Contents lists available atScienceDirect
Food and Chemical Toxicology
journal homepage:www.elsevier.com/locate/foodchemtoxUtilisation of Rhododendron luteum Sweet bioactive compounds as valuable
source of enzymes inhibitors, antioxidant, and anticancer agents
Mohamad Fawzi Mahomoodally
a, Elwira Sieniawska
b, Kouadio Ibrahime Sinan
c,
Marie Carene Nancy Picot-Allain
a, Serife Yerlikaya
d, Mehmet Cengiz Baloglu
d,e,
Yasemin Celik Altunoglu
d, Ismail Senkardes
f, Kannan RR. Rengasamy
g, Gokhan Zengin
c,∗aDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius
bChair and Department of Pharmacognosy, Medical University of Lublin, Lublin, Poland
cDepartment of Biology, Faculty of Science, Selcuk University, Campus, Konya, Turkey
dDepartment of Genetics and Bioengineering, Faculty of Engineering and Architecture, Kastamonu University, Kastamonu, Turkey
eAgronomy Department, University of Florida - IFAS, Gainesville, USA
fDepartment of Pharmaceutical Botany, Pharmacy Faculty, Marmara University, Istanbul, Turkey
gDepartment of Bioresources and Food Science, Konkuk University, Seoul, 05029, South Korea
A R T I C L E I N F O Keywords: Yellow azalea Honeysuckle azalea Enzyme inhibitory Anticancer PCA analysis A B S T R A C T
Ethnobotanical evidences report the use of Rhododendron luteum Sweet (Ericaceae) in traditional medicinal
systems. However, R. luteum has been associated to the occurrence of‘mad honey’ poisoning. In the present
study, the ethyl acetate, methanol, and water extracts of R. luteum were investigated for their in vitro antioxidant, enzyme inhibition, and cytotoxic properties. The cytotoxicity of R. luteum extracts on A549 lung cancer cell line
was evaluated using MTT cell viability assay. Besides, HPLC-ESI-MSnapproach was employed to elucidate the
secondary metabolite profiles of R. luteum in order to establish any structure-activity relationship. Methanol and
water extracts of R. luteum possessed highest radical scavenging and reducing properties while the ethyl acetate extract showed highest metal chelating properties. In terms of enzyme inhibition, the methanol and ethyl acetate
extracts of R. luteum, possessing epigallocatechin, were active inhibitors of cholinesterase enzymes,
α-glucosi-dase, and tyrosinase. Water extract caused growth inhibition of A549 cells with 207.2μg/ml IC50value. Though
R. luteum has received little scientific attention due to the occurrence of grayanotoxins in the plant, however,
data presented in this work shows promising biological activity of R. luteum and highlighted its role as a potential source of antioxidant and key enzyme inhibitors.
1. Introduction
The Rhododendron genus (Ericaceae) is considered as the most di-verse group of the wood plant with more than 1200 species which are famous for their colourful flower (Shrestha et al., 2017). It has been reported that the nectar produced by Rhododendronflowers was related to mad honey poisoning, caused by grayanotoxins provoking choli-nergic toxidrome which manifests as incapacity, bradycardia, hypo-tension, and altered mental status (Gunduz et al., 2008). However, ethnobotanical evidence report the use of Rhododendron species in traditional medicinal systems for the management of inflammatory conditions, gastrointestinal disorders, skin disease, common cold, and asthma (Popescu and Kopp, 2013;Shrestha et al., 2017).
Rhododendron luteum Sweet or Rhododendron flavum G. Don,
commonly known as yellow azalea or honeysuckle azalea, is described as a deciduous plant, measuring between 3 and 4 m height, possessing ovate to lanceolate leaves with strigose glandular hairs on both surfaces and yellow, funnel-shapedflowers (Küçük et al., 2010). This species is native to southeastern Europe (southern Poland and Austria south through the Balkans) and southwest Asia to southern Russia. R. luteum leaves have been used in Turkish traditional medicine as a diuretic, analgesic against rheumatic pains, and fungal foot infection (Popescu and Kopp, 2013). The biological activity of R. luteum extracts has been demonstrated previously. The chloroform: methanol (1:1, 1 mg/ml) extract of R. luteum inhibited acetylcholinesterase (76.32%) and bu-tyrylcholinesterase (69.14%) activity (Orhan et al., 2004). R. luteum flowers extracted with DMSO was rich in phenolics (54.2 mg gallic acid equivalents), showed reducing activity (164.2 mg Trolox equivalents
https://doi.org/10.1016/j.fct.2019.111052
Received 25 October 2019; Received in revised form 2 December 2019; Accepted 8 December 2019
∗Corresponding author.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin).
Available online 16 December 2019
0278-6915/ © 2019 Elsevier Ltd. All rights reserved.
per g sample), and exhibited non-selective cytotoxic effect against MCF-7 cells (IC50203.2 μg/mL) compared to chemotherapeutic drug, cis-platin (IC500.53 μg/mL) (Demir et al., 2016). Besides, it should be noted that other plant secondary metabolites naturally occurring in the plant, such as flavonoids, have been claimed to possess biological properties, such as anti-inflammatory and anti-diabetic activities (Bilir et al., 2018). Therefore, it can be claimed that R. luteum might serve as a source of promising biologically-active plant secondary metabolites, thus supporting the need for further scientific assessments.
In the light of the above statements, the present study was designed and aimed to define the phytochemical profiles of the ethyl acetate, methanol, and water extracts of R. luteum aerial parts. Besides, cyto-toxicity assay was used for determination of the antiproliferative ac-tivity of plant extracts and the antioxidant profile of the different R. luteum extracts has been established using radical scavenging, reducing potential, and metal chelating assays. The possible inhibition of R. lu-teum extracts against enzymes, such as acetylcholinesterase (AChE), butyrylcholinesterase (BChE), α-amylase, α-glucosidase, and tyr-osinase, was also determined using standard in vitro assays. Finally, the principal component analysis (PCA) was applied to provide additional insights on the biological activities with respect to the difference among the extracting solvent.
2. Materials and methods
2.1. Plant material and preparation of extracts
Aerial parts of R. luteum were collected from Taskopru (Kastamonu), Turkey. Taxonomic identification was performed at Marmara University, Istanbul, Turkey (by Dr. Ismail Senkardes, voucher number: MARE-19872). Aerial parts of the plant were dried until a constant weight was recorded. The dried material was then ground and stored in a cool, dry place.
Maceration in ethyl acetate and methanol, and infusion in water were used for the extraction of a wider range of secondary metabolites from R. luteum aerial parts. These methods are known as traditional extraction methods (Belwal et al., 2018;WHO, 2018). With regards to maceration, 5 g of plant material was mixed with 100 ml of ethyl acetate and methanol for 24 h. Extracts so obtained were concentrated using vacuum evaporator. For infusion, 5 g of plant material was kept in 100 ml boiling water (100 °C) for 20 min. The water extract then was lyophilized. All extracts were stored at 4 °C until use.
