Effects of endogenous molasses carbon dots on macrophages
and their potential utilization as anti‑inflammatory agents
Emine Yavuz1 · Saliha Dinc2 · Meryem Kara3
Received: 29 August 2019 / Accepted: 27 November 2019 / Published online: 12 December 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract
The biological effect of endogenous food-borne carbon dots (CDs) is one of the controversial issues of current research areas in food biotechnology. In this study, the biocompatibility and biofunctionality of sugar beet molasses-derived fluorescent carbon dots (CDs) were investigated for the first time in mammalian macrophage and fibroblast cells. The molasses CDs were nearly spherical in shape and monodispersed with a typical amorphous structure and a particle size distribution in the range of 1.3–3.8 nm. The anionic molasses CDs could easily enter the cells and exhibited excellent biocompatibility. Importantly, CDs with high photostability not only enabled the intracellular tracking of the nanomaterials by confocal microscopy, but also could reduce the LPS-induced NO production in RAW 264.7 cells. Altogether, endogenous CDs with dual functionality, bioimaging and anti-inflammatory effect, are believed to have great potential as macrophage-mediated theranostic agents.
1 Introduction
Since their discovery in 2004 [1], fluorescent and zero-dimensional carbon dots (CDs) with sizes below 10 nm have drawn significant attention due to their unique char-acteristics including excellent water solubility, low toxicity, excellent biocompatibility, high photostability, chemical inertness and facile, green and low-cost synthesis [2–11]. Hence, carbon dots have found many applications in the fields of sensors [12–14], nanomedicine [15–17], optoelec-tronics [18, 19], electrocatalysis [20], photocatalysis [21,
22], bacterial labeling [23, 24], and bio-imaging [14, 25–27]. In addition to their production by a variety of methods such
as hydrothermal carbonization [28–30], combustion [31], microwave-assisted synthesis [32–34], and chemical oxida-tion [35, 36], carbon dots can also be extracted from various food sources exposed to heating process [37–40]. Recently, Song et al. showed the formation of fluorescent carbon nano-particles during the roasting of chicken breasts which was attributed to the Maillard and a series of chemical reactions [41]. Similarly, thermal food processing in sugar production from sugar beet resulted in the natural formation of carbon dots as well [37, 42].
Toxicity and possible biological drawbacks of endog-enous nanoparticles generated during food manufacturing are a controversial issue of ongoing academic discussions [39, 43–46]. The ultra-small endogenous CDs can easily penetrate the cell membrane. Thus, the assessment of the biocompatibility and biofunctionality of CDs has signifi-cant importance for biomedical applications [47, 48]. Very recently, Dongmei et al. showed that endogenous CDs from a food source (i.e. canned yellow croaker) caused the sup-pression of the mitochondrial respiration and glycolysis in HepG2 cells [49]. Factually, carbon dots of different sources may have different cellular reactions depending on the cell type [50]. To our knowledge, there have been a few studies performed on the effects of CDs on macrophages [51–53]. For example, Furkan et al. showed that undoped carbon nanodots had a mild anti-inflammatory property [53] and Thoo et al. discussed the uptake efficiency of carbon dots as a drug delivery vehicle to immune cells [51]. However, the
Electronic supplementary material The online version of this article (https ://doi.org/10.1007/s0033 9-019-3189-1) contains supplementary material, which is available to authorized users. * Emine Yavuz
emineyavuz@selcuk.edu.tr * Saliha Dinc
salihadinc@gmail.com
1 Advanced Technology Research and Application Center,
Selcuk University, Konya, Turkey
2 Cumra School of Applied Sciences, Selcuk University,
Konya, Turkey
3 Cumra Vocational High School, Selcuk University, Konya,
effects of endogenous CDs on mammalian macrophages and fibroblast cells have not been studied yet.
Upon exposure, nanoparticles first encounter mac-rophages since they are strategically located against foreign materials as guards [54]. Being major inflammatory cells of the immune system, macrophages can activate the expres-sion of several genes that are responsible for the synthesis of reactive oxygen and nitrogen species (NO, O2−, H
2O2, etc.)
[55, 56]. However, irregular nitric oxide (NO) production generally causes various disorders including hypertension, atherosclerosis, diabetes, neurodegenerative diseases and tumor growth [57, 58]. Currently, anti-inflammatory/anti-oxidative therapies of nanoparticles which include the sys-temic modulation of macrophage activities have been a key research area for nanomedicine [59, 60]. Furthermore, the successful translation of nanomaterials into clinical applica-tions often requires an in-depth analysis of the immunologic reactions of these nanoproducts [61]. However, depending on the carbon source and preparation methods the immuno-logical responses of CDs may vary [38, 62–64]. For exam-ple, Yang et al. showed that carbon dots synthesized from garlic by microwave heating decreased LPS-induced NO production in macrophages [65]. On the other hand, Lat-egan et al. demonstrated that carbon dots synthesized from glycerol and NaH2PO4 via domestic microwave had no effect
on NO production of unstimulated macrophages [62]. To the best of our knowledge, the potential utilization of endog-enous CDs as anti-inflammatory agents through inhibition of NO production has not been explored until now.
Herein, endogenous carbon dots simply extracted from sugar beet molasses were used to explore their effects on RAW 264.7 macrophages and L929 mouse fibroblast cells for the first time. Importantly, the potential utilization of molasses CDs as anti-inflammatory agents was also inves-tigated. The in vitro immunotoxicological risk assessment of carbon dots were conducted on mammalian macrophage cells using XTT-based cell viability assay, real-time cell analysis assay (i.e. xCELLigence RTCA DP) and NO deter-mination assay. Furthermore, uptake and cellular tracking potential of sugar beet molasses CDs were also investi-gated by laser scanning confocal microscopy. Overall, the results of this study provide valuable information for the possible utilization of food-borne endogenous carbon dots as macrophage-mediated theranostic agents for inflamma-tory diseases.
