO is spread on the rest of the molecule. Thus, the photo-excitation of BC involved ICT from the benzothiazole moiety to the acetyl coumarine moiety. In BPN, both the HOMO and the LUMO are nearly spread on the entire molecule. However, accompany with desolvation and basicity effect of hydrazine, the contour maps changed remarkably. In BPN−, the negative charge of the fenolate ion interact by resonant with the pyrazolone ring, causing a planarity establishment in the chromophore. The dihedral angle be-tween fenol and pyrazolone rings changed from −32.40 (BPN) to +7,23° (BPN−). Therefore, the ICT from the ionized hydroxyl of the fenolate to the pyrazolone refers a dramatic decrease in the internal
conversion probability explaining the emergence of fluorescence re-sponse in basic media. These effects are also seen from that the calcu-lated energy gaps of the HOMOs and LUMOs become smaller over BPN.
3.4. Application in water samples
The practical application of BC by high sensitivity and selectivity for hydrazine was observed by simple test strip kits. The test strips were prepared by immersing a TLC plate (3 × 1 cm) into the BC solution (10−4M, 3 mL) for 2 min and then dried in air. As shown inFig. 7, test kit coated with BC were colorless and had no fluorescence emission under 365 nm ultraviolet light in the absence of hydrazine. This test kit was then interacted by hydrazine solution (2 × 10−4µM), and the color of test kit was changed obviously from colorless to purple which can be detected by the naked eye. Besides, when the purple colored test kit was again immersed into the hydrazine solution (10−3M), the obvious color change from purple to light yellow was observed with the strong fluorescent under 365 nm. As a result, the development of such test strips was extremely attractive for “on-site” analysis without any in-strumental analyses. Employing these excellent properties, the hy-drazine vapor sensing ability of BC was visually tested with TLC plates (Fig.S14). Blank and BC-soaked TLC plates were placed to the top of a conical flask including a hydrazine solution. After 10 min., the color of BC-soaked TLC plates was gradually turned from the colorless to yellow. Moreover, distinct fluorescence response was observed through the emergence of cyan color on TLC, indicating that the detection of hydrazine vapor. Therefore, the results showed that BC could be ap-plied for the sensing hydrazine vapor in practical applications. On the other hand, the practicability of the proposed method was also tested Fig.6. Frontier molecular orbital diagrams and energy levels of BC, BPN and BPN-.
Fig. 7. Photographs of the test kits with BC for detecting hy-drazine in aqueous solution; (a) naked-eye; (b) under 365 nm UV light.
Table. 1
Determination of hydrazine in spiked water samples with BC.
Sample Amount of spiked
hydrazine (µM) Found (µM) Recovery (%) RSD (n == 3)(%) TW1 5.0 4.89 ± 0.2 97.8 2.8 TW2 10.0 9.72 ± 0.3 97.2 3.2 TW3 20.0 20.25 ± 0.2 101.2 2.1 MW1 5.0 4.86 ± 0.5 97.2 3.0 MW2 10.0 10.37 ± 0.7 103.7 3.9 MW3 20.0 19.89 ± 0.5 99.4 3.0
TW1-TW3: Tap water samples, MW1-MW3: Mineral water samples.
by using the spiked mineral and tap water samples with the different concentration of hydrazine. For this, a solution of BC was interacted with the hydrazine spiked water or mineral samples, and then the emission spectra of the resulting solution were recorded. Hydrazine concentrations in samples were calculated by using the fitted linear fluorescence calibration curve. As seen inTable 1, we observed that the recovery values were 97.2–103.7% with RSD in the range of 2.1–3.9%, which indicates that BC is feasible and practical for the determination of hydrazine in real water samples.
4. Conclusion
We have developed a new high selective fluorescent probe BC (benzothiazole coumarin derivative) for hydrazine over various amines, anions and cations. Probe BC showed distinct naked eye colorimetric and visual fluorescence sensing towards hydrazine over the formation of pyrazolone and hydrazide by subsequent ring-opening and closing reactions. The ring-opening and closing reactions were supported by1H and13C NMR spectra. The fluorescence intensity of BC enhanced ob-viously with the addition of hydrazine with low limit of detection (8.85 ppb), owing to the formation pyrazolone and its deprotonation form, which affected the intramolecular electron density distribution. Additionally, it showed excellent performance in test paper strips, which allows a simple and effective way for on-site detection of hy-drazine without any instrument. Also, the proposed sensor was suc-cessfully used to detect hydrazine in tap and mineral water samples.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influ-ence the work reported in this paper.
Acknowledgment
We thank the Research Foundation of Selcuk University for financial support (Grant No: 18601031) of this work.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, atdoi:10.1016/j.microc.2019.104375.
References
[1] K. Jones, The Chemistry of Nitrogen: Pergamon Texts in Inorganic Chemistry, Elsevier, 2013.
[2] S.A. Lawrence, Amines: Synthesis, Properties and Applications, Cambridge University Press, 2004.