2.2. Profile of bioactive compounds
Total bioactive compounds namely, total phenolic (TPC) and fla-vonoid content (TFC) were assessed by colorimetric methods (Folin-Ciocalteu and AlCl3 methods, respectively) (Zengin and Aktumsek, 2014). The standards of gallic acids and rutin were used for inter-pretation of the results.
Phytochemical analysis was performed using Agilent 1200 Infinity HPLC apparatus coupled with Agilent 6530B QTOF Accurate-Mass QTOF system equipped with Dual Agilent Jet Stream spray source (ESI) (Agilent Technologies, Santa Clara, CA, USA) following the conditions described previously (Zengin et al., 2019b). Obtained fragmentation patterns were used for tentative identification, supported by literature and Metlin database (Metlin database)
2.3. Determination of antioxidant and enzyme inhibitory effects
The antioxidant and enzyme inhibitory properties of the different extracts were determined using in vitro assays. All procedures are de-scribed in our previous papers (Zengin, 2016;Zengin and Aktumsek, 2014). The obtained results were expressed by using appropriate standards, for instance, galantamine (GALAE, for cholinesterase), kojic acid (KAE, for tyrosinase), acarbose (ACAE, for amylase and
α-glucosidase), Trolox (TE, for ABTS, DPPH, FRAP, CUPRAC, and phos-phomolybdenum), and EDTA (EDTAE, for metal chelating).
2.4. Antiproliferative activity assay
MTT-based cytotoxicity assay was used for determination of the antiproliferative activity of plant extracts. Ethyl acetate and methanol extracts were prepared in 0.1% of DMSO solution, and water extract was dissolved in PBS before treatment of cells. A549 lung cancer cells were cultured in high glucose DMEM including L-glutamine, 10% FBS, 0.01 mg/ml human insulin, 1% non-essential amino acid and 0.1% penicillin/streptomycin at 37 °C in a 5% CO2 humidified incubator. Routine passages of cells were carried out when cells reached ~80% confluency. A total of 5000 cells were seeded to 96-well plates and allowed to fasten for 24 h. Thereafter, the cells were treated with var-ious doses (62.5-125-250-500-1000 μg/ml) of extracts for 24 h and 48 h. Control cells were treated with 0.1% DMSO/PBS. Antiproliferative or cell viability analysis was performed as reported previously (Yerlikaya et al., 2019). Formazan crystals of MTT dye was dissolved with 3% SDS, and HCI/isopropanol solution and sample plates were measured under a microplate reader at 570 nm. After the calculation of cell viability percentage, half-maximal inhibitory con-centrations of extracts against A549 cells were calculated based on log (inhibitor) vs normalized response– variable slope analysis function by using GraphPad Prism 7 software.
2.5. Statistical analysis
One-way ANOVA, together with Tukey's test, with statistical sig-nificance considered as p < 0.05, were used to characterize each samples. Principal component analysis (PCA) was applied on biological activities to assess the effect of the extracting solvent, and hierarchical cluster analysis (HCA) was done on the result of PCA for the classifi-cation. All calculations were done using Xlstat 2018 and R software v. 3.6.1 with FactoMineR package.
3. Results and discussion
The total phenolic andflavonoid contents of R. luteum extracts as-sessed using standard colorimetric methods are presented inTable 1. Phenolic and flavonoid contents determination have been routinely carried out in plant extracts studies since these parameters have been associated with antioxidant activity and other biological activities. Data presented inTable 1, showed that the water extract (110.25 mg GAE/g extract) possessed the highest phenolic content while the highest fla-vonoid content was present in the methanol extract (89.71 mg RE/g extract). The higher capacity of methanol to extract flavonoids as compared to ethyl acetate and water has been previously reported (Dhawan and Gupta, 2017). Although the Folin-Ciocalteu and alumi-nium chloride assays are widely used, rapid, and simple spectro-photometric means of determining phenolic and flavonoid contents, respectively, a group of researchers recently highlighted several loop-holes (Granato et al., 2018). Due to inadequacies with spectro-photometric determinations, advanced chromatographic techniques Table 1
Total bioactive components of Rhododendron luteum extracts.
Extracts Total phenolic content (mg
GAE/g extract)
Totalflavonoid content (mg
RE/g extract)
Ethyl acetate 22.45 ± 0.24c 47.02 ± 0.89b
Methanol 105.47 ± 0.15b 89.71 ± 1.07a
Water 110.25 ± 0.11a 46.45 ± 0.47b
*Values expressed are means ± S.D. of three parallel measurements. GAE:
Gallic acid equivalent; RE: Rutin equivalent. Different superscripts indicate
