Contents lists available atScienceDirect
Dyes and Pigments
journal homepage:www.elsevier.com/locate/dyepig
Dual-emissive
fluorescent probe based on phenolphthalein appended
diaminomaleonitrile for Al
3+
and the colorimetric recognition of Cu
2+
Serkan Erdemir
a,∗, Sait Malkondu
baSelcuk University, Science Faculty, Department of Chemistry, Konya, 42031, Turkey
bGiresun University, Faculty of Engineering, Department of Environmental Engineering, Giresun, 28200, Turkey
A R T I C L E I N F O Keywords: ESIPT Dual emissive Fluorescent Colorimetric A B S T R A C T
A novel dual emissivefluorescent probe was designed and synthesized by linking phenolphthalein and diami-nomaleonitrile units. The present probe (PDM) can be used not only forfluorogenic detection of Al3+by way of the excited enol-keto forms but also colorimetric detection of Cu2+.PDM probe showed a selectivefluorescence enhancement with dual channel emissions for Al3+in a mixture of EtOH/H
2O (9/1).1H NMR and DFT methods were also carried out to support the complexation betweenPDM and Al3+ion. In addition,PDM presented highly selective colorimetric response for Cu2+over other metal ions. The reversibility ofPDM-Al3+and PDM-Cu2+complexes was successively established with the addition of TBAF and EDTA, respectively. The detection limits were determined to be 92.0 nM for Al3+and 2.81μM for Cu2+. The obtained results revealed that the designedPDM probe could be suitable for monitoring Al3+under UV lamp and Cu2+in water samples with the naked eye, which was rapid, convenient, low-cost and environmental friendly.
1. Introduction
Among all elements, aluminum is the third most abundant after oxygen and silicon and is found in most animal and plant tissues as well as in natural water owing to acidic rain and human activities [1–5]. Nearly 8% of aluminum components are present in the atmosphere [6]. Aluminum and its compounds are greatly used in textile industries, paper industries, in making utensils, in alloys [7], water purification, automobiles [8] etc. Even though it is quite useful but it has got some negative effects too. Al3+
may induce neurodegenerative disease like Alzheimer and Parkinson diseases when consumed in the surplus amount [9], bone abnormalities etc. The permissible amount of alu-minum in human is 7 mg kg−1per week declared by the world health organization (WHO) [10].
Copper is one of the abundant transition metals in the human body and actively participates in various biological processes and tends to be an integral part of a number of metalloenzymes covering the whole range of functionality [11]. Nevertheless, copper is a significant metal pollutant due to it displays high toxicity to some organisms and leads neurodegenerative diseases such as Alzheimer and prion diseases under overloading conditions [12]. Especially, exposure to a high concentra-tion of Cu2+ions can induce gastrointestinal disturbance and can da-mage to the liver and kidney [13]. The safe limit of copper has been set as 20μM in drinking water by the U.S. Environmental Protection
Agency (EPA) [14]. Considering that the potential impact of Al3+and Cu2+ ions can threaten human health and the environment, the de-velopment of highly sensitive and selective probes, which are able to detect and estimate trace levels of Al3+and Cu2+ions, are very es-sential.
Unlike other analytical techniques, fluorescence and colorimetric methods have some advantages such as simplicity, rapid response, handy, cost-effectiveness and high sensitivity [15–20]. Therefore, the development offluorescent and colorimetric probes to detect Al3+and Cu2+is also becoming more common. Recently, simple Schiff base li-gands have gained attention asfluorescent sensor for the detection of various metal ions including Al3+and Cu2+owing to their easy one or two steps synthesis [21–27]. The classical design of afluorescent sensor includes the presence of afluorophore and receptor units where they are either intrinsically attached or extrinsically separated by spacer [28]. As well as other common fluorophores [16,29–33], the phe-nolphthalein has been also used as afluorophore in the detection of Al3+, however, its examples are still very seldom [34,35]. Herein, we report the design and synthesis of a new simple and efficient sensor possessing phenolphthalein as signaling unit and diaminomaleonitrile as receptor unit. In the presence of Al3+,PDM displayed strong dual emission bands (λem= 491 and 525 nm) owing to the inhibition of PET and the excited state C=N isomerization. In addition, the dual emis-sions were justified in terms of enol-keto tautomerization as a result of
https://doi.org/10.1016/j.dyepig.2018.12.017
Received 11 November 2018; Received in revised form 10 December 2018; Accepted 10 December 2018 ∗Corresponding author.
E-mail address:serdemir82@gmail.com(S. Erdemir).
Available online 11 December 2018
0143-7208/ © 2018 Elsevier Ltd. All rights reserved.