2 Experimental procedures
2.1 MaterialsDulbecco’s modified Eagle’s medium (DMEM) with high glucose and stable glutamine, trypsin/EDTA 0.05%/0.02%
(w/v), antibiotic mixture of penicillin (100 U/mL)/strep-tomycin (100 µg/mL), and fetal bovine serum (FBS) were purchased from Biochrom (Berlin, Germany). Dulbecco’s phosphate-buffered saline solution (1 × DPBS, Ca and Mg free) and aqueous nonradioactive cell proliferation kit (XTT- (sodium3′-[1-[(phenylamino)-carbony]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate)-) were obtained from Biological Industries (Israel). Mouse macrophage cell line RAW 264.7 cells (ATTC ® TIB-71™)
was supplied from American Type Culture Collection (ATCC) (LGC Standards GmbH, Wesel, Germany). Alexa Fluor 680 Phalloidin and Griess reagent kit were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Lipopolysaccharide (LPS) from Escherichia coli 055:B5 was supplied from Sigma–Aldrich, Inc. (St. Louis, MO, USA). Ultrapure water (18.2 MΩ cm at 25 °C, Merck Millipore, Germany) was used as needed.
2.2 Extraction and characterization of carbon dots
Carbon dots were extracted with water from sugar beet molasses (from Konya Sugar Factory) as previously pub-lished by Dinc [37]. Briefly, a homogenous solution of 5 g of sugar beet molasses was obtained with 10 mL of deionized water. After 5 min centrifugation at 5000 rpm, supernatant rich in CDs was diluted with water (1:80). In the previous study [37], CDs have been characterized using UV–Vis spectroscopy, scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX), FT-IR, atomic force microscopy (AFM) and fluorescence spectroscopy. The photostabilities of CDs and DAPI were measured using a fluorescence spectrophotometer (Hitachi F-7000, Japan). Photostability data were collected every 3 min. Transmission electron microscope (JEOL, JEM-2100 UHR, Japan) was used to examine the morphologies of molasses CDs. Fol-lowing the dilution of CDs with ethanol, CDs were dropped onto the carbon-coated copper grid and dried to be analyzed by TEM. Selected area electron diffraction (SAED) analysis was performed in TEM to understand the crystallographic properties of molasses CDs. X-ray diffraction (XRD) pat-terns of molasses CDs were taken with Bruker D8-Advance diffractometer operating at 40 kV using CuKα radiation (λ = 1.5418 Å). The sample for XRD analysis was prepared by dropping CD onto cleaned glass wafer. Zeta potential of CDs was recorded by Zetasizer Nano-ZS (Malvern Instru-ments Pvt. Ltd., UK).
2.3 Cell culture
Murine macrophage cell line, RAW 264.7, (ATCC TIB-71, LGC Standards GmbH, Wesel, Germany) was grown in DMEM supplemented with 10% non-heated-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells
were incubated under the standard culture conditions (37 °C in 5% of CO2 and 95% humidity). RAW 264.7 cells were cultured
to 80% confluency, detached with a cell scraper and passaged. RAW 264.7 cells of the lower passages were used through-out the study (passage number less than 10). L929 cells, an immortal non-cancerous mouse fibroblast cell line, were cultured in DMEM media supplemented with high-glucose,
l-glutamine, sodium pyruvate, 1% penicillin/streptomycin
and 10% heat-inactivated FBS in an incubator of 5% CO2 at 37 °C. Confluent L929 cells were detached with 0.05% trypsin. Microscopic images of RAW 264.7 cells and L929 cells were taken by an inverted light microscope (Leica DM IL LED, Leica, Germany).
2.4 XTT‑based cell viability assay
The XTT assay is a colorimetric method based on the cleavage of the tetrazolium salt XTT into orange colored compounds of formazan in viable cells. The carbon nanodots were sonicated with cell culture medium before in vitro studies. The protocol supplied with the assay kit was used. Briefly, RAW 264.7 cells and L929 cells (with an initial seeding density of 104 cells per
well in 100 μL growth medium) were seeded into flat-bottom 96-well plates. At the logarithmic growth phase, the medium in each well was replaced with 100 μL C-dots solution (freshly prepared in serum-free medium) at different concentrations of 0–4.3 mg/mL and the cells were incubated for 24 h. After incu-bation, the XTT (Biological Industries, Israel) solution was added to the wells. The plates were incubated for another 4 h at 37 °C. Absorbance was recorded at 460 nm for formazan and 670 nm for background by a multiwell plate reader (Epoch, BioTek, Winooski, VT, USA). The results were presented as a relative value to the control in percent.
The relative cell viability was calculated using the follow-ing formula:
where Atreated is absorbance of a well with cells, XTT solu-tion, and CDs; Acontrol is absorbance of a well with cells and XTT solution, without CDs; Ablank is absorbance of well with
medium and XTT solution, without cells. The results were expressed as relative cell viability of at least two independ-ent measuremindepend-ents, ten repetitions for each sample and con-trol per assay and the values are given as mean ± standard error (SE). GraphPad Prism 6.01 (GraphPad Software, La Jolla, CA, USA) was used for statistical analyses.
2.5 Real‑time analysis of cell proliferation (RTCA) assay
xCELLigence real-time cell analyzer dual-plate instru-ment (RTCA DP, ACEA Biosciences, San Diego, CA) is
Cell viability(%) = (Atreated−Ablank∕Acontrol− Ablank) x100%,
an impedance-based dynamic microfluidic system designed for continuous real-time monitoring of changes in cell pro-liferation, morphology, and adhesion in vitro. The cell sen-sor impedance was given as an arbitrary unit termed the cell index (CI). The producer’s directions were followed to carry out the study. First, before adding cells to the wells a background measurement was performed and a minimum of triplicate wells per condition was set. Second, RAW 264.7 cells were seeded at a density of 4 × 104 cells/well into the
E-plate View 16 (ACEA Biosciences, San Diego, CA, USA) with a total volume of 100 μL in each well. Finally, when the cells entered the log phase, CDs were added at final concen-trations of 0.13, 0.27, 0.54, 1.08 and 2.15 mg/mL, and the impedance was measured every 15 min for at least 140 h at 37 °C in a 5% CO2 atmosphere. Control groups were treated
with medium only. The impedance signal was evaluated by normalizing data of each single well to the first measurement of analysis: CI (normalized) = CItime x/CInorm time. The RTCA
software v. 2.0.0 was used to obtain viability charts and IC50
value. All experiments were carried out in duplicate.
