Contents lists available atScienceDirect
Journal of Photochemistry & Photobiology A: Chemistry
journal homepage:www.elsevier.com/locate/jphotochemCo-catalyst-free photocatalytic hydrogen evolution by Laponite D clay
Emre Aslan
Selcuk University, Department of Biochemistry, Konya, Turkey
A R T I C L E I N F O Keywords:
Energy storage and conversion Solar energy materials Hydrogen evolution Clay
A B S T R A C T
Laponite D is known as a zwitterionic synthetic clay having disc-shaped with negatively charged top faces and positively charged lateral edges, which is photochemically inactive and used to increase catalytic active surfaces. Herein, photocatalytic hydrogen evolution over Laponite D, which known as low cost inorganic clay, isfirstly reported in a system containing Eosin-Y (EY) and triethanolamine (TEOA) as a photosensitizer and electron donor, respectively, under visible light illumination. This reaction system shows excellent stability due to the good charge transport and separation efficiency thanks to zwitterionic property of Laponite D.
1. Introduction
Recently, photochemically inert layered clay materials have been drawn attention in energy conversion reactions due to the increasing catalytic active surfaces and suppressing the backward reaction by electron accumulation on itself [1,2]. Of particular interest to the pre-sent study is the use of Laponite D as a catalytically active electron transfer mediator, because of its disc-shaped structure with a diameter of approximately 25 nm and 1 nm thickness and asymmetric charge distribution with net positive and negative charges on its lateral edges and faces, respectively. Some researchers found high photocatalytic activity for hydrogen evolution reaction (HER) under visible light ir-radiation using sensitized natural silicate-based minerals [3–5]. Pho-tocatalytic HER properties of EY-sensitized clay-like materials as the hybrid photocatalyst (Ti-MCM41/EY/Pt) was displayed good stability in the presence of Pt [3]. Recently, montmorillonite and Hangjin2 clays were used in the photocatalytic HER under both ultraviolet and visible light [6,7]. Also, kaolinite and halloysite clays decorated with Pd were used in the electrocatalytic HER [8]. Natural silicate based Attapulgite (ATP) clay was mostly used in the photocatalytic and photoelec-trochemical HER thanks to the highly crystalline 1-D properties, which supplies the good charge transport and separation process by acid treatment or sensitizing with Erythrosine B (ErB) and CdS [4,5,9,10]. Photochemically inactive disc-shaped Laponite clays have been also used in the photocatalytic HER as the catalytic active surfaces. Nakato et al. were reported photocatalytic HER by niobate-sensitized Laponite in the presence of Pt [11,12]. There is no photocatalytic HER applica-tion by using metal free organic dye sensitized Laponite and/or not using noble metal co-catalyst in the literature.
Here it is reported a cheap and convenient route for visible light-induced hydrogen from water by using Laponite D clay (BYK Additives)
by means of metal free dye sensitization for thefirst time. EY and TEOA were used as the photosensitizer and electron donor, respectively. Laponite D displayed superb photocatalytic activity and its activity was barely decreased during the photocatalytic HER.
2. Experimental section
The photocatalytic HER experiments were performed in a Pyrex flask in the anaerobic glovebox system. Laponite D (10 mg), oxygen-free TEOA (5%) and EY (0.33 mM) solutions were mixed in theflask. Before photocatalytic experiments, the mixing solution were sonicated 10 min for homogenization. The well-dispersed mixture was stirred under the visible light (Solar Light– XPS 300™). The headspace gas was analyzed to determine of hydrogen amount with gas chromatograph (Shimadzu GC-2010Plus).