Table 2 Chemical characterization of Rhododendron luteum extracts. Comp. No Tentative identi fi cation R time Molecular formula Molecular weight [M -H] -Fragments (m/z) Extracts References 1C aff eic acid-O -hexoside derivative 1.782 388.1202 387.1202 341.1143; 179.0131 E, W Shrestha (2016) 2 Quinic acid 2.120 C7 H12 O6 192.0205 191.0205 173.0034; 127.0437; 111.0463 E, M, W ( Cli ff ord et al., 2006 ; METLIN Database ) 3 Quinic acid dimethylgallate hexoside 2.444 C22 H30 O15 534.1868 533.1968 353.5524; 191.0211 E, M Shrestha (2016) 4 Quinic acid dimer 2.510 C14 H24 O12 384.1311 383.1311 191.0199 E, M, W ( Cli ff ord et al., 2006 ; METLIN Database ) 5C aff eic acid-O -hexoside derivative 2.905 430.1346 429.1346 383.1264; 341.1162; 179.0082 E Shrestha (2016) 6 Unknown 3.691 196.7751 195.7751 160.7863 E 7 Gallic acid 4.349 C7 H6 O5 170.0128 169.0128 125.0232 W ( METLIN Database ) 8 Ferulic acid derivative 5.359 338.1165 337.1165 235.0696; 193.0377 W Shrestha (2016) 9 Vanillic acid derivative 6.780 392.1328 391.1328 229.0347; 185.0234; 167.0076; 149.0235 W Shrestha (2016) 10 2,3-Dihydroxybenzoic acid 11.114 C7 H6 O4 154.0440 153.0440 109.0311 W ( METLIN Database ) 11 2,3-Dihydroxybenzoic acid hexoside 15.386 C13 H16 O9 316.0741 315.0741 153.0440; 109.0311 W ( METLIN Database ) 12 (Epi)gallocatechin 17.088 C15 H14 O7 306.0684 305.0684 287.0581; 261.0662; 219.0459; 179.0551; 167.0587; 125.0521 M, W ( METLIN Database ; Wu et al., 2012 ) 13 Gallic acid hexoside 17.620 C7 H6 O5 332.0711 331.0711 169.0128; 151.0562; 125.0232 W ( METLIN Database ) 14 Unknown 17.915 468.1526 467.1526 421.1881; 259.0953 W 15 (Epi)gallocatechin 18.422 C15 H14 O7 306.0664 305.0664 287.0520; 261.0711; 219.0413; 179.0551; 125.0521 E, M ( METLIN Database ; Wu et al., 2012 ) 16 (Epi)gallocatechin-(epi)catechin 18.684 C30 H26 O13 594.1406 593.1406 425.1016; 407.0832; 289.0702; 245.0743; 205.0210 M, W Shrestha (2016) 17 (Epi)catechin-O -hexoside 18.841 C21 H24 O11 452.1372 451.1372 289.0711; 245.0719; 205.0207 W Shrestha (2016) 18 Ca ff eic acid derivative 18.898 468.1512 467.1512 421.1344; 259.0645; 227.0168; 179.0088 E Shrestha (2016) 19 (Epi)catechin-O -hexoside derivative 19.037 488.1135 487.1135 451.1234; 289.0625; 245.0533 W Shrestha (2016) 20 3-O -Ca ff eoylquinic acid 19.468 C16 H18 O9 354.0950 353.0950 191.0042; 179.0036 W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 21 Vanillic acid-O -hexoside 20.336 C14 H18 O9 330.1195 329.1195 167.0206; 122.9556 E, M, W Shrestha (2016) 22 Unknown 20.517 452.1209 451.1209 259.0706; 147.0785 W 23 (Epi)catechin-O -hexoside 21.105 C21 H24 O11 452.1606 451.1606 289.0329; 245.5217; 125.0399 E, M, W Shrestha (2016) 24 (Epi)catechin-(epi)catechin (Procyanidin dimer B1) 21.585 C30 H26 O12 578.1457 577.1457 451.1423; 425.1210; 407.1064; 289.0910; 287.0755 W Shrestha (2016) 25 3-O -p -Coumaroylquinic acid 21.826 C16 H18 O8 338.0984 337.0984 163.0168; 191.0579; 173.0002 W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 26 (Epi)catechin-O -hexoside 21.938 C21 H24 O11 452.1360 451.1360 289.0779; 245.0709; 205.0189 E Shrestha (2016) 27 Gambiriin A/D 22.400 C30 H28 O12 580.1605 579.1605 289.0671; 245.0722; 205.0272 W Shrestha (2016) 28 (Epi)catechin 22.458 C15 H14 O6 290.0704 289.0704 245.0830; 205.0278; 125.0241 E, M, W ( METLIN Database ; Wu et al., 2012 ) 29 (Epi)catechin derivative 22.531 326.0518 325.0518 289.1342; 245.1244; 125.0241 W Shrestha (2016) 30 5-O -Ca ff eoylquinic acid 22.935 C16 H18 O9 354.0950 353.0950 191.0191 M, W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 31 Vanilloyl-O -hydroxybenzoylhexoside 23.263 C21 H22 O11 450.1202 449.1202 359.0895; 329.0741; 301.0933; 167.0235 E Shrestha (2016) 32 (Epi)catechin 23.602 C15 H14 O6 290.0678 289.0678 245.0580; 205.0178; 125.0241 E ( METLIN Database ; Wu et al., 2012 ) 33 4-O -Ca ff eoylquinic acid 23.975 C16 H18 O9 354.0950 353.09750 191.0211; 179.0245; 173.0052 E, M, W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 34 Ca ff eic acid 24.473 C9 H8 O4 180.04228 179.04228 135.0437; 107.0502 E ( METLIN Database ) 35 Unknown 24.513 370.0902 369.0902 207.0033; 191.9662 E 36 5-O -Ca ff eoylquinic acid 24.568 C16 H18 O9 354.0973 353.0973 191.0216 M, ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 37 3-O -p -Coumaroylquinic acid 24.575 C16 H18 O8 338.0984 337.0984 191.0194; 173.0027; 163.0169 M, W ( Cli ff ord et al., 2006 ; METLIN Database ) 38 p-Coumaroylquinic acid derivative 24.645 668.1941 667.1941 337.0992; 173.0051 M, W ( METLIN Database ) 39 (Epi)catechin 24.944 C15 H14 O6 290.0440 289.0440 245.0466; 205.0101; 125.0241 W ( METLIN Database ; Wu et al., 2012 ) 40 Unknown 24.948 C16 H16 O7 320.0514 319.0514 301.0243; 193.0023; 175.0747 M, W 42 5-O -p -Coumaroylquinic acid derivative 25.592 658.1588 657.1588 337.0588; 191.0120; 173.0009; 119.0258 M, W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 43 3-O -p -Coumaroylquinic acid 25.736 C16 H18 O8 338.1008 337.1008 191.0005; 173.0009; 163.0169 M, W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 44 Unknown 25.832 448.2266 447.2266 329.0683; 192.9711 E 45 Myricetin-O -hexoside 26.258 C21 H20 O13 480.0963 479.0963 317.0249; 178.9496; 151.0238; 137.0234 12 Shrestha (2016) 46 Dihydromyricetin 26.359 C16 H16 O7 320.0514 319.0514 192.9810 E, M, W Tong et al. (2015) 47 3-O -p -Coumaroylquinic acid derivative 26.528 658.1535 657.1535 337.0578; 191.0000; 173.0009; 119.0258 M, W Shrestha (2016) 48 5-O -p -Coumaroylquinic acid 26.602 C16 H18 O8 338.0984 337.0984 191.0197 M, W ( Cli ff ord et al., 2006 ; Shrestha, 2016 ) 49 Naringenin-O -hexoside 26.940 C21 H22 O10 434.1269 433.1269 271.0540; 177.0480; 151.0589; 107.0235 W Shrestha (2016) 50 Naringenin-O -hexoside 27.132 C21 H22 O10 434.1296 433.1296 271.0540; 177.0480; 151.0589 M, W Shrestha (2016) (continued on next page )