2.6 Measurement of nitric oxide production
The amount of nitrite ion produced by the macrophage cells was measured in the culture supernatant as indicia of NO production. The RAW 264.7 cells (4 × 104 cells/well) were
seeded in a 96-well plate and incubated overnight. The cells were treated with 1 μg/mL LPS (E. coli 055:B5; Sigma, MO, USA) in the presence or absence of CDs at different con-centrations (0, 1.08, and 2.15 mg/mL) for 24 h or first with C-dots for 4 h and then stimulated with LPS (1 μg/mL) for 18 h. Afterward, culture supernatants were taken and mixed with Griess reagent (G-7921; Thermo Fisher Scientific) according to the protocol of the assay kit and the absorb-ance was read at 548 nm against a standard curve of sodium nitrite with a microplate reader (Epoch, BioTek, Winooski, VT, USA). The amount of NO produced by macrophage cells was quantified using Gen5 data analysis software ver-sion 2.00 (BioTek, VT, USA). The positive control was the cells only stimulated by LPS (1 μg/mL) without the presence of carbon dots.
2.7 In vitro CLSM imaging
For seeking the bioimaging potential of molasses CDs and investigating their intracellular distribution in macrophages, cells were stained with Alexa Fluor 680 phalloidin (red channel; 679 nm/702 nm) to label actin filaments of the cells. Briefly, RAW 264.7 cells (4 × 105 cells/well) were
seeded in special glass-bottom 24-well plates (SensoPlate, Greiner Bio-One, USA) and incubated overnight at 37 °C in a 5% CO2 atmosphere. Then, cells were treated with different doses of CDs (0.27–2.15 mg/mL) for 24 h. The
non-internalized carbon dots were removed by washing cells with DPBS and the cells were fixed with 4% paraformalde-hyde (PFA) for 15 min at room temperature (RT). For cell membrane staining the adhering cells were permeabilized in 0.5% TritonX-100 for 5 min. Cells were subsequently stained with Alexa Fluor 680 phalloidin for 30 min. Cellular uptake images of CDs in vitro were taken by an inverted confocal laser scanning microscope (CLSM) (Nikon A1R1, Japan) equipped with an Ar (457/488/514 nm) and solid-state diode lasers (405/561/640 nm).
2.8 Statistical analysis
The statistical parameters (i.e., mean ± standard deviation (SD), Student’s t test) of experimental data were analyzed for three independent experiments using GraphPad Prism 6.01 (GraphPad Software, La Jolla, CA, USA) unless stated oth-erwise. Student’s t test was performed between the selected groups and differences were considered statistically signifi-cant at p value < 0.05, whereas p value < 0.0001 was con-sidered as highly significant.
3 Results and discussion
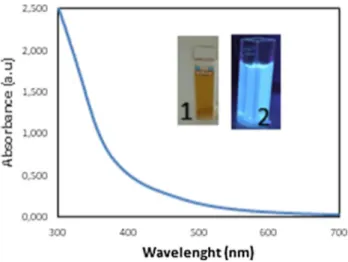
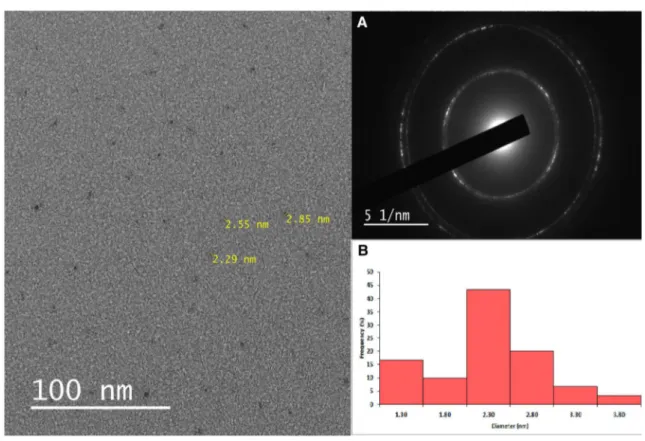
3.1 Characterization of CDsTo the best of our knowledge, this is the first study examin-ing the effect of endogenous CDs on macrophage functions and their in vitro imaging. In the present study, a light brown aqueous solution of carbon dots was obtained from sugar beet molasses, primary byproducts of sugar beet process-ing, via green, simple and low-cost extraction method [37]. Other than water, no surface passivation or additive was used during the extraction. Molasses CDs were further diluted (1:80) with water for in vitro cell applications. Upon irra-diation by UV lamp at 365 nm, molasses CDs gave strong blue photoluminescence and the highest emission intensity was achieved at 465 nm under the excitation wavelength of 370 nm (Fig. 1). For this reason, 370 nm was determined as the best excitation wavelength for imaging studies. Molasses CDs had absorption peaks owing to O–H and C–H, C=C, C–C, C–O stretchings according to FTIR data (Fig. 2). Molasses CDs had a maximum absorbance at 300 nm [37]. The absorption band at 300 nm may be associated with n–π* and π–π* electron transitions of C=C, C=O and O–H bonds. Figure 3 and Fig. S4 show a representative TEM image of molasses CDs as uniform ultra-small black dots. CDs were nearly spherical in shape and monodispersed. CDs had relatively narrow diameter distribution within 1.3–3.8 nm, resulting in average particle sizes of ca. 2.3 nm—estimated from statistical distributions (Fig. 3b). According to TEM results, spherical shape and narrow diameter distribution of
molasses CDs were similar to our previously synthesized yogurt CDs [66]. The XRD patterns of CDs in Fig. 4 exhib-ited abroad peak centered at ca. 20° (2Ɵ) which showed the amorphous structure of CDs. XRD patterns of molas-ses CDs are well correlated with those previously reported [67, 68]. The amorphous structure of CDs supported TEM-SAED analysis which is shown in Fig. 3a. Halo-like view of SAED pattern shows a typical amorphous structure of CDs [69]. However, bright diffraction spots in SAED figure also imply some crystallinity in our CDs which may be attrib-uted to other larger-sized particles [70, 71]. Zeta potential of molasses CDs was measured as negative (− 5.43 mV at pH 5.09 and − 12.9 mV at pH 8.67) at room temperature (25 °C). The surface functional groups determine most of the CD characteristics; thus, zeta (ζ) potentials are informa-tional. Negative zeta potential values of molasses CDs may
Fig. 1 UV absorbance of carbon dots (CDs) a: CDs under sunlight; b:
CDs under 365 nm UV light. Reproduced with permission from Ref. [24]
Fig. 2 FTIR spectrum of sugar beet molasses carbon dots. Repro-duced with permission from Ref. [24]
be due to carboxyl and hydroxyl groups on the surface of CDs in accordance with the elemental analysis and FTIR data [37]. It is noteworthy that the negative zeta potential of molasses CDs may be responsible for the good water disper-sity and solubility of the particles. The good hydrophilicity of the carbon dots is favorable for biomedical applications as well [72]. To investigate the optical photostability of sugar molasses-derived CDs, their photoluminescence was
compared with a commercially available fluorescence dye, DAPI (4′,6-diamidino-2-phenylindole) which is a frequently utilized nuclear and chromosome counterstain. DAPI trans-mits blue fluorescence upon binding to DNA [73]. As seen in Fig. 5, molasses CDs were as photostable as DAPI for the time measured with preserved fluorescence intensity at least for 20 min. The photostability of fluorescence nanomate-rial is an important feature in cell imaging [74]. Factually,
Fig. 3 TEM image of molasses CDs. a SAED pattern of molasses CDs, b the size distribution histogram of CDs as determined by TEM
it was stated that carbon dots possess high resistance to photobleaching [75–77] as supported by the photostability of our molasses CDs within the cells, and they were also reported photostable under the two-photon imaging condi-tions [25].