ESIPT (excited-state intramolecular proton transfer). PDM also de-monstrated a reversible colorimetric response to Cu2+over other metal ions.
2. Experimental 2.1. General
All the chemicals and solvents were purchased from commercial suppliers. Bruker FTIR instrument was used to FTIR spectra analysis. NMR spectra were measured on a Varian 400 MHz instrument in CDCl3 and DMSO‑d6as solvent. Thefluorescence and UV/Visible spectra of PDM in the absence and presence of metal ions were recorded in Perkin Elmer LS 55 and Shimadzu 1280 instruments, respectively. Elemental analysis results for PDM were obtained by using a Leco CHNS 932 in-strument. The solutions of metal ions and anions were prepared from their perchlorate and tetrabutylammonium salts, respectively.
2.2. Synthesis
The intermediate (compound 1) was synthesized according to the previously reported procedure [36]. A solution of phenolphthalein (0.5 g, 1.57 mmol) and hexamethylenetetramine (HMTA, 0.66 g, 4.71 mmol) in trifluoroacetic acid (40 mL) was refluxed for 8 h. After the reaction mixture was cooled to room temperature, a solution of HCl (1.0 M, 100 mL) was added to resulting mixture, and then extracted with dichloromethane (100 mL). The organic layer was washed with water three times and saturated brine once, and was dried over sodium sulfate. Removal of the solvent gave white solid. The crude product was recrystallized from a mixture of ethanol/water. Yield: 75%;1H NMR (400 MHz, 25 °C, DMSO‑d6): δ 11.04 (s, 2H, ArOH), 10.20 (s, 2H, CHO),7.91 (d, 1H, J = 7.74 Hz, ArH), 7.79–7.76 (m, 2H, ArH), 7.65 (t, 1H, J = 7.52 Hz, ArH), 7.51 (d, 2H, J = 2.60 Hz, ArH), 7.43 (d, 1H, J = 2.60 Hz, ArH), 7.41 (d, 1H, J = 2.60 Hz, ArH), 7.02 (d, 2H, J = 8.73 Hz, ArH).
2.2.1. Synthetic procedure forPDM
An ethanolic solution of compound1 (0.375 g, 1 mmol) was added dropwise to a solution of diaminomaleonitrile (0.217 g, 2 mmol) in 20 mL of ethanol in presence of one drop glacial acetic acid (Scheme 1). After the stirring for 2 h, the yellow coloured product was precipitated. The obtainedPDM wasfiltered, washed with ethanol and then dried. The crude product was recrystallized from ethanol. Yield: 76%; Mp: 236–238 °C; FTIR (ATR): 2237, 2200 cm−1(CN), 1758 cm−1(C=O), 1625 cm−1(C=N); 1H NMR (400 MHz, 25 °C, DMSO‑d 6):δ 10.77 (s, 2H, ArOH), 8.53 (s, 2H, CHN), 8.05 (d, 1H, J = 7.36 Hz, ArH), 7.82–7.91 (m, 4H, ArH), 7.64 (t, 1H, J = 7.36 Hz, ArH), 7.49 (s, 4H, NH2), 7.29 (d, 2H, J = 8.61 Hz, ArH), 6.94 (d, 1H, J = 8.61 Hz, ArH). 13C NMR (100 MHz, 25 °C, DMSO‑d 6)δ 169.27, 158.72, 154.48, 152.15, 135.41, 132.51, 131.85, 130.26, 127.98, 126.68, 125.96, 125.22, 124.94, 121.06, 117.23, 114.94, 114.45, 103.75, 90.89; Anal. Calcd for C30H18N8O4(554.53): C, 64.98; H, 3.27; N, 20.21. Found: C, 65.03; H, 3.31; N, 20.32.
2.3. Preparation of solutions
A stock solution ofPDM (0.01 M) was prepared in DMSO. Then, it was diluted to 1 × 10−5M for UV–vis and 1 × 10−6M forfluorescence studies with a mixture of EtOH/H2O (9/1, v/v) at 25 °C. The solutions of the guest cations as their perchlorate salts (0.01M) were prepared in deionized water. Fluorescence and Uv–vis titrations were performed by adding of the appropriate amount of metal ion to 3 mL of a solution of PDM using micropipette. To monitor the chemical shifts arising from the interaction of PDM with Al3+, 1H NMR experiments were also realized by addition of the known quantity of Al3+ion to a solution of PDM (0.054 M).
3. Results and discussion 3.1. Synthesis of PDM
The synthesis started with the formylation of phenolphthalein via Duff reaction [36] inScheme 1. Then, condensation of dialdehyde de-rivative of phenolphthalein (1) with diaminomaleonitrile afforded probePDM in 76% yield, which was fully characterized by1H NMR, 13C NMR, COSY, APT, DEPT, elemental analysis and FT-IR spectra (Figs. S1–S7).