Table 2 (continued ) Comp. No Tentative identi fi cation R time Molecular formula Molecular weight [M -H] -Fragments (m/z) Extracts References 51 Myricetin-O -hexoside 27.508 C21 H20 O13 480.0983 479.0983 317.0273; 271.0164; 178.9496; 151.0238 M, W Shrestha (2016) 52 Vanilloyl-O -hydroxybenzoylhexoside 27.543 C21 H22 O11 450.1247 449.1247 359.0869; 329.0801; 301.1204; 167.0306 E Shrestha (2016) 53 Apigenin derivative 28.010 414.1572 413.1572 269.0970 W ( METLIN Database ) 54 p -Coumaric acid 28.158 C9 H8 O3 164.0261 163.0261 119.0230 E ( METLIN Database ; Shrestha, 2016 ) 55 Methoxy Eriodictyol-O -hexoside 28.172 C22 H24 O12 480.0988 479.0988 317.0256; 287.0126; 125.0825 M Fabre et al. (2001) 56 Naringenin-O -hexoside 28.246 C21 H22 O10 434.1246 433.1246 271.0573; 151.0069; 106.9243 E Shrestha (2016) 57 Eriodictyol-O -hexoside 28.502 C21 H22 O11 450.1243 449.1243 287.0560; 151.0526; 135.0756; 125.0825; 106.9500 M, W Fabre et al. (2001) 58 Eriodictyol-O -hexoside derivative 28.516 486.0976 485.0976 449.1293; 287.0604; 151.0526; 135.0756 W Fabre et al. (2001) 59 Myricetin-O -pentoside 28.762 C20 H18 O12 450.0859 449.0859 317.0308; 316.0199; 271.0177 W Shrestha (2016) 60 Quinic acid derivative-O-acetylhexoside 28.870 540.2429 539.2429 335.2412; 191.0224 M Shrestha (2016) 61 Myricetin-O -hexoside 28.832 C21 H20 O13 480.0957 479.0957 317.0293; 316.0281; 271.0236; 179.0177; 150.9469 E Shrestha (2016) 62 Myricetin-O -rhamnoside 29.089 C21 H20 O12 464.1052 463.1052 317.0143; 316.0281; 271.0141; 179.0149; 151.0139 M, W Shrestha (2016) 63 Myricetin-O -pentoside 29.306 C20 H18 O12 450.0903 449.0903 317.0310; 316.0281; 271.0187; 179.0425 M, W Shrestha (2016) 64 Eriodictyol-O-hexoside 29.511 C21 H22 O11 450.1202 449.1202 287.0588; 151.0526 E Fabre et al. (2001) 65 Quercetin-O -hexoside 29.593 C21 H20 O12 464.1052 463.1052 301.0172; 179.0202; 151.2736 M, W Shrestha (2016) 66 Laricitrin-O -hexoside 29.821 C22 H22 O13 494.1109 493.1109 331.0405; 316.0183; 287.0113; 271.0117 E Shrestha (2016) 67 Myricetin-O -pentoside 29.964 C20 H18 O12 450.0846 449.0846 317.0293; 316.0199; 287.0383; 271.0102; 179.0177; 150.9469 E Shrestha (2016) 68 Myricetin-O -rhamnoside 30.003 C21 H20 O12 464.1003 463.1003 317.0313; 316.0300; 271.0102; 179.0382; 151.0525 E Shrestha (2016) 69 Quercetin-O -hexoside 30.411 C21 H20 O12 464.1003 463.1003 301.0603; 178.9662; 150.9424 E Shrestha (2016) 70 Unknown 30.546 428.1773 427.1773 325.1458; 222.0531; 124.9457 M, W 71 Quercetin-O -pentoside 30.912 C20 H18 O11 434.0950 433.0950 301.0318; 179.0285; 151.0258 E, M, W Shrestha (2016) 72 Quercetin-O -pentoside 31.023 C20 H18 O11 434.0950 433.0950 301.0341; 179.0285; 151.0258 E, M, W Shrestha (2016) 73 Quercetin-O -rhamnoside 31.381 C21 H20 O11 448.1105 447.1105 301.0353; 179.0285; 151.0258 E, M, W Shrestha (2016) 74 Eriodictyol-O -hexoside 31.599 C21 H22 O11 450.1243 449.1243 287.0560; 151.0526; 135.0756; 125.0825; 106.9500 M Fabre et al. (2001) 75 Phloridzin 32.150 C21 H24 O10 436.1463 435.1463 273.0981; 167.0324 M, W ( METLIN Database ) 76 Myricetin 32.758 C15 H10 O8 318.0354 317.0354 179.0801; 151.0130 M, W Shrestha (2016) 77 Amburoside B 32.921 C21 H24 O10 436.1412 435.1412 273.0699; 167.0172; 123.0106 E Shrestha (2016) 78 Kaempferol-O -pentoside 33.654 C20 H18 O10 418.0945 417.0945 285.0322; 255.0083; 150.9798 E Shrestha (2016) 79 Unknown 34.050 260.0150 259.0150 248.1207; 191.0280; 171.0359 W 80 Quercetin-O -acetylpentoside 34.193 C22 H20 O12 476.1011 475.1011 301.0379; 300.0308; 271.0167; 198.9482; 150.9440 E Shrestha (2016) 81 Quercetin-O -rhamnoside-O -hexoside 34.687 C27 H30 O16 610.1322 609.1322 463.1026; 301.0338; 300.0207; 272.0279 W Shrestha (2016) 82 Eriodictyol 35.071 C15 H12 O6 288.0557 287.0557 287.0560; 151.0526; 135.0756; 125.0825; 106.9500 E, M, W Fabre et al. (2001) 83 Laricitrin-O -glucuronide 35.199 C22 H20 O14 508.0919 507.0919 331.0565; 316.0249; 271.0186 W Shrestha (2016) 84 Methylquercetin-O -hamnoside 35.498 C22 H22 O11 462.1261 461.1261 315.0572 300.0279 M Fabre et al. (2001) ; Metlin Database 85 Quercetin 36.867 C15 H10 O7 302.0350 301.0350 273.0455; 229.0311; 179.0284; 151.0162; 120.9409; 106.9212 E, M, W Fabre et al. (2001) 86 Vanillic acid derivative 37.492 328.2250 327.2250 211.1088; 229.1282; 167.0990; 123.0128 E, M, W Shrestha (2016) 87 Phloretin 38.406 C15 H14 O5 274.0725 273.0725 167.0322; 125.0436 M, ( METLIN Database ) 88 Naringenin 38.704 C15 H12 O5 272.0548 271.0548 177.0562; 151.0073 E Shrestha (2016) 89 Sinapoyl Quercetin-O -hexoside 38.936 C32 H30 O16 670.1937 669.1937 463.1003; 301.0364 M, W Shrestha (2016) 90 Kaempferol 39.599 C15 H10 O6 286.0389 285.0389 151.0015 M ( METLIN Database ) 91 Unknown 46.693 488.3571 487.3571 – E 92 Unknown 47.130 310.2089 309.2089 291.1974; 197.0920; 171.0577 E 93 Unknown 47.790 488.3571 487.3571 – E E – ethyl acetate extract, M – methanol extract, W – aqueous extract.
coupled with mass spectrometry might be used as a more accurate method to evaluate the secondary metabolite composition of plant ex-tracts, including R. luteum extracts. In our study, HPLC-ESI-MSn was employed and results are presented inTable 2.