3.2 Biocompatibility of CDs
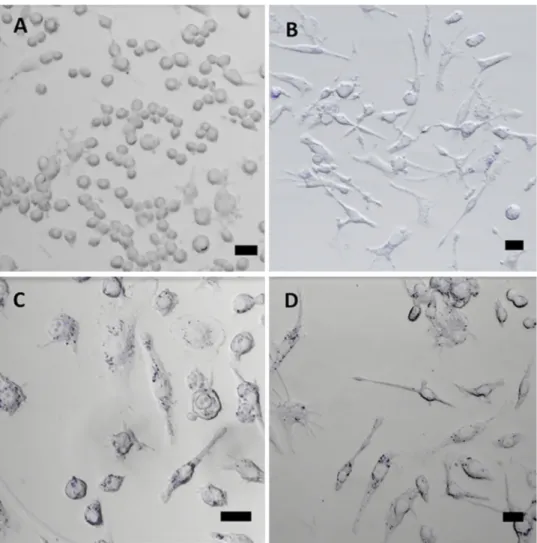
The biocompatibility of nanoparticles is one of the most crucial requirements for biological applications. Hence, the potential cytotoxicity of carbon nanoparticles is always an important issue to be considered before in vitro and in vivo studies [78]. Here, we evaluated the effects of varying con-centrations of molasses CDs on RAW 264.7 cells and L929 cells. Only at the highest concentration used (4.3 mg/mL) exposure of carbon dots to RAW 264.7 cells and L929 cells caused significant changes in the morphologies of the cells as seen in microscope images (Fig. S1). The adhered cells got disengaged and took a tiny ball-shaped form. To fur-ther prove that, the cytotoxicity of the molasses CDs was evaluated against RAW 264.7 cells and L929 cells using
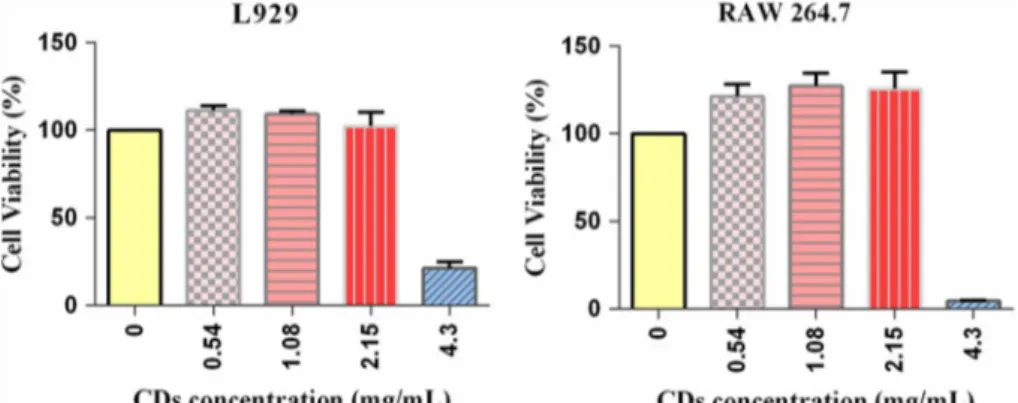
XTT-based cell viability assay for 24 h. The plot of percent-age viability of cells to the changing concentration of CDs (0.54–4.3 mg/mL) was shown in Fig. 6 and as expected no cytotoxicity was apparent except at the highest concentration of CDs applied (4.3 mg/mL). Therefore, the following exper-iments were performed using concentrations of 2.15 mg/mL or lower. According to the results of acute in vitro toxicity investigation, CDs were found to have no obvious effect on the viability of RAW 264.7 and L929 cells due to metabolic stress (i.e. by measuring the ability of reduction of the tetra-zolium salt XTT to formazan by succinic dehydrogenase in the mitochondria of metabolically active cells). The cell via-bility did not display any notable decline up to 2.15 mg/mL which is in accordance with the cytotoxicity results of other CDs derived from foods [44, 50, 64, 65]. Since the cells were exposed to CDs at higher concentrations and longer exposure times than those required for potential applications (i.e. imaging applications) [78, 79], it was concluded that molasses CDs can be used in high concentrations for various biomedical applications’ safely.