3.2. Absorption studies of PDM towards metal ions
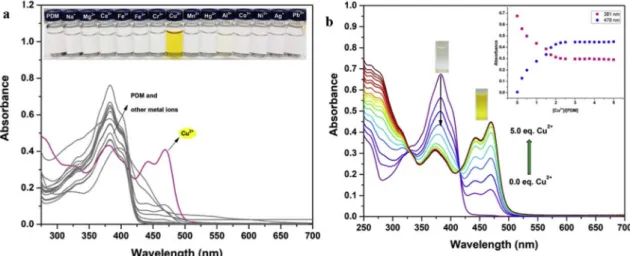
Absorption properties ofPDM towards different metal ions were realized in a mixture of EtOH/H2O (9/1) (Fig. 1a). When 5.0 equiv. of various metal ions such as Na+, Mg2+, Ca2+, Al3+, Zn2+, Cd2+, Cu2+, Fe2+, Fe3+, Cr3+, Hg2+, Ag+, Co2+, Ni2+, Mn2+and Pb2+were added to PDM solution, only Cu2+displayed distinct spectral changes at 440 and 470 nm and instant color changed from colorless to yellow, while other metal ions could not show any color change (Fig. 1a). These re-sults showed thatPDM could be used as a“naked-eye” sensor for the detection of Cu2+in aqueous media (Fig. 1a, inset).
UV–vis titration experiments were also performed to examine the concentration-dependent signaling of PDM toward Cu2+ (Fig. 1b). PDM exhibited a strong absorption band at 380 nm. After the addition of Cu2+(0.0–5.0 eq.) to a solution of PDM (10 μM) in a mixture of EtOH/H2O (9:1), absorption of the band at 380 nm was significantly decreased, and two new bands at 440 and 470 nm were developed and gradually reached a maxima with the addition 2.0 equiv. of Cu2+, suggesting that the binding stoichiometry is 1:2 in the formed complex. In addition, two clear isosbestic points appeared at 330 and 414 nm which clearly indicated the presence of the formed complex in equili-brium with the receptor. In combination with the UV–vis titration, the binding constant of PDM for Cu2+was calculated from the modern non-linear regression fitting method from the freely software tool available in the Bind Fit v0.5 module [37], and found to be 9.36 (logK). The detection limit was calculated and it was found to be 2.81μM (Fig. S8). Furthermore, the colorimetric behavior of interaction ofPDM with Cu2+was found to be reversible and the reversibility experiments were performed by adding ethylenediaminetetraacetate (EDTA). For this, a solution of EDTA to a solution ofPDM-Cu2+complex was gradually added, and it was seen that the absorbance was fully inverted to that of PDM (Fig. 2). These results showed that EDTA provides the reversibility
conditions, which is an important parameter for the practical applica-tions of a sensor.
3.3. Emission studies ofPDM towards metal ions
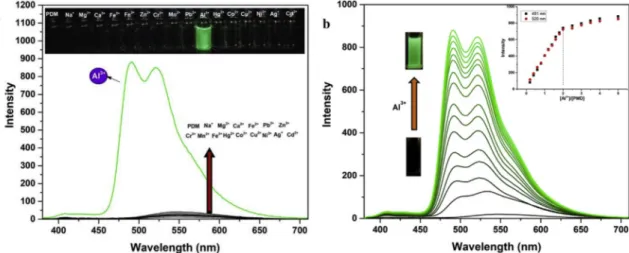
The emission properties ofPDM were explored by observing the fluorescence changes in the presence of various metal ions in a mixture of EtOH/H2O (9/1, v/v). As seen inFig. 3a, upon excitation at 365 nm, PDM demonstrated weak emissive behavior at 547 nm owing to PET (Photo-induced electron transfer) and the excited state C=N iso-merization processes. Apart from these effects, the electron with-drawing effect of the nitrile groups in PDM may also support the weak-fluorescent feature of PDM. Nevertheless, a distinct change in fluores-cence intensity was monitored with the addition of only Al3+ with respect to the all tested metal ions. WhenPDM was treated with Al3+, PDM showed dual emission bands at 491 and 525 nm withfluorescent enhancement due to ICT and ESIPT processes. Other metal ions could not induce any obvious changes in emission properties ofPDM. Also, upon addition of Al3+toPDM solution, dramatic visualfluorescence change was produced under UV lamb (Fig. 3a, inset).