Studied extracts were abundant in phenolic compounds. Monomers and dimers of phenolic acids (2,3-dihydroxybenzoic, caffeic, gallic, ferulic, vanillic, and quinic acids) were detected in free and glycosy-lated forms. Galloyglycosy-lated or glycosyglycosy-lated catechins and catechin reglycosy-lated compounds were present as well as several classes offlavonoid com-pounds (flavonols, flavanonol, flavanones, flavone and dihy-drochalcones), their aglycones and O-glycosides. The major difference between studied extracts was observed in terms of intensity of detected compounds. In general, all samples contained very similar molecules. However, the highest abundance and intensity of compounds were detected in the aqueous extract (Fig. 1). Major constituents of this sample were (epi)catechin (28) and its derivatives ((epi)gallocatechin, 12; procyanidin dimer B1, 12; gambiriin A/D, 27; (epi)catechin deri-vative, 29), caffeoylquinic acids (20, 25, 30, 33) and glycosides of myricetin (51, 62, 63) and quercetin (65, 72, 73). Flavonol glycosides (myricetin-O-hexoside, 51; myricetin-O-rhamnoside, 62; quercetin-O-hexoside, 65; myricetin-O-rhamnoside, 68; quercetin-O-pentoside, 72) were prominent in the methanolic extract. Myricetin (76) and quercetin (85) in free form was also detected in significant amounts. Dihy-dromyricetin (46) and again glycosides of myricetin (61, 67, 68) and quercetin (69, 72, 73) were found in large amounts in ethyl acetate extract.
Compounds identified in studied samples were previously reported in various Rhododendron species (Shrestha, 2016). As shown inFig. 1 andTable 2, caffeoylquinic acids, glycosides of myricetin and quercetin were characteristic for R. luteum. Vanilloyl-O-hydroxybenzoyl-hexoside was indicated as a chemotaxonomic marker for R. luteum (Shrestha, 2016). Interestingly, this molecule was detected in small amount in ethyl acetate extract of this species.
The selection of appropriate extracting solvent is necessary to re-cover main bioactive compounds and to obtain higher pharmacological activity. As an example, polar solvents are commonly employed for the extraction of phenolic compounds, while non-polar solvents are ade-quate for the recovery of steroids and fatty acids. Indeed, phenolic compounds are one of the most common compounds in medicinal herbals and represent a crucial part of the human diet due to their beneficial health properties (Balasundram et al., 2006). Several studies reported the influence of solvents used during extraction process on the biological activities of numerous plant species (Dirar et al., 2019; Zengin et al., 2019a). However, there is no report on the impact of extracting solvent on the biological activities of R. luteum. With the view of the possible usefulness of R. luteum to manage/prevent some human's health problems, R. luteum extracts were investigated to in-dicate their antioxidant properties and inhibitory activity against some key enzymes targeted for the management of diabetes (α-amylase and α-glucosidase), Alzheimer's diseases (butyrylcholinesterase [BChE] and acetylcholinesterase [AChE]), and skin disorders (tyrosinase).
Table 3 summarises the data for different evaluated antioxidant assays. In general, the methanol and water extracts of R. luteum pos-sessed the highest total antioxidant capacity, radical scavenging, and reducing properties. However, the water extract showed the highest radical scavenging in the ABTS assay and the highest reducing ability in both the FRAP and CUPRAC assays. Regarding to the DPPH assay, the highest activity was exhibited by the methanol extract. The reduction of molybdenum (VI) to molybdenum (V) by electron transfer, also known as the phosphomolybdenum assay, was employed to evaluate the total antioxidant activity of R. luteum extracts (Rocchetti et al., 2019). The methanol and water extracts (3.42 mmol TE/g extract) showed highest total antioxidant activity and the ethyl acetate extract (1.58 mmol TE/g extract) was the least active (Table 3). Regarding the metal chelating potential, the ethyl acetate extract of R. luteum exhibited the highest activity.
Fig. 1. LC chromatograms of analysed samples. Numbers of compounds refer toTable 2. From above: ethyl acetate extract, methanolic extract, aqueous extract.
Table 3
Antioxidant activities of Rhododendron luteum extracts.
DPPH (mg TE/g extract) ABTS (mg TE/g extract) CUPRAC (mg TE/g extract)
FRAP (mg TE/g extract) Phosphomolybdenum (mmol
TE/g)
Metal chelating ability (mg EDTAE/g)
p-value 0.0001 0.0001 0.0001 0.0001 0.0001 0.0001
Ethyl acetate 41.94 ± 0.96c 38.96 ± 0.50c 108.82 ± 1.59c 42.22 ± 0.50c 1.58 ± 0.09b 45.19 ± 3.76a
Methanol 480.07 ± 0.85a 454.66 ± 6.83b 606.30 ± 3.52b 348.37 ± 13.51b 3.42 ± 0.06a 22.20 ± 0.49c
Water 381.07 ± 3.08b 525.32 ± 6.63a 687.00 ± 6.47a 412.32 ± 1.10a 3.42 ± 0.10a 28.72 ± 0.29b
* Values expressed are means ± S.D. of three parallel measurements. TE: Trolox equivalent; EDTAE: EDTA equivalent. Different superscripts indicate significant
Other studies have observed and reported the relationship between high phenolic/flavonoid content and pronounced antioxidant activity. The antioxidant potential of secondary plant metabolites relates to their chemical structures (Kubalt, 2016). Previous assessment of the struc-ture-activity relationship of catechin and its derivatives revealed that galloylated derivatives presented relatively higher radical scavenging activity due to the presence of more hydroxyl groups as well as the ester function at C-3 (Es-Safi et al., 2007). Myricetin, identified in R. luteum methanol and water extracts, was previously reported to possess ex-cellent reducing capacity owing to its unique chemical structure, in-cluding pyrone and two phenyl rings (Barzegar, 2016). Dihy-drochalcones, such as phloretin and its glucoside, phloridzin, were found to exhibit antioxidant action through radical adduct formation, as well as redox-based reactions, including electron and hydrogen atom transfer (Li et al., 2018).
The inhibitory action of R. luteum extracts against enzymes are presented inTable 4. Dementia, an umbrella term for several conditions characterised by the progressive deterioration of memory, cognitive abilities, and behaviour, includes Alzheimer's disease, which accounts for 60–70% of cases (Organization, 2017). Indeed, Alzheimer's disease is one of the most common geriatric complications. It has been postu-lated that cholinesterase inhibitors could significantly delay the
progress of Alzheimer's disease by preventing the hydrolysis of acet-ylcholine, a neurotransmitter. Evidences from clinical studies claim the involvement of AChE and BChE in the pathogenesis of Alzheimer's disease (Mesulam, 1994;Mushtaq et al., 2014;Sokolow et al., 2017), though other factors such as oxidative stress, might also be involved. The role of these cholinesterase enzymes supports the quest for novel therapeutic candidates possessing both AChE and BChE inhibitory ac-tivity. Methanol and ethyl acetate extracts showed the highest AChE and BChE inhibitions, respectively (Table 4). The ability of R. luteum extracts to inhibitα-amylase and α-glucosidase was also determined. These enzymes have been targeted in the management of diabetes type II, the most common form of diabetes which results from insulin dys-function/resistance provoked by environmental and genetic factors (Shahbazian et al., 2019). Over the past decades, substantial effort has been made to unveil the relationship between diabetes type II and Alzheimer's disease. Research studies have established that diabetes type II and Alzheimer's disease were intertwined complications and that insulin, which controls neuronal and synaptic functions of the brain, was observed to play a pivotal role (Riaz et al., 2015). The statement mentioned above highlights the need for the development of drug candidates possessing dual action on enzymes related to Alzheimer's disease and diabetes type II. In the present study, it was observed that Table 4
Enzyme inhibitory properties of Rhododendron luteum extracts.