3.3 Cell proliferation
A more comprehensive investigation of cell proliferation uti-lizing a real-time impedance-based system was performed to further prove the biocompatibility of carbon dots stated above. The xCELLigence Real-Time Cell Analyzer (RTCA) system measures cell viability as a change of electrical impedance of gold microelectrodes covering the bottoms of microwells. Impedance is displayed as a dimensionless cell index (CI) which is dependent on the cell number, cell adhesion, cell morphology and cell viability [80–83]. Ini-tially, cell calibration on the RTCA DP system had been performed to determine the optimum cell seeding num-ber (data not shown). The real-time effect of five different concentrations (0.13, 0.27, 0.54, 1.08, and 2.15 mg/mL) of molasses CDs on the viability of RAW 264.7 macrophages was evaluated. Based on the results obtained from xCELLi-gence data, cell control group exhibited normal cell growth of mammalian macrophages and CDs alone did not influence
Fig. 5 The effect of time on the fluorescence intensity of molasses
CDs compared to DAPI. The fluorescence intensity of CDs at differ-ent time points with continuous excitation at 365 nm
Fig. 6 Cytotoxicity of CDs against RAW 264.7 cells and L929 cells. Relative cell viabili-ties of RAW 264.7 cells and L929 cells incubated with CDs at 0.54, 1.08, 2.15 and 4.3 mg/ mL for 24 h. The values repre-sent percentages of cell viability (mean ± SD, n = 10)
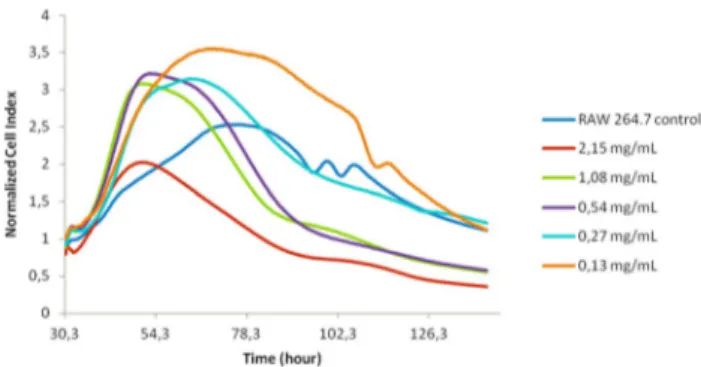
the impedance. The effect of CDs on RAW 264.7 cells was monitored in real-time for about 120 h. Figure 7 shows a sharp increase in the normalized CI of CDs-treated groups for the first 24 h of incubation. However, after longer treat-ment hours DNA-damaging effect triggering apoptosis was seen for the highest dose of CDs (2.15 mg/mL). The concen-tration which is necessary for lowering the CI measurement by 50% compared to the control after 24 h of CDs appli-cation (i.e. IC50 value) was obtained from dose–response curve (CI at a time point vs concentration) and calculated as 25,817.7 g/mL. Figure S2 displays the cellular responses as a function of the log concentration of the CDs after 24 h for IC50 data.
A sharp increase in the normalized CI of CDs-treated groups for the first 24 h of incubation may be ascribed to the fact that molasses carbon dots activated the prolifera-tion or phagocytosis of RAW 264.7 macrophages. Besides, higher IC50 value justifies CDs biocompatibility after 24 h
of treatment. Moreover, the kinetic responses of CDs were evaluated to understand the cytotoxicity mechanism of CDs which revealed the long-term DNA-damaging effects of CDs at high doses [84, 85]. This definite information of molasses CDs, provided by this new technology analysis system here, was not discussed elsewhere.
3.4 Effect of CDs on the production of NO in RAW 264.7 cells
Macrophage-derived nitric oxide (NO) played an important role in inflammatory responses [86]. The RAW 264.7 cell
line has been widely used in studies for the in vitro assess-ment of biomaterial-induced immune activation since it represents a stable macrophage phenotype in vitro model [87–89]. To investigate the inhibitory effect of CDs on NO production in lipopolysaccharide (LPS)-induced mac-rophages (i.e. the classically activated macmac-rophages (M1) secreting pro-inflammatory mediators), RAW 264.7 cells were treated with CDs at different concentrations in the presence of LPS. The level of NO in the culture media was measured as its stable break down product nitrite by Griess reaction. RAW 264.7 cells were treated with CDs at differ-ent concdiffer-entrations (0, 1.08 and 2.15 mg/mL) in the presence of LPS (1 μg/mL) for 24 h or the cells were pretreated for 4 h with CDs, then treated with LPS for 18 h. LPS alone markedly induced NO production, whereas CDs signifi-cantly (p < 0.0001) diminished the degree of NO generation in LPS-induced RAW 264.7 cells in both CDs-treated groups (Fig. 8). This is consistent with the previous result where carbon nanodots showed mild anti-inflammatory activity [90]. Thus, CDs may successfully promote free radical inhi-bition in cellular systems [91].
3.5 In vitro optical cellular imaging
As seen in Fig. 9b–d, after incubation with CDs (2.15 mg/ mL, 1.08 mg/mL, and 0.54 mg/mL, respectively) for 24 h, blue luminescent spots appeared inside the cells upon excitation at 405 nm, similar to most of the reported CDs [92], though no noticeable fluorescence was identified in control cells (Fig. 9a). CDs were viewed well distributed in the cell membrane and cytoplasmic space, indicat-ing the entry of CDs, especially around the cell nucleus. Even in experimental groups where the cells were fixed
Fig. 7 Kinetics of cytotoxic responses of RAW 264.7 cells to carbon dots (particle size 1.3–3.8 nm). The initial RAW 264.7 cells (4 × 104
cells/well) were seeded into the E-plates, and cell proliferation was monitored hourly. Once the cells reached the exponential growth phase, they were treated with a CDs suspension at different concen-trations as indicated and data was recorded every 15 min. The control here indicates cells without CDs treatment. Different compound con-centrations are indicated by different colors. Data are normalized to the time of compound addition at 30 h of cell culture. A minimum of triplicate wells per condition was set
Fig. 8 Inhibition of NO production by CDs in LPS-stimulated RAW
264.7 cells. RAW 264.7 cells were treated with LPS (1 μg/mL) in the presence or absence of CDs or pretreated for 4 h with CDs, then treated cells with LPS (1 μg/mL) for 18 h. The supernatant from the cells stimulated by LPS was collected for nitrite measurement using the colorimetric Griess reaction. Results are the mean ± SD of the two independent experiments. ****p < 0.0001 compared to control (LPS alone group)
with paraformaldehyde followed by treatment with Tri-tonX-100, strong labeling of nucleus has not occurred (Fig. S3).