[3] B. Timmer, W. Olthuis, A. Van Den Berg, Ammonia sensors and their applications – a review, Sens. Actuators B: Chem. 107 (2005) 666–677.
[4] N.V. Rees, R.G. Compton, Carbon-free energy: a review of ammonia-and hydrazine-based electrochemical fuel cells, Energy Environ. Sci. 4 (2011) 1255–1260. [5] A. Serov, C. Kwak, Direct hydrazine fuel cells: a review, Appl.Catal. B: Environ. 98
(2010) 1–9.
[6] J. Kroschwitz, A. seidel, hydrazine and its derivatives, Kirk-Othmer Encycl. Chem. Technol. 13 (2005) 562–607.
[7] R. Bunker, D. Baker, J. Lee, Explosive decomposition of hydrazine by rapid com-pression of a gas volume, dynamics of detonations and explosions: detonations, Prog. Astronaut. Aeronaut. 133 (1991) 325–341.
[8] Á. Costoyas, J. Ramos, J. Forcada, Hydrazine‐functionalized latexes, J. Polym. Sci. Part A: Polym. Chem. 47 (2009) 6201–6213.
[9] G. De Matteis, E. Agostinelli, B. Mondovì, L. Morpurgo, The metal function in the reactions of bovine serum amine oxidase with substrates and hydrazine inhibitors, JBIC J. Biol. Inorg. Chem. 4 (1999) 348–353.
[10] A. Furst, R.C. Berlo, S. Hooton, Hydrazine as a reducing agent for organic com-pounds (catalytic hydrazine reductions), Chem. Rev. 65 (1965) 51–68. [11] X. Wu, W. Xing, L. Zhang, S. Zhuo, J. Zhou, G. Wang, S. Qiao, Nickel nanoparticles
prepared by hydrazine hydrate reduction and their application in supercapacitor, Powder Technol 224 (2012) 162–167.
[12] K. Yamada, K. Yasuda, N. Fujiwara, Z. Siroma, H. Tanaka, Y. Miyazaki, T. Kobayashi, Potential application of anion-exchange membrane for hydrazine fuel
cell electrolyte, Electrochem. Commun. 5 (2003) 892–896.
[13] A. Umar, M.M. Rahman, S.H. Kim, Y.-.B. Hahn, Zinc oxide nanonail based chemical sensor for hydrazine detection, Chem. Commun. (2008) 166–168.
[14] S. Sanins, J. Timbrell, C. Elcombe, J. Nicholson, Proton nmr spectroscopic studies on the metabolism and biochemical effects of hydrazine in vivo, Arch. Toxicol. 66 (1992) 489–495.
[15] E. Vernot, J. MacEwen, R. Bruner, C. Haun, E. Kinkead, D. Prentice, A. Hall III, R. Schmidt, R. Eason, G. Hubbard, Long-term inhalation toxicity of hydrazine, Fundam. Appl. Toxicol. 5 (1985) 1050–1064.
[16] S. Garrod, M.E. Bollard, A.W. Nicholls, S.C. Connor, J. Connelly, J.K. Nicholson, E. Holmes, Integrated metabonomic analysis of the multiorgan effects of hydrazine toxicity in the rat, Chem. Res. Toxicol. 18 (2005) 115–122.
[17] G. Choudhary, H. Hansen, Human health perspective of environmental exposure to hydrazines: a review, Chemosphere 37 (1998) 801–843.
[18] U.E.P. Agency, Integrated risk information system (IRIS) on formaldehyde, national center for environmental assessment, Office of Research and Development (1999) 1–16.
[19] J. Du, J. Lu, Hydrazine‐induced post‐chemiluminescence phenomenon of perman-ganate–luminol reaction and its applications, Lumin.: J. Biological Chem. Lumin. 19 (2004) 328–332.
[20] W. Stillwell, M.S. Bryant, J.S. Wishnok, GC/MS analysis of biologically important aromatic amines. Application to human dosimetry, Biomed. Environ. Mass Spectrom. 14 (1987) 221–227.
[21] M.T. Salazar, T.K. Smith, A. Harris, High-performance liquid chromatographic method for determination of biogenic amines in feedstuffs, complete feeds, and animal tissues, J. Agric. Food Chem. 48 (2000) 1708–1712.
[22] H.E. Malone, The Determination of Hydrazino-Hydrazide Groups: Monographs in Organic Functional Group Analysis, Elsevier, 2013.
[23] Z. He, B. Fuhrmann, U. Spohn, Coulometric microflow titrations with chemilumi-nescent and amperometric equivalence point detection: bromimetric titration of low concentrations of hydrazine and ammonium, Anal. Chim. Acta 409 (2000) 83–91.
[24] İ. Teoman, S. Karakaya, Y. Dilgin, Sensitive and rapid flow injection amperometric hydrazine sensor using an electrodeposited gold nanoparticle graphite pencil electrode, Anal. Lett. (2019) 1–16.