AChE (mg GALAE/g extract) BChE (mg GALAE/g extract) Tyrosinase (mg KAE/g extract)
α-amylase (mmol ACAE/g extract)
α-glucosidase (mmol ACAE/g extract)
p-value 0.0001 0.0001 0.0001 0.0001 0.0001
Ethyl acetate 3.84 ± 0.44b 2.74 ± 0.10a 122.44 ± 1.23b 0.84 ± 0.05b 24.19 ± 00.07b
Methanol 4.87 ± 0.28a 2.07 ± 0.19b 149.04 ± 0.37a 0.94 ± 0.02a 25.02 ± 00.05a
Water 3.80 ± 0.06b 0.48 ± 0.09c 85.39 ± 2.64c 0.18 ± 0.01c na
* Values expressed are means ± S.D. of three parallel measurements. GALAE: Galantamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active. Different superscripts indicate significant differences in the tested extracts (p < 0.05).
Fig. 2. Principal component analysis and hierarchical clustering on biological activities of Rhododendron luteum. A: scree plot of percentage of explained variances by
each component. B&C: loading plot and bar plot describing the link of evaluated biological activities with thefirst two principal components. D: Typology of studied
R. luteum methanol and ethyl acetate extracts showed the highest in-hibition against α-glucosidase (Table 4) while the water extract ex-hibited no activity.
On the other hand, all R. luteum extracts displayed low inhibition against α-amylase. In the quest for new anti-diabetic candidates, moderateα-amylase inhibition and pronounced α-glucosidase is highly desired since high inhibition ofα-amylase has been linked to gastro-intestinal disturbances caused by undigested food. Interestingly, epi-gallocatechin ([M-H]-at m/z 305) identified in the ethyl acetate and methanol extracts of R. luteum has been previously found to inhibit AChE, BChE, andα-glucosidase (Barbosa et al., 2015;Jabir et al., 2018; Zhu et al., 2010). Inhibitory action of R. luteum extracts against tyr-osinase was assessed in vitro. Tyrtyr-osinase is targeted to modulate the biosynthesis of melanin, a brown pigment which protects the human skin from harmful UV rays (Sut et al., 2019). However, excessive pro-duction and accumulation of melanin lead to the development of der-matological conditions and epidermal hyperpigmentation problems. Therefore, tyrosinase inhibitors find application in the cosmetic and dermatological fields for the treatment of skin hyperpigmentation conditions, such as acne scar, age spots, and melisma (Mollica et al., 2018) R. luteum extracts showed inhibition against tyrosinase in the following order methanol extract > ethyl acetate extract > water extract. Through the use of statistical methods, our data revealed that polar solvent, particularly methanol, was the appropriate solvent having allowed leading the best biological activity of R. luteum.
As can be observed, a significant difference was obtained between samples for each bioassays conducted. In order to evaluate the effect of three extracting solvents, a dataset, including enzymatic inhibitory and antioxidant activities, was submitted to multivariate analysis in
substance, PCA. PCA is a valuable multivariate method for complex datasets by reducing the dimension of data in a few principal compo-nents, which describe variation and account for the varied influences of the original variables. Graphical results of PCA are displayed inFig. 2. Firstly, by examining the quality of the projection, it was observed that two principal components (PCs) explained the more significant varia-bility; thefirst two PCs explained 98.02% of total variance with 65.20% and 32.82% of the total variance was represented by PC1 and PC2, respectively (Fig. 2A). The loading plot of biological activities, and how each of them influenced a first two requested principal component were shown inFig. 2B–C. As observed, all biological activities overall were well represented and influenced the model (Fig. 2B). Although, PC1 was mainly characterized by CUPRAC, FRAP, DPPH, ABTS, and phospho-molydenum (positive side), BChE and metal chelating (negative side) while along with PC2 the major influenced biological was AChE, tyr-osinase, α-amylase, and α-glucosidase (positive side) (Fig. 2B). Ac-cordingly, PC1 could be seen as the relation of the increase in radical scavenging, reducing power, and total antioxidant properties of sam-ples and the decrease of their ability to suppress BChE enzyme and to chelate iron metal. PC2 could be interpreted as the increase of samples potentialities to manage diabetes mellitus, skin disorders, and Alzhei-mer's disease by targeting acetylcholinesterase.
On the other hand, based on similarity of the biological activities of the samples, the PCA score plot discriminated the samples in three different groups (Fig. 2D). Similarly, a heat map was performed based on the PCA, provided the classification of samples inside three clusters following a factorial plan PC1 and PC2 (Fig. 2E). For instance, the solvents used for the extraction had a significant influence on the overall biological activities of R. luteum.
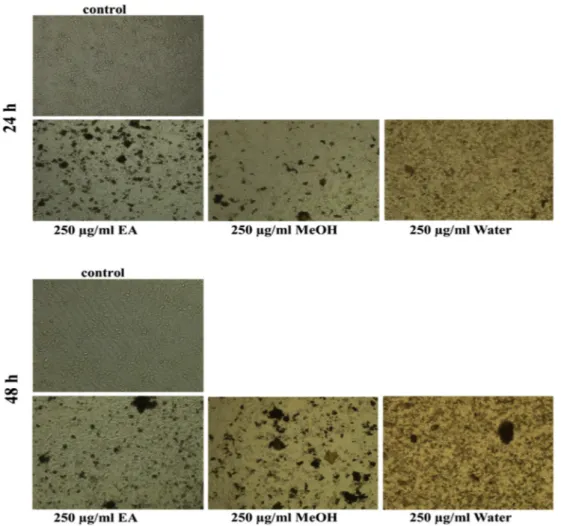
Antiproliferative activity assay of three types of extracts was also evaluated by MTT-based cytotoxic analysis. A549 lung cancer cells were treated with varying concentrations of ethyl acetate, methanol, and water extracts for 24 and 48 h. Results showed that water extracts exhibited the lowest inhibitory effective concentration against A549 cancer cell line. Fig. 3 illustrates cell images after treatment with 250μg/ml of all three extracts that was the beginning concentration of cytotoxicity, under an inverted microscope. Also, as shown inFig. 4, cell viability decreased notably after treatment with water extracts in a dose and time-dependent manner.