CDs were introduced into RAW 264.7 cells for in vitro bioimaging using confocal microscopy [93]. With the potential of being used as optical labels for in vitro and in vivo cell imaging, carbon dots are basically internal-ized into the various type of cells (i.e. MCF-7 cells, A549 cells, L929 cells, HeLa cells, Vero cells, etc.) mainly through endocytosis [25, 72, 76, 92, 94–97], yet the details of internalization mechanism still needs to be explored. The fluorescence of the CDs was detected in the cytosol, demonstrating the potential of CDs to be used for in vitro phagocytic cell labeling via a simple incubation method. Yet no CDs were imaged within the nucleus which revealed that genetic modification of the cells following treatment may be ignored [72, 91, 98]. Having shown the biocompatibility, with no fluorescence blinking and low photobleaching, the above results indicated that the pho-toluminescence properties of CDs may allow easy tracking of their cellular localization.
4 Conclusion
In summary, the results demonstrated that sugar beet molasses-derived fluorescent carbon nanodots are neither toxic to the selected cell lines, nor do they impose any NO-related toxic effects on macrophages. More importantly, CDs showed significant anti-inflammatory effects by free radical inhibition in the LPS-induced conditions, which may be favorable for the treatment of chronic wounds. In addition to their apparent biocompatibility and immune modulation activity, CDs exhibited fluorescence imaging capability with potential for both in vitro and in vivo appli-cations. The distribution of CDs in the cytoplasm of the cells has shown that they could easily penetrate the cells via receptor-mediated and/or non-receptor-mediated endo-cytosis, even though the exact mechanism for the endocy-tosis of CDs into the cell is not clear. The internalization of nanoparticles is affected by surface charge, particle size and shape and the type of cells. Hence, analyzing the cel-lular uptake pathways is an important issue to consider.
Fig. 9 Cellular imaging. Subcellular localization of CDs aggregates monitored by using an inverted Nikon Eclipse Ti-E microscope integrated with A1/A1R multispectral laser scanning confocal imag-ing system. RAW 264.7 cells were incubated with DMEM (control) (a) or CDs at a con-centration of 2.15 mg/mL (b), 1.08 mg/mL (c), and 0.54 mg/ mL (d) for 24 h at 37 °C. Scare bars = 25 μm
Carbon dots have great potential for preclinical research (i.e. imaging-guided therapy). For specific labeling, func-tionalizing CDs with ligands for specific targets would be effective. In this study, we tested immunotoxicity of CDs using mammalian macrophages. Yet in vivo studies and other studies related to genotoxicity and mutagenic-ity could be applied to certify the clinical application of molasses carbon dots.
Compliance with ethical standards
Conflict of interest The authors declare no competing financial inter-est.
References
1. X. Xu, R. Ray, Y. Gu, H.J. Ploehn, L. Gearheart, K. Raker, W.A. Scrivens, J. Am. Chem. Soc. 126(40), 12736–12737 (2004) 2. Y. Zu, J. Bi, H. Yan, H. Wang, Y. Song, B.W. Zhu, M. Tan,
Nano-materials (Basel) 6(7), 130 (2016)
3. P. Huang, J. Lin, X. Wang, Z. Wang, C. Zhang, M. He, K. Wang, F. Chen, Z. Li, G. Shen, D. Cui, X. Chen, Adv. Mater. 24(37), 5104–5110 (2012)
4. P.-C. Hsu, P.-C. Chen, C.-M. Ou, H.-Y. Chang, H.-T. Chang, J. Mater. Chem. B 1(13) (2013)
5. K. Qu, J. Wang, J. Ren, X. Qu, Chemistry 19(22), 7243–7249 (2013)
6. A. Sachdev, P. Gopinath, Analyst 140(12), 4260–4269 (2015) 7. S.L. D’Souza, B. Deshmukh, J.R. Bhamore, K.A. Rawat, N.
Lenka, S.K. Kailasa, RSC Adv. 6(15), 12169–12179 (2016) 8. N. Parvin, T.K. Mandal, RSC Adv. 6(22), 18134–18140 (2016) 9. B. De, N. Karak, J. Mater. Chem. A 5(5), 1826–1859 (2017) 10. M. Tuerhong, Y. Xu, X.-B. Yin, Chin. J. Anal. Chem. 45(1),
139–150 (2017)
11. Z.L. Wu, Z.X. Liu, Y.H. Yuan, J. Mater. Chem. B 5(21), 3794– 3809 (2017)
12. L.-S. Li, X.-Y. Jiao, Y. Zhang, C. Cheng, K. Huang, L. Xu, Sens. Actuators B Chem. 263, 426–435 (2018)
13. X. Sun, Y. Lei, Trends Anal. Chem. 89, 163–180 (2017) 14. W. Liu, H. Diao, H. Chang, H. Wang, T. Li, W. Wei, Sens.
Actua-tors B Chem. 241, 190–198 (2017)
15. C. Liu, P. Zhang, X. Zhai, F. Tian, W. Li, J. Yang, Y. Liu, H. Wang, W. Wang, W. Liu, Biomaterials 33(13), 3604–3613 (2012) 16. L.M. Shen, J. Liu, Talanta 156–157, 245–256 (2016)
17. A. Pramanik, S. Jones, F. Pedraza, A. Vangara, C. Sweet, M.S. Williams, V. Ruppa-Kasani, S.E. Risher, D. Sardar, P.C. Ray, ACS Omega 2(2), 554–562 (2017)
18. S. Sahu, B. Behera, T.K. Maiti, S. Mohapatra, Chem. Commun. (Camb). 48(70), 8835–8837 (2012)
19. X. Guo, C.F. Wang, Z.Y. Yu, L. Chen, S. Chen, Chem. Commun. (Camb). 48(21), 2692–2694 (2012)
20. C. Zhu, J. Zhai, S. Dong, Chem. Commun. (Camb). 48(75), 9367– 9369 (2012)
21. X. Qin, W. Lu, A.M. Asiri, A.O. Al-Youbi, X. Sun, Catal. Sci. Technol. 3(4) (2013)
22. H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C.H. Tsang, X. Yang, S.T. Lee, Angew Chem. Int. Ed. Engl. 49(26), 4430–4434 (2010)
23. M.M.F. Baig, Y.C. Chen, J Colloid Interface Sci. 501, 341–349 (2017)
24. C.I. Weng, H.T. Chang, C.H. Lin, Y.W. Shen, B. Unnikrishnan, Y.J. Li, C.C. Huang, Biosens. Bioelectron. 68, 1–6 (2015) 25. L. Cao, X. Wang, M.J. Meziani, F. Lu, H. Wang, P.G. Luo, Y. Lin,
B.A. Harruff, L.M. Veca, D. Murray, S.-Y. Xie, Y.-P. Sun, J. Am. Chem. Soc. 129(37), 11318–11319 (2007)
26. P. Roy, P.-C. Chen, A.P. Periasamy, Y.-N. Chen, H.-T. Chang, Mater. Today 18(8), 447–458 (2015)
27. D. Wang, Z. Wang, Q. Zhan, Y. Pu, J.-X. Wang, N.R. Foster, L. Dai, Engineering 3(3), 402–408 (2017)
28. Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang, Y. Liu, Chem. Commun. (Camb). 48(3), 380–382 (2012)