[25] M. Koupparis, T. Hadjiioannou, Indirect potentiometric determination of hydrazine, isoniazid, sulphide and thiosulphate with a chloramine-T ion-selective electrode, Talanta 25 (1978) 477–480.
[26] Y. He, B. Xu, W. Li, H. Yu, Silver nanoparticle-based chemiluminescent sensor array for pesticide discrimination, J. Agric. Food Chem. 63 (2015) 2930–2934. [27] Y. Zhou, W. Huang, Y. He, pH-Induced silver nanoprism etching-based
multi-channel colorimetric sensor array for ultrasensitive discrimination of thiols, Sens. Actuators B: Chem. 270 (2018) 187–191.
[28] W. Huang, Y. Deng, Y. He, Visual colorimetric sensor array for discrimination of antioxidants in serum using MnO2 nanosheets triggered multicolor chromogenic system, Biosens. Bioelectron. 91 (2017) 89–94.
[29] J. Du, M. Zhao, W. Huang, Y. Deng, Y. He, Visual colorimetric detection of tin (II) and nitrite using a molybdenum oxide nanomaterial-based three-input logic gate, Anal. Bioanal. Chem. 410 (2018) 4519–4526.
[30] M.V.R. Raju, E.C. Prakash, H.-.C. Chang, H.-.C. Lin, A facile ratiometric fluorescent chemodosimeter for hydrazine based on Ing–Manske hydrazinolysis and its appli-cations in living cells, Dyes Pigment. 103 (2014) 9–20.
[31] S.I. Reja, N. Gupta, V. Bhalla, D. Kaur, S. Arora, M. Kumar, A charge transfer based ratiometric fluorescent probe for detection of hydrazine in aqueous medium and living cells, Sens. Actuators B: Chem. 222 (2016) 923–929.
[32] M. Sun, J. Guo, Q. Yang, N. Xiao, Y. Li, A new fluorescent and colorimetric sensor for hydrazine and its application in biological systems, J. Mater. Chem. B 2 (2014) 1846–1851.
[33] M.H. Lee, B. Yoon, J.S. Kim, J.L. Sessler, Naphthalimide trifluoroacetyl acetonate: a hydrazine-selective chemodosimetric sensor, Chem. Sci. 4 (2013) 4121–4126. [34] Y.-.D. Lin, T.J. Chow, A pyridomethene–BF 2 complex-based chemosensor for
de-tection of hydrazine, RSC Adv. 3 (2013) 17924–17929.
[35] G.E. Collins, S.L. Rose-Pehrsson, Fluorescent detection of hydrazine, mono-methylhydrazine, and 1, 1-dimethylhydrazine by derivatization with aromatic di-carbaldehydes, Analyst 119 (1994) 1907–1913.
[36] S. Goswami, S. Paul, A. Manna, A highly reactive (<1 min) ratiometric chemodo-simeter for selective “naked eye” and fluorogenic detection of hydrazine, RSC Adv. 3 (2013) 18872–18877.
[37] J. Fan, W. Sun, M. Hu, J. Cao, G. Cheng, H. Dong, K. Song, Y. Liu, S. Sun, X. Peng, An ICT-based ratiometric probe for hydrazine and its application in live cells, Chem. Commun. 48 (2012) 8117–8119.
[38] J. Cui, G. Gao, H. Zhao, Y. Liu, H. Nie, X. Zhang, A highly sensitive and selective fluorescent probe for N 2 H 4 in air and living cells, New J. of Chem. 41 (2017) 11891–11897.
[39] L. Xiao, J. Tu, S. Sun, Z. Pei, Y. Pei, Y. Pang, Y. Xu, A fluorescent probe for hy-drazine and its in vivo applications, RSC Adv. 4 (2014) 41807–41811. [40] A.A. Ensafi, B. Rezaei, Flow injection determination of hydrazine with fluorimetric
detection, Talanta 47 (1998) 645–649.
[41] Y. He, Z. Li, B. Shi, Z. An, M. Yu, L. Wei, Z. Ni, A new near-infrared ratiometric fluorescent probe for hydrazine, RSC Adv. 7 (2017) 25634–25639.
[42] Y.-.H. Xiao, G. Xi, X.-.X. Zhao, S. Zhou, Z.-.Q. Zhou, B.-.X. Zhao, A novel coumarin-based fluorescent probe for the detection of hydrazine both in aqueous solution and vapor state, J. Fluoresc. 25 (2015) 1023–1029.
[43] S. Goswami, S. Das, K. Aich, D. Sarkar, T.K. Mondal, A coumarin based chemodo-simetric probe for ratiometric detection of hydrazine, Tetrahedron Lett. 55 (2014) 2695–2699.
[44] S. Erdemir, B. Tabakci, M. Tabakci, A highly selective fluorescent sensor based on calix [4]arene appended benzothiazole units for Cu2+, S2− and HSO4− ions in aqueous solution, Sens. Actuators B: Chem. 228 (2016) 109–116.