To gain an insight into the interaction properties ofPDM for Al3+, a fluorescence titration of the probe was performed up to 5.0 equiv. of
Al3+due to its weak coordination ability (as a hard acid).PDM ex-hibited increasingfluorescence intensity at 491 and 525 nm with the addition of increasing concentration of Al3+(Fig. 3b). Emerging two bands at 491 and 525 nm are due to the presence of equilibrium of both enol and keto forms observed in phenolic Schiff bases and may be at-tributed to the excited enol (E*) and keto tautomer (K*) forms arising due to ESIPT process (Fig. 4). In the presence of Al3+, while the keto form of PDM emits at 525 nm, the enol form shows emission at 485 nm. If the binding between Al3+ andPDM induce deprotonation of the phenolic-OH,PDM would emit at single wavelength because the ESIPT process is prevented [38,39]. However, the deprotonation could not be observed in the phenolic-OH ofPDM and thus ESIPT is not inhibited and PDM emitted dual channels. This phenomenon was further sup-ported by1H NMR and DFT studies. The stoichiometric ratio between PDM and Al3+was found as 1:2 by Job analysis (Fig. 5a). The asso-ciation constant (logK) ofPDM towards Al3+is found to be 8.78, in the Bind Fit v0.5 module [37]. From the fluorescence titration data, the detection limit ofPDM for Al3+is also determined to be 92 nM, using the equation DL = 3s/k, where s is the standard deviation of the blank solution and k is the slope of the calibration curve (Fig. S9). Detection limit ofPDM for Al3+is comparable to those of other ESIPT based fluorescent sensors (Table 1). The quantum yield (Φ) measurements for PDM and PDM-Al3+complex were also carried out at different con-centrations in EtOH/H2O (v/v, 9/1). PDM-Al3+complex (Φ = 0.329) indicated about 18 times higher Φ value than that of PDM (Φ = 0.0176) (Fig. S10). In addition, the binding stoichiometry be-tweenPDM and Al3+was supported by performing UV–vis. titration experiments ofPDM at different amounts of Al3+. As seen inFig. 5b, the UV–vis spectra of PDM exhibits two bands at 381 and 398 nm as-signed toπ-π* and n-π* transitions of PDM, respectively. Upon addition of Al3+ion, two bands at 381 and 398 nm were slightly decreased and new absorption bands were weakly developed at 438 and 469 nm which suggested the formation of a new species in solution. The addition of more than two equiv. of Al3+could not induce any change in the ab-sorption spectra ofPDM which means that PDM and Al3+
were com-plexed in a ratio of 1:2 (Fig. 5b, inset).
To better understand the nature of the coordination of Al3+with PDM, geometric optimizations and theoretical calculations of PDM and PDM-Al3+ complexes (enol-keto forms) were realized by applying density functional theory (DFT) with B3LYP and 3-21G/Lanl2dz basis set using Gaussian 16 program [40–42]. The optimized geometries of PDM and PDM-Al3+complex in enol-keto forms are shown inFig. S11. In addition, the dispersions and energies of HOMO and LUMO orbitals of PDM and the corresponding Al3+ complexes were calculated. As shown in Fig. 6, the HOMO and LUMO orbitals are distributed in Fig. 1. (a) The UV–vis absorption spectra of PDM (10.0 μM) in the presence of various metal ions and (b) the UV–vis titration of PDM with Cu2+in EtOH/H
2O (9/1, v/v).
Fig. 2. The absorbance spectra of PDM-Cu2+complex in presence of EDTA (inset: the sequential reversibile behavior ofPDM with EDTA.
different parts of PDM and PDM-Al3+complexes. The energy gap be-tween HOMO and LUMO inPDM was 3.3290 eV, whereas the energy gaps ofPDM-Al3+(enol) andPDM-Al3+(keto) were calculated to be 2.4812 and 2.0921 eV, respectively. These calculations indicate that the
interaction betweenPDM and Al3+decreases the HOMO-LUMO energy gap of the complex and stabilizes the system. Also, it was found that the HOMO-LUMO energy gap inPDM was quite larger than that of PDM-Al3+(enol), confirming the strengthening of ICT. The energy gap of Fig. 3. (a) Effect of different metal ions on the fluorescence spectra of PDM (1.0 μM) (inset: visual fluorescence color changes of PDM after addition of metal ions); (b) Fluorescence titration spectra ofPDM upon incremental addition of Al3+in EtOH/H
2O (9/1, v/v). (For interpretation of the references to color in thisfigure legend, the reader is referred to the Web version of this article.)
Fig. 4. Proposed dual emissive and binding mode of PDM with Al3+in the enol (E) and keto (K) forms ofPDM by the ESIPT process.