Half maximal inhibitory concentrations (IC50) against cells were calculated as 732.5 μg/ml and 207.2 μg/ml for water extracts and 805.2μg/ml and 584.2 μg/ml for ethyl acetate extracts for 24 and 48 h, respectively. Besides, inhibitory concentration against cells was de-termined as 308μg/ml for methanol extracts for 48 h. Results showed that 207.2μg/ml of water extracts would be used for further experi-mental analyses in order to investigate intracellular biological process on A549 cancer cells since the minimum effective dose is important for highlighting mechanism in therapy in vitro. Cytotoxic activity of ethyl acetate and water extracts against A549 cancer cells can be associated to caffeic acid and some epicatechin derivatives. According to some reports, caffeic acid derivatives increased the cytotoxicity against some breast cancer cells (Serafim et al., 2011). Caffeic acid phenethyl esters were found to be the most bioactive compound with antiproliferative activity against cancer cells including breast, prostate, lung, cervical, head and neck cancer cells (Kuo et al., 2015). Water extract also in-cluded naringenin, apigenin, ferulic acid, and myricetin compounds different from other extracts. Among these compounds, lots of drug design researches focused on ferulic acid. Ferulic acid is a good anti-oxidant compound found in vegetables and fruits (Srinivasan et al.,
2007). Also, some researches have revealed anti-apoptotic effects of ferulic acid in normal human peripheral blood mononuclear cells ex-posed H2O2-induced oxidative stress (Khanduja et al., 2006).
4. Conclusion
In summary, the methanol and water extracts of R. luteum, posses-sing highestflavonoid and phenolic contents respectively, showed the highest antioxidant activities in all assays carried out except for the metal chelation assay, where the ethyl acetate extract was the most active. The observed antioxidant activity might be caused by the sy-nergistic action of secondary metabolites present in the extracts. In terms of enzyme inhibition, the ethyl acetate and methanol extracts of R. luteum were active against AChE, BChE, α-glucosidase, and tyr-osinase. Advanced chromatographic techniques coupled with mass spectrometry used to obtain more accurate profiles of R. luteum extracts revealed the presence of secondary metabolites known to possess an-tioxidant and enzyme modulatory abilities. The tested extracts, espe-cially water extract, exhibited notable anticancer effect correlated with higher levels of bioactive compounds. Data presented in this study advocates for further studies to isolate and assess the enzyme inhibitory action of epigallocatechin naturally occurring in R. luteum.
Author contribution
Mohamad Fawzi Mahomoodally: Conceptualization; Data curation; Investigation: Writing - original draft/revision. Elwira Sieniawska: Conceptualization; Data curation; Investigation; Writing - original draft. Kouadio Ibrahime Sinan: Conceptualization; Methodology; Software. Marie Carene Nancy Picot-Allain: Writing - original draft. Serife Yerlikaya: Conceptualization; Data curation; Investigation: Writing -original draft/revision. Mehmet Cengiz Baloglu: Conceptualization; Data curation; Investigation. Yasemin Celik Altunoglu: Conceptualization; Data curation; Investigation. Ismail Senkardes: Methodology. Kannan RR Rengasamy: Conceptualization; Data cura-tion; Investigation. Gokhan Zengin: Conceptualizacura-tion; Data curacura-tion; Investigation: Writing - original draft/revision.
Declaration of competing interest
The authors declare that they have no known competingfinancial interests or personal relationships that could have appeared to in flu-ence the work reported in this paper.
References
Balasundram, N., Sundram, K., Samman, S., 2006. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 99, 191–203.
Barbosa, J.S., Almeida, V.M., Marcal, R.M., Branco, A., 2015. RP-HPLC-DAD-MS(n) Analysis and Butyrylcholinesterase inhibitory activity of Barbacenia blanchetii ex-tracts. Nat. Prod. Commun. 10, 983–986.
Barzegar, A., 2016. Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Mol. Biol. Res. Commun. 5, 87–95.
Belwal, T., Ezzat, S.M., Rastrelli, L., Bhatt, I.D., Daglia, M., Baldi, A., Devkota, H.P., Orhan, I.E., Patra, J.K., Das, G., 2018. A critical analysis of extraction techniques used for botanicals: trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 100, 82–102.
Bilir, E.K., Tutun, H., Sevin, S., Kismali, G., Yarsan, E., 2018. Cytotoxic effects of Rhododendron ponticum L. extract on prostate carcinoma and adenocarcinoma cell line (DU145, PC3). Kafkas Üniversitesi Veteriner Fakültesi Derg. 24.
Clifford, M.N., Knight, S., Surucu, B., Kuhnert, N., 2006. Characterization by LC-MS n of four new classes of chlorogenic acids in green coffee beans:
dimethox-ycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxdimethox-ycinnamoylquinic acids, and feruloyl-dimethoxycinnamoylquinic acids. J. Agric. Food Chem. 54, 1957–1969.
Demir, S., Turan, I., Aliyazicioglu, Y., 2016. Selective cytotoxic effect of Rhododendron luteum extract on human colon and liver cancer cells. J. BUON 21, 883–888. Dhawan, D., Gupta, J., 2017. Comparison of different solvents for phytochemical
ex-traction potential from Datura metel plant leaves. Int. J. Biol. Chem. 11, 17–22. Dirar, A., Alsaadi, D., Wada, M., Mohamed, M., Watanabe, T., Devkota, H., 2019. Effects Fig. 4. Cell survival (%) after treated with various doses of extracts for 24 and
48 h. Cell viability initiated to reduce when exposed to higher dose from
of extraction solvents on total phenolic andflavonoid contents and biological activ-ities of extracts from Sudanese medicinal plants. South Afr. J. Bot. 120, 261–267. Es-Safi, N.-E., Bauhaire, J., Guerneve, C., Ducrot, P.-H., 2007. Synthesis and antioxidant
activity of modified (+)-catechin derivatives. Strucure-activity relationship. Am. J. Food Technol. 2, 618–629.
Fabre, N., Rustan, I., de Hoffmann, E., Quetin-Leclercq, J., 2001. Determination of fla-vone,flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 12, 707–715. Granato, D., Shahidi, F., Wrolstad, R., Kilmartin, P., Melton, L.D., Hidalgo, F.J.,
Miyashita, K., Camp, J.v., Alasalvar, C., Ismail, A.B., Elmore, S., Birch, G.G., Charalampopoulos, D., Astley, S.B., Pegg, R., Zhou, P., Finglas, P., 2018. Antioxidant activity, total phenolics andflavonoids contents: should we ban in vitro screening methods? Food Chem. 264, 471–475.
Gunduz, A., Suleyman, T., Russell, R., Ayaz, F., 2008. Clinical review of grayanotoxin/ mad honey poisoning past and present. Clin. Toxicol. 46, 437–442.
Jabir, N.R., Khan, F.R., Tabrez, S., 2018. Cholinesterase targeting by polyphenols: a therapeutic approach for the treatment of Alzheimer's disease. CNS Neurosci. Ther. 24, 753–762.
Khanduja, K.L., Avti, P.K., Kumar, S., Mittal, N., Sohi, K.K., Pathak, C.M., 2006. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: a Bcl-2 independent mechanism. Biochim. Biophys. Acta Gen. Subj. 1760, 283–289.
Kubalt, K., 2016. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 80 (2), 97–108.