29. W.J. Niu, R.H. Zhu, S. Cosnier, X.J. Zhang, D. Shan, Anal. Chem.
87(21), 11150–11156 (2015)
30. A. Kumar, A.R. Chowdhuri, D. Laha, T.K. Mahto, P. Karmakar, S.K. Sahu, Sens. Actuators B Chem. 242, 679–686 (2017) 31. H. Liu, T. Ye, C. Mao, Angew Chem. Int. Ed. Engl. 46(34), 6473–
6475 (2007)
32. S. Chandra, P. Das, S. Bag, D. Laha, P. Pramanik, Nanoscale 3(4), 1533–1540 (2011)
33. W. Guan, W. Gu, L. Ye, C. Guo, S. Su, P. Xu, M. Xue, Int. J. Nanomed. 9, 5071–5078 (2014)
34. M. Bayati, J. Dai, A. Zambrana, C. Rees, M. Fidalgo de Cor-talezzi, J. Environ. Sci. (China). 65, 223–235 (2018)
35. Y. Fang, S. Guo, D. Li, C. Zhu, W. Ren, S. Dong, E. Wang, ACS Nano 6(1), 400–409 (2012)
36. G. Zhongcai, G. Shen, X. Zhao, N. Dong, P. Jia, J. Wu, D. Cui, Y. Zhang, Y. Wang, Nanoscale Res. Lett. 8 (2013)
37. S. Dinç, Sugar Ind. 141(9), 560–564 (2016)
38. S. Dinç, M. Kara, M. Demirel Kars, F. Aykül, H. Çiçekci, M. Akkuş, Appl. Phys. A 123(9), 572 (2017)
39. C. Jiang, H. Wu, X. Song, X. Ma, J. Wang, M. Tan, Talanta 127, 68–74 (2014)
40. M.P. Sk, A. Jaiswal, A. Paul, S.S. Ghosh, A. Chattopadhyay, Sci. Rep. 2, 383–383 (2012)
41. X. Song, L. Cao, S. Cong, Y. Song, M. Tan, J. Agric. Food Chem.
66(28), 7522–7530 (2018)
42. B. Das, P. Dadhich, P. Pal, P.K. Srivas, K. Bankoti, S. Dhara, J. Mater. Chem. B. 2(39), 6839–6847 (2014)
43. D. Li, X. Na, H. Wang, Y. Xie, S. Cong, Y. Song, X. Xu, B.-W. Zhu, M. Tan, J. Agric. Food Chem. 66(6), 1569–1575 (2018) 44. J. Bi, Y. Li, H. Wang, Y. Song, S. Cong, D. Li, D. Zhou, B.-W.
Zhu, M. Tan, New J. Chem. 41(16), 8490–8496 (2017)
45. H. Liao, C. Jiang, W. Liu, J.M. Vera, O.D. Seni, K. Demera, C. Yu, M. Tan, J. Agric. Food Chem. 63(38), 8527–8533 (2015) 46. A.M. Al-Hadi, V.S. Periasamy, J. Athinarayanan, A.S. Al-Khalifa,
A.A. Alshatwi, Process Biochem. 52, 250–258 (2017)
47. Z. Gao, G. Shen, X. Zhao, N. Dong, P. Jia, J. Wu, D. Cui, Y. Zhang, Y. Wang, Nanoscale Res. Lett. 8(1), 276–276 (2013) 48. K. Lategan, J. Fowler, M. Bayati, M. Fidalgo de Cortalezzi, E.
Pool, Nanomaterials (Basel, Switzerland) 8(6), 388 (2018) 49. D. Li, X. Na, W. Zhou, C. Wang, Y. Li, B.W. Zhu, M. Tan, Food
Funct. 10(2), 1123–1131 (2019)
50. L. Thoo, M.Z. Fahmi, I.N. Zulkipli, N. Keasberry, A. Idris, Cent. Eur. J. Immunol. 42(3), 324–330 (2017)
51. L. Thoo, M.Z. Fahmi, I.N. Zulkipli, N. Keasberry, A. Idris, Cent. Eur. J. Immunol. 42(3), 324–330 (2017)
52. K. Lategan, J. Fowler, M. Bayati, M. Fidalgo de Cortalezzi, E. Pool, Nanomaterials (Basel). 8(6) (2018)
53. F. Ayaz, M.O. Alas, M. Oguz, R. Genc, Mol. Biol. Rep. (2019) 54. H.H. Gustafson, D. Holt-Casper, D.W. Grainger, H. Ghandehari,
Nano Today 10(4), 487–510 (2015)
55. L. Boscá, M. Zeini, P.G. Través, S. Hortelano, Toxicology 208(2), 249–258 (2005)
56. J. MacMicking, Q. Xie, C. Nathan, Ann. Rev. Immunol. 15(1), 323–350 (1997)
58. S. Bhattacharya, R. Sarkar, B. Chakraborty, A. Porgador, R. Jelinek, ACS Sens. 2(8), 1215–1224 (2017)
59. F. Nagatoshi, K. Kazuo, Curr. Drug Targets Inflamm. Allergy 4(3), 281–286 (2005)
60. S.K. Patel, J.M. Janjic, Theranostics 5(2), 150–172 (2015) 61. L. Xu, Y. Dai, Z. Wang, J. Zhao, F. Li, J.C. White, B. Xing, Part
Fibre Toxicol. 15(1), 45 (2018)