Fig. 5. (a) Job plot of PDM-Al3+complexation; (b) UV–vis titration of PDM (10 μM) with Al3+
ions in EtOH/H2O (9/1, v/v) (inset: the molar ratio plot ofPDM with Al3+ion).
PDM-Al3+(enol) is higher than that ofPDM-Al3+(keto). This di ffer-ence may be attributed to dual emission wavelength of PDM upon binding with Al3+due to ESIPT process.
3.4. 3.4.1H NMR experiments
To understand the interaction ofPDM with Al3+,1H NMR spectra of PDM-Al3+was recorded by addition of Al3+to a solution ofPDM in DMSO‑d6. As seen inFig. 7, the signals atδ 10.77, 8.53 and 7.49 ppm are ascribed to the phenolic-OH, aldimine and freeeNH2protons, re-spectively. When the 2.0 equiv. of Al3+was added to a solution ofPDM in DMSO‑d6, the freeeNH2signal atδ 7.49 ppm disappeared and si-multaneously a new signal was formed at 9.10 ppm which clearly in-dicated the deprotonation of eNH2 by interaction with Al3+. In
addition, the phenolic-OH signal atδ 10.77 ppm upfield shifted to δ 8.56 ppm, whereas the aldimine (eCHN) signal at δ 8.53 ppm slightly downfield shifted. The aromatic protons of PDM did not exhibited significant changes in the presence of Al3+. These results supported that phenolic-OH,eNH2and aldimine groups are effective in the complex formation betweenPDM and Al3+.
3.5. Competition and reversibility studies for Al3+
The competition effect of other analytes such as some anions, ca-tions and amino acids on the selectivity ofPDM towards Al3+, was also investigated. Firstly, it was tested a series of metal ion in the presence of Al3+andPDM. As seen inFig. 8a, other metal ions could not induced significant emission change on the detection of Al3+
byPDM, except for Cu2+ which decreased thefluorescence intensity. The interfering effect of Cu2+could be eliminated by using a masking reagent. It was found that DMG (dimethylglyoxime) could completely mask Cu2+for the determination of Al3+[43]. Then, the interference of a series of anion (F−, Cl−, Br−, I−, S2−, AcO−, NO3−, CN−, ClO4−, HSO4−, NO2−) and amino acids (Phe, Ala, Thr, Ser, Arg, Cys, Hcy, GSH) on the detection of Al3+was explored by adding of them to a solution con-tainingPDM–Al3+complex.Fig. 8b shows that no significant difference in the intensity ofPDM-Al3+ complex was noticed except F−. Al3+ detection was interrupted byfluoride because it can interact with Al3+ to give the complex of AlF63−. Exploiting from this result, we used TBAF (tetrabutylammoniumfluoride) as reversibility agent, which is desired property in practical applications. The reversibility experiments were performed by consecutive additions of Al3+and F− ions to a solution ofPDM (1.0μM) in EtOH/H2O (9/1). As seen inFig. 9, the green fluorescence of PDM in the presence of Al3+ was completely quenched by the addition of F−ion (OFF), but it was regenerated by the addition of Al3+ion (ON), which clearly showed the reversible prop-erty of PDM in sensing of Al3+ ion. Moreover, the time-dependent emission intensity ofPDM with Al3+was realized to view the stability of thePDM-Al3+complex. The emission intensity ofPDM-Al3+was increased gradually and reached a stable level within about 2 min (Fig. S12), revealing thatPDM has a high potential for real-time and highly selective sensing of Al3+in practical applications.
The pH value has great effect on the detection procedure. To de-termine the convenient pH condition of PDM–Al3+
complex, pH ex-periments was examined at a pH range from 3.0 to 10.0 (Fig. S13). As seen inFig. S13, the emission intensity of the complex betweenPDM and Al3+reached to the maximum a pH range of 5.5–7.5. The lower pH values (pH < 5.5) causes the dissociation of the complex and hydro-lysis of thePDM, therefore the emission intensity decreases. At higher pH values (pH > 7.5), thePDM competes with OH−ions for Al3+ion, resulting in the metal ion precipitation, therefore the emission intensity Table 1
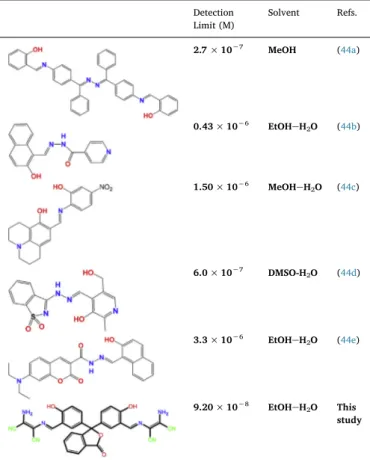
Comparision of some ESIPT basedfluorescent sensors for Al3+detection.