Kuo, Y.-Y., Jim, W.-T., Su, L.-C., Chung, C.-J., Lin, C.-Y., Huo, C., Tseng, J.-C., Huang, S.-H., Lai, C.-J., Chen, B.-C., 2015. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 16, 10748–10766.
Küçük, S., Kurkcuoglu, M., Goger, F., Baser, K.H.C., 2010. Morphological, chemical and indumentum characteristics of Rhododendron luteum Sweet (Ericaceae). Pak. J. Bot. 42, 3729–3737.
Li, X., Chen, B., Xie, H., He, Y., Zhong, D., Chen, D., 2018. Antioxidant structure activity relationship analysis offive dihydrochalcones. Molecules 23, 1162.
Mesulam, M.-M., 1994. Butyrylcholinesterase in Alzheimer's Disease. Birkhäuser Boston, Boston, MA, pp. 79–83.
METLIN Database, "Scripps Center for Metabolomics.".
Mollica, A., Zengin, G., Locatelli, M., Picot-Allain, C.M.N., Mahomoodally, M.F., 2018. Multidirectional investigations on different parts of Allium scorodoprasum L. subsp. rotundum (L.) Stearn: phenolic components, in vitro biological, and in silico pro-pensities. Food Res. Int. 108, 641–649.
Mushtaq, G., Greig, N.H., Khan, J.A., Kamal, M.A., 2014. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer's disease and type 2 diabetes mellitus. CNS Neurol. Disord. - Drug Targets 13, 1432–1439.
Organization, W.H., 2017. Global Action Plan on the Public Health Response to Dementia 2017–2025.
Orhan, I.,Şener, B., Choudhary, M.I., Khalid, A., 2004. Acetylcholinesterase and butyr-ylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 91, 57–60.
Popescu, R., Kopp, B., 2013. The genus Rhododendron: an ethnopharmacological and toxicological review. J. Ethnopharmacol. 147, 42–62.
Riaz, S., Khan, I.U., Bajda, M., Ashraf, M., Qurat ul, A., Shaukat, A., Rehman, T.U., Mutahir, S., Hussain, S., Mustafa, G., Yar, M., 2015. Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and Alzheimer's disease: in vitro studies against yeastα-glucosidase, acetylcholinesterase and butyrylcholinesterase. Bioorg. Chem. 63, 64–71.
Rocchetti, G., Senizza, B., Zengin, G., Senkardes, I., Bibi Sadeer, N., Fawzi Mahomoodally, M., Lucini, L., 2019. Metabolomics-based profiling with chemometric approach to delineate the bio-pharmaceutical properties of fruit extracts from Ligustrum vulgare L. Ind. Crops Prod. 140, 111635.
Serafim, T.L., Carvalho, F.S., Marques, M.P., Calheiros, R., Silva, T., Garrido, J., Milhazes, N., Borges, F., Roleira, F., Silva, E.T., 2011. Lipophilic caffeic and ferulic acid deri-vatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 24, 763–774.
Shahbazian, H., Aleali, A.M., Rashidi, H., Latifi, S.M., Rashidi, M., Yazdanpanah, L., Zaman, F., Payami, S.P., Moradi, L., Jahanshahi, A., Sedaghat, A., Zakerkish, M., Moradi, M., 2019. Frequency of type I and II diabetes in newly diagnosed diabetic patients: measuring C-Peptide level. Diabetes, Metab. Syndrome 13, 1833–1835. Shrestha, A., 2016. Phytochemical Analysis of Rhododendron Species. IRC-Library,
Information Resource Center der Jacobs University Bremen.
Shrestha, A., Hakeem Said, I., Grimbs, A., Thielen, N., Lansing, L., Schepker, H., Kuhnert, N., 2017. Determination of hydroxycinnamic acids present in Rhododendron species. Phytochemistry 144, 216–225.
Sokolow, S., Li, X., Chen, L., Taylor, K.D., Rotter, J.I., Rissman, R.A., Aisen, P.S., Apostolova, L.G., 2017. Deleterious effect of butyrylcholinesterase K-Variant in do-nepezil treatment of mild cognitive impairment. J. Alzheimer's Dis. 56, 229–237. Srinivasan, M., Sudheer, A.R., Menon, V.P., 2007. Ferulic acid: therapeutic potential
through its antioxidant property. J. Clin. Biochem. Nutr. 40, 92–100.
Sut, S., Dall'Acqua, S., Zengin, G., Senkardes, I., Bulut, G., Cvetanović, A., Stupar, A., Mandić, A., Picot-Allain, C., Dogan, A., Ibrahime Sinan, K., Mahomoodally, F., 2019. Influence of different extraction techniques on the chemical profile and biological properties of Anthemis cotula L.: multifunctional aspects for potential pharmaceutical applications. J. Pharm. Biomed. Anal. 173, 75–85.
Tong, Q., Hou, X., Fang, J., Wang, W., Xiong, W., Liu, X., Xie, X., Shi, C., 2015. Determination of dihydromyricetin in rat plasma by LC–MS/MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 114, 455–461.
WHO, 2018. World Health Organization & WHO Expert Committee on Specifications for Pharmaceutical Preparations. World Health Organization Fifty-second report of the WHO Expert Committee on Specifications for Pharmaceutical Preparations. Wu, C., Xu, H., Héritier, J., Andlauer, W., 2012. Determination of catechins andflavonol
glycosides in Chinese tea varieties. Food Chem. 132, 144–149.
Yerlikaya, S., Baloglu, M.C., Diuzheva, A., Jeko, J., Cziáky, Z., Zengin, G., 2019. Investigation of chemical profile, biological properties of Lotus corniculatus L. extracts and their apoptotic-autophagic effects on breast cancer cells. J. Pharm. Biomed. Anal. 174, 286–299.
Zengin, G., 2016. A study on in vitro enzyme inhibitory properties of Asphodeline ana-tolica: new sources of natural inhibitors for public health problems. Ind. Crops Prod. 83, 39–43.
Zengin, G., Aktumsek, A., 2014. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: an endemic plant to Turkey. Afr. J. Tradit., Complementary Altern. Med. 11, 481–488.
Zengin, G., Ferrante, C., Gnapi, D.E., Sinan, K.I., Orlando, G., Recinella, L., Diuzheva, A., Jekő, J., Cziáky, Z., Chiavaroli, A., 2019a. Comprehensive approaches on the che-mical constituents and pharmacological properties offlowers and leaves of American basil (Ocimum americanum L). Food Res. Int. 125, 108610.
Zengin, G., Sieniawska, E., Senkardes, I., Picot-Allain, M.C.N., Sinan, K.I., Mahomoodally, M.F., 2019b. Antioxidant abilities, key enzyme inhibitory potential and phyto-chemical profile of Tanacetum poteriifolium Grierson. Ind. Crops Prod. 140, 111629. Zhu, Y., Ding, H., Wang, Z., Yang, Y., Xiang, X., 2010. Effect of epigallocatechin-3-gallate on the activity of alpha-glucosidase in vitro. Wei Sheng Yan Jiu 39, 168–171 176.