62. K. Lategan, J. Fowler, M. Bayati, M. Fidalgo de Cortalezzi, E. Pool, Nanomaterials 8(6), 388 (2018)
63. Y. Zu, J. Bi, H. Yan, H. Wang, Y. Song, B.-W. Zhu, M. Tan, Nano-materials (Basel, Switzerland) 6(7), 130 (2016)
64. A.-M. Alam, B.-Y. Park, Z.K. Ghouri, M. Park, H.-Y. Kim, Green Chem. 17(7), 3791–3797 (2015)
65. C. Yang, R. Ogaki, L. Hansen, J. Kjems, B.M. Teo, RSC Adv.
5(118), 97836–97840 (2015)
66. S. Dinç, M. Kara, M. Demirel Kars, F. Aykül, H. Çiçekci, M. Akkuş, Appl. Phys. A 123(9) (2017)
67. M.L. Bhaisare, A. Talib, M.S. Khan, S. Pandey, H.-F. Wu, Micro-chim. Acta 182(13–14), 2173–2181 (2015)
68. J.B. Essner, C.H. Laber, S. Ravula, L. Polo-Parada, G.A. Baker, Green Chem. 18(1), 243–250 (2016)
69. A.B. Siddique, A.K. Pramanick, S. Chatterjee, M. Ray, Sci Rep.
8(1), 9770 (2018)
70. P. Yang, Z. Zhu, T. Zhang, M. Chen, Y. Cao, W. Zhang, X. Wang, X. Zhou, W. Chen, Carbon 146, 636–649 (2019)
71. P. Yang, Z. Zhu, T. Zhang, W. Zhang, W. Chen, Y. Cao, M. Chen, X. Zhou, Small 15(44), e1902823 (2019)
72. Q. Liang, W. Ma, Y. Shi, Z. Li, X. Yang, Carbon 60, 421–428 (2013)
73. B. Chazotte, Cold Spring Harb. Protoc. 2011(1), pdb.prot5556 (2011)
74. J. Wang, J. Mater. Sci. 51(10), 4728–4738 (2016)
75. A. Zhao, Z. Chen, C. Zhao, N. Gao, J. Ren, X. Qu, Carbon 85, 309–327 (2015)
76. C.-L. Li, C.-M. Ou, C.-C. Huang, W.-C. Wu, Y.-P. Chen, T.-E. Lin, L.-C. Ho, C.-W. Wang, C.-C. Shih, H.-C. Zhou, Y.-C. Lee, W.-F. Tzeng, T.-J. Chiou, S.-T. Chu, J. Cang, H.-T. Chang, J. Mater. Chem. B 2(28), 4564–4571 (2014)
77. P.D. Alexander, O.D. Mariia, Methods Appl. Fluoresc. 1(4), 042001 (2013)
78. S.-T. Yang, X. Wang, H. Wang, F. Lu, P.G. Luo, L. Cao, M.J. Meziani, J.-H. Liu, Y. Liu, M. Chen, Y. Huang, Y.-P. Sun, J. Phys. Chem. C Nanomater. Interfaces 113(42), 18110–18114 (2009) 79. S.C. Ray, A. Saha, N.R. Jana, R. Sarkar, J. Phys. Chem. C 113(43),
18546–18551 (2009)
80. J.M. Boyd, L. Huang, L. Xie, B. Moe, S. Gabos, X.-F. Li, Anal. Chim. Acta 615(1), 80–87 (2008)
81. L. Huang, L. Xie, J.M. Boyd, X.-F. Li, Analyst 133(5), 643–648 (2008)
82. M. Xia, R. Huang, K.L. Witt, N. Southall, J. Fostel, M.-H. Cho, A. Jadhav, C.S. Smith, J. Inglese, C.J. Portier, R.R. Tice, C.P. Austin, Environ. Health Perspect. 116(3), 284–291 (2008)
83. C. Zeng, C. Nguyen, S. Boitano, J.A. Field, F. Shadman, R. Sierra-Alvarez, Environ. Res. 164, 452–458 (2018)
84. J.Z. Xing, L. Zhu, J.A. Jackson, S. Gabos, X.-J. Sun, X.-B. Wang, X. Xu, Chem. Res. Toxicol. 18, 154–161 (2005)
85. Y.A. Abassi, B. Xi, W. Zhang, P. Ye, S.L. Kirstein, M.R. Gay-lord, S.C. Feinstein, X. Wang, X. Xu, Chem. Biol. 16(7), 712–723 (2009)
86. J.S. Duffield, Clin. Sci. 104(1), 27–38 (2003)
87. M. Kim, S. Lee, C.S. Ki, ACS Biomater. Sci. Eng. (2019) 88. B. Taciak, M. Białasek, A. Braniewska, Z. Sas, P. Sawicka, Ł.
Kiraga, T. Rygiel, M. Król, PLoS ONE 13(6), e0198943 (2018) 89. V.B. Konkimalla, M. Blunder, B. Korn, S.A. Soomro, H. Jansen,
W. Chang, G.H. Posner, R. Bauer, T. Efferth, Nitric Oxide Biol. Chem. 19(2), 184–191 (2008)
90. F. Ayaz, M.Ö. Alaş, M. Oğuz, R. Genç, Mol. Biol. Rep. (2019) 91. B. Das, P. Dadhich, P. Pal, P.K. Srivas, K. Bankoti, S. Dhara, J.
Mater. Chem. B 2(39), 6839–6847 (2014)
92. P.-C. Hsu, P.-C. Chen, C.-M. Ou, H.-Y. Chang, H.-T. Chang, J. Mater. Chem. B 1(13), 1774–1781 (2013)
93. A.P. Demchenko, M.O. Dekaliuk, Methods Appl. Fluoresc. 1(4), 042001 (2013)
94. X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai, W. Liu, Chem. Commun. 48(64), 7955–7957 (2012)
95. J. Zhang, S.-H. Yu, Mater. Today 19(7), 382–393 (2016) 96. J. Zhou, W. Deng, Y. Wang, X. Cao, J. Chen, Q. Wang, W. Xu, P.
Du, Q. Yu, J. Chen, M. Spector, J. Yu, X. Xu, Acta Biomater. 42, 209–219 (2016)
97. M. Dekaliuk, K. Pyrshev, A. Demchenko, J. Nanobiotechnol.
13(1), 86 (2015)
98. X. Cao, J. Wang, W. Deng, J. Chen, Y. Wang, J. Zhou, P. Du, W. Xu, Q. Wang, Q. Wang, Q. Yu, M. Spector, J. Yu, X. Xu, Sci. Rep.
8(1), 7057 (2018)
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.