Detection Limit (M) Solvent Refs. 2.7 × 10−7 MeOH (44a) 0.43 × 10−6 EtOHeH2O (44b) 1.50 × 10−6 MeOHeH2O (44c) 6.0 × 10−7 DMSO-H2O (44d) 3.3 × 10−6 EtOHeH2O (44e) 9.20 × 10−8 EtOHeH2O This study
decreases. Thus, detection of Al3+ion withPDM is shown to be proper within a pH range (5.5–7.5).
The potential utility ofPDM was also checked in the detection of Al3+and Cu2+ions in tap water samples. Accordingly, a portion of the tap water sample was spiked with known amounts of Al3+or Cu2+. Briefly, the Cu2+or Al3+spiked water samples were interacted with a solution ofPDM, and then the fluorescence and UV–vis spectra were recorded. Al3+and Cu2+concentrations in samples were found from the fitted linear fluorescence and UV–vis calibration curves, respec-tively. According to the results shown inTable 2, the recoveries of Al3+ and Cu2+ions were between 88.7 and 104.8% with RSD in the range of 0.9–2.0%, which shows that PDM is quite suitable for detecting Cu2+ and Al3+ions.
4. Conclusion
We successfully developed a simplefluorescent for Al3+ and the colorimetric sensor for Cu2+based phenolphthalein appended diami-nomaleonitrile (PDM). PDM displayed selectively colorimetric response for Cu2+through 1:2 of complexation. Alongside, the addition of Al3+ toPDM solution generated a noticeablefluorescence enhancement by dual channel emissions due to the formation of enol-keto structures in the excited state. The detection limits (LOD) ofPDM for sensing Al3+ and Cu2+were calculated to be 92 nM and 2.81μM, which makes it promising to these ions at nano- and micro-molar concentration levels in practical samples, respectively. In addition, Al3+and Cu2+ were successively detected by PDM in water samples. The results demon-strated that PDM can be used as an efficient sensor for selective Fig. 7.1H NMR spectra ofPDM (A) and PDM-Al3+(B) complex in DMSO‑d
6
Fig. 8. The interference effect of other competition metal ions (a) and anions/amino acids (b) in fluorescence intensity of PDM (1.0 μM) for Al3+in EtOH/H 2O (9/1).
Fig. 9. Reversible visualfluorescence changes after sequential addition of Al3+ and F−ions toPDM solution.
Table 2
Determination of Cu2+and Al3+ions in the spiked tap water samples byPDM.
Metal ion Spiked water sample (μM) Fluorescence Method (found Al3+) UV–vis method (found Cu2+) Recovery (%) RSD (n = 3) (%) Al3+ 5 5.24 – 104.8 1.1 10 9.71 – 97.1 0.9 Cu2+ 15 – 13.31 88.7 2.9 20 – 19.52 97.6 1.8
detection of Al3+and Cu2+. Acknowledgment
We thank the Research Foundation of Selcuk University forfinancial support of this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.dyepig.2018.12.017.
References
[1] Jeyanthi D, Iniya M, Krishnaveni K, Chellappa D. A ratiometricfluorescent sensor for selective recognition of Al3+ions based on a simple benzimidazole platform.
RSC Adv 2013;3(43):20984–9.
[2] Kumar A, Kumar V, Upadhyay K. An Al3+and H
2PO4−/HSO4−selective
con-formational arrest and bail to a pyrimidine-naphthalene anchored molecular switch. Analyst 2013;138(6):1891–7.
[3] Ding W-H, Cao W, Zheng X-J, Fang D-C, Wong W-T, Jin L-P. A highly selective fluorescent chemosensor for Al(III) ion and fluorescent species formed in the solu-tion. Inorg Chem 2013;52(13):7320–2.
[4] Tria J, Butler EC, Haddad PR, Bowie AR. Determination of aluminium in natural water samples. Anal Chim Acta 2007;588(2):153–65.
[5] Sen S, Mukherjee T, Chattopadhyay B, Moirangthem A, Basu A, Marek J, et al. A water soluble Al3+selective colorimetric andfluorescent turn-on chemosensor and
its application in living cell imaging. Analyst 2012;137(17):3975–81.
[6] Godbold D, Fritz E, Hüttermann A. Aluminum toxicity and forest decline. Proc Natl Acad Sci Unit States Am 1988;85(11):3888–92.
[7] Choi YW, Park GJ, Na YJ, Jo HY, Lee SA, You GR, et al. A single schiff base molecule for recognizing multiple metal ions: afluorescence sensor for Zn (II) and Al (III) and colorimetric sensor for Fe (II) and Fe (III). Sensor Actuator B Chem
2014;194:343–52.
[8] Mergu N, Singh AK, Gupta VK. Highly sensitive and selective colorimetric and off-onfluorescent reversible chemosensors for Al3+based on the rhodamine
fluor-ophore. Sensors 2015;15(4):9097–111.
[9] Kumar V, Kumar A, Diwan U, Srivastava S, Upadhyay K. Salicylideneimines as ef-ficient dual channel emissive probes for Al3+: harnessing ESIPT and ICT processes.
Sensor Actuator B Chem 2015;207:650–7.
[10] Valeur B, Leray I. Design principles offluorescent molecular sensors for cation re-cognition. Coord Chem Rev 2000;205(1):3–40.
[11] Cardona MA, Kveder M, Baisch U, Probert MR, Magri DC. Water-soluble β-amino-bisulfonate building blocks for pH and Cu2+indicators. RSC Adv
2016;6(88):84712–21.
[12] Multhaup G, Schlicksupp A, Hesse L, Beher D, Ruppert T, Masters CL, et al. The amyloid precursor protein of Alzheimer's disease in the reduction of copper (II) to copper (I). Science 1996;271(5254):1406–9.
[13] Georgopoulos PG, Roy A, Yonone-Lioy MJ, Opiekun RE, Lioy PJ. Environmental copper: its dynamics and human exposure issues. J Toxicol Environ Health B Crit Rev 2001;4(4):341–94.
[14] Lan G-Y, Huang C-C, Chang H-T. Silver nanoclusters asfluorescent probes for se-lective and sensitive detection of copper ions. Chem Commun 2010;46(8):1257–9. [15] Wang Y-W, Yu M-X, Yu Y-H, Bai Z-P, Shen Z, Li F-Y, et al. A colorimetric and
fluorescent turn-on chemosensor for Al3+and its application in bioimaging.
Tetrahedron Lett 2009;50(45):6169–72.
[16] Manjunath R, Hrishikesan E, Kannan P. A selective colorimetric andfluorescent sensor for Al3+ion and its application to cellular imaging. Spectrochim Acta Mol
Biomol Spectrosc 2015;140:509–15.
[17] Narayanaswamy N, Govindaraju T. Aldazine-based colorimetric sensors for Cu2+
and Fe3+. Sensor Actuator B Chem 2012;161(1):304–10.
[18] An R, Zhang D, Chen Y, Cui Y-z. A“turn-on” fluorescent and colorimetric sensor for selective detection of Cu2+in aqueous media and living cells. Sensor Actuator B
Chem 2016;222:48–54.
[19] Wang YW, Hua YX, Wu HH, Sun X, Peng Y. A solvent-tuningfluorescence sensor for In(III) and Al(III) ions and its bioimaging application. Chin Chem Lett
2017;28:1994–6.
[20] Wang YW, Liu SB, Ling WJ, Peng Y. Afluorescent probe for relay recognition of homocysteine and Group IIIA ions including Ga(III). Chem Commun
2016;52:827–30.
[21] Dutta K, Deka RC, Das DK. Thefirst enhanced ring planarity based fluorescent
“off–on” sensor for Ca2+ion. J Lumin 2014;148:325–9.
[22] Kumar J, Sarma MJ, Phukan P, Das DK. A new simple Schiff base fluorescence “on” sensor for Al3+and its living cell imaging. Dalton Trans 2015;44(10):4576–81.
[23] Kumar J, Bhattacharyya PK, Das DK. New duelfluorescent “on–off” and colori-metric sensor for copper (II): copper (II) binds through N coordination and pi cation interaction to sensor. Spectrochim Acta Mol Biomol Spectrosc 2015;138:99–104. [24] Sharma D, Kuba A, Thomas R, Kumar R, Choi HJ, Sahoo SK. An aqueous friendly
chemosensor derived from vitamin B6 cofactor for colorimetric sensing of Cu2+and
fluorescent turn-off sensing of Fe3+. Spectrochim Acta Mol Biomol Spectrosc
2016;153:393–6.
[25] Sahoo SK, Sharma D, Moirangthem A, Kuba A, Thomas R, Kumar R, Kuwar A, Choi HJ, Basu A. Pyridoxal derived chemosensor for chromogenic sensing of Cu2+and
fluorogenic sensing of Fe3+in semi-aqueous medium. J Lumin 2016;172:297–303.
[26] Torawane P, Tayade K, Bothra S, Sahoo SK, Singh N, Borse A, Kuwar A. A highly selective and sensitivefluorescent ‘turn-on’ chemosensor for Al3+based on CN
isomerisation mechanism with nanomolar detection. Sensor Actuator B Chem 2016;222:562–6.
[27] Fu ZH, Yan LB, Zhang X, Zhu FF, Han XL, Fang J, Wang YW, Peng Y. A fluorescein-based chemosensor for relayfluorescence recognition of Cu(II) ions and biothiols in water and its applications to a molecular logic gate and living cell imaging. Org Biomol Chem 2017;15:4115–21.
[28] Patra C, Bhanja AK, Sen C, Ojha D, Chattopadhyay D, Mahapatra A, et al. Vanillinyl thioether Schiff base as a turn-on fluorescence sensor to Zn2+ion with living cell
imaging. Sensor Actuator B Chem 2016;228:287–94.
[29] Tiwari K, Mishra M, Singh VP. A highly sensitive and selectivefluorescent sensor for Al3+ions based on thiophene-2-carboxylic acid hydrazide Schiff base. RSC Adv
2013;3(30):12124–32.
[30] Lu Y, Huang S, Liu Y, He S, Zhao L, Zeng X. Highly selective and sensitive fluor-escent turn-on chemosensor for Al3+based on a novel photoinduced electron
transfer approach. Org Lett 2011;13(19):5274–7.
[31] Yue X-l, Li C-r, Yang Z-y. A novel Schiff-base fluorescent probe based on 1, 8-naphthyridine and naphthalimide for Al3+. Inorg Chim Acta 2017;464:167–71.
[32] Ma TH, Dong M, Dong YM, Wang YW, Peng Y. A unique water‐tuning dual‐channel fluorescence‐enhanced sensor for aluminum ions based on a hybrid ligand from a 1,1′‐binaphthyl scaffold and an amino acid. Chem Eur J 2010;16:10313–8. [33] Anand T, Ashok Kumar SK, Sahoo SK. A new Al3+selectivefluorescent turn-on
sensor based on hydrazide-naphthalic anhydride conjugate and its application in live cells imaging. Spectrochim Acta 2018;204:105–12.
[34] Erdemir S, Kocyigit O. A novel dye based on phenolphthalein-fluorescein as a fluorescent probe for the dual-channel detection of Hg2+and Zn2+. Dyes Pigments
2017;145:72–9.
[35] Li Y, Liao C, Huang S, Xu H, Zheng B, Du J, et al. A selectivefluorescent probe based on bis-Schiff base for “turn-on” detection of Al3+and cysteine by different
me-chanisms. RSC Adv 2016;6(30):25420–6.
[36] Guo Y, Huo F, Yin C, Kang J, Li J. A highly selective and sensitive turn-on fluor-escent probe for the detection of holmium ion and its bioimaging. RSC Adv 2015;5(14):10845–8.
[37] http://supramolecular.org(accessed October 2018).
[38] Liu S, Zhang L, Zan W, Yao X, Yang Y, Liu X. A novel HBT-based Schiff base for colorimetric detection of aluminum: synthesis, characterization, spectral and DFT computational studies. Sensor Actuator B Chem 2014;192:386–92.
[39] Wang J, Pang Y. A simple sensitive ESIPT on-off fluorescent sensor for selective detection of Al3+in water. RSC Adv 2014;4(12):5845–8.
[40] Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, et al. Gaussian 16, revision A. 03. Wallingford CT: Gaussian Inc; 2016.
[41] Becke AD. Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys 1993;98(7):5648–52.
[42] Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 1988;37(2):785. [43] Porrawatkul P, Pimsen R, Kuyyogsuy A, Nuengmatcha P. Simple and selective
naked-eye and visual detection of Cu2+and Al3+ions using Hibiscus rosa-sinensis
linnflower extract. Orient J Chem 2018;34(1):188–95.
[44] (a) Kumar G, Paul K, Luxami V. Aggregation induced emission-excited state in-tramolecular proton transfer based“off-on” fluorescent sensor for Al3+ions in
li-quid and solid state. Sensor Actuator B 2018;263:585–93;
(b) Yue XL, Wang ZQ, Li CR, Yang ZY. Naphthalene-derived Al3+-selective
fluor-escent chemosensor based on PET and ESIPT in aqueous solution. Tetrahedron Lett 2017;58:4532–7;
(c) Singhal D, Gupta N, Singh AK. Fluorescent sensor for Al3+ion in partially
aqueous media using julolidine based probe. New J Chem 2016;40:7536–41; (d) Kejík Z, Kaplánek R, Havlík M, Bříza T, Vavřinová D, Dolenský B, Martásek P, Král V. Aluminium(III) sensing by pyridoxal hydrazone utilising the chelation en-hancedfluorescence effect. J Lumin 2016;180:269–77;
(e) Qin JC, Yang ZY. Ratiometricfluorescent probe for Al3+based on coumarin