3. Results and discussion
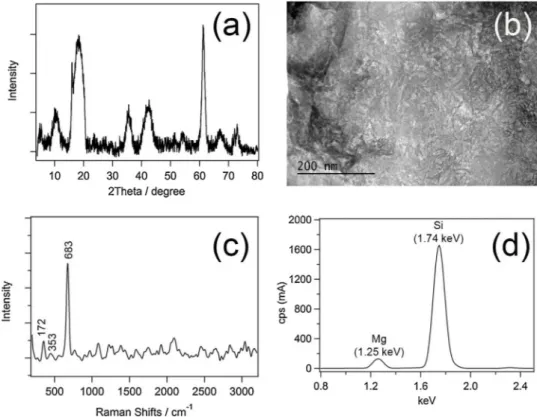
The XRD pattern shows characteristic broad diffraction peaks of the Laponite clay structure (Fig. 1a) [13]. The broad (001) diffraction band of Laponite is centered at 2θ = 7.14°, corresponding to d-spacing of 12.37 Å [14]. The structural unit, composed of the layer thickness and the interlayer space, is given by the d-value. TEM observation confirms both the spherical morphology and the size polydispersity (Fig. 1b). Due to electrostatic interactions, it shows aggregated spherical particles and not shown thefingerprint lattice fringes [15,16]. TEM image also shows a partial delamination of clay layers [17]. The bands at 683, 353 and 172 cm−1assigned to SieOeSi vibration, SieO bending and MgeO vibration, respectively, in the Raman spectrum (Fig. 1c) [18]. The Si4+
and Mg2+contents of Laponite D were measured by the XRF (Fig. 1d), which is containing highly Si and Mg elements [19]. XRF result also
https://doi.org/10.1016/j.jphotochem.2019.112335
Received 4 September 2019; Received in revised form 6 December 2019; Accepted 12 December 2019 E-mail address:emreaslan89@gmail.com.
Journal of Photochemistry & Photobiology A: Chemistry 390 (2020) 112335
Available online 20 December 2019
1010-6030/ © 2019 Elsevier B.V. All rights reserved.
shows that Laponite D is consisted of 31.3 %wt Si, 15.2 %wt Mg and little amount of (3.34 %wt) Na. Elemental analysis is also carried out by using energy dispersive X-ray (EDX) spectroscopy technique. EDX spectrum and its corresponding SEM images of Laponite D is given in the SI as a Figure S1. A series of elemental analyses have been carried out different areas of samples (Table S1) and it has been also in har-mony with XRF results. Moreover, the optical property of Laponite D was examined for the first time by UV–vis absorption (Fig. S2) and diffuse reflectance (Fig. S3) spectroscopies to the best of our knowledge. As illustrated infigure S4 optical band gap values are shifted from 4 to 5.2 by changing m = 1/2, 3/2, 2 and 3 correspond to allowed direct, forbidden direct, allowed indirect and forbidden indirect transitions, respectively (see SI for the details of calculations) [20,21].
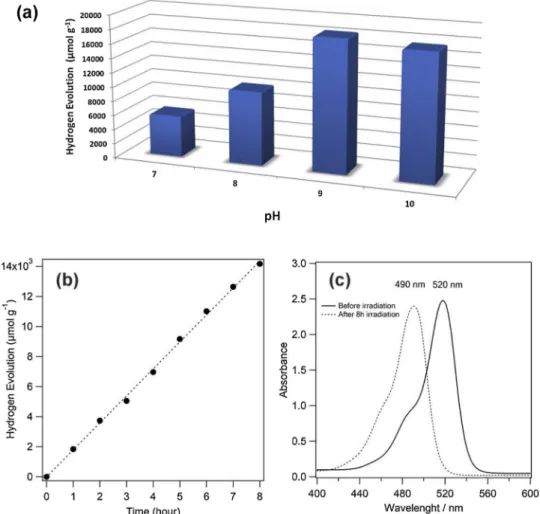
The photocatalytic HER was evaluated under visible light irradia-tion employing EY, TEOA and Laponite D as photosensitizer, sacrificial donor and electron transfer mediator, respectively. No hydrogen pro-duction is observed in the absence of dye molecule, sacrificial electron donor or Laponite D. First of all, the photocatalytic HER was in-vestigated by dye sensitized Laponite D in TEOA solution at different pH values (7–10) under the illumination of visible light source as dis-played inFig. 2a. The most efficient hydrogen evolution rate was found to be pH 9 and drops off remarkably at more basic or acidic conditions. The protonation of TEOA at more acidic pH values leads to diminished effectiveness of TEOA as a sacrificial reagent. H2production from water
becomes thermodynamically unfavorable at more basic pH values, which is in accordance with previous works [22–24].
The HER rates of Laponite D by dye sensitization were found to be 1.84 mmolg−1h−1at pH 9 in aqueous TEOA solution. No hydrogen production was observed in the absence of EY/TEOA. Fig. 2b, which shows HER rate against time, indicates steady increasing over 8 h. The total amount of H2after 8 h was turned out to be 13.8 mmol g−1. Also,
the shift of the characteristic absorption peak of EY (520 nm to 490 nm) without any intensity decrease was observed in the UV–vis spectra after 8 h, indicating that the sensitizer EY could barely degraded in this photocatalytic system (Fig. 2c). Bromine atoms in the EY is eliminated under illumination in the alkaline solution media and resulted in
shifting peak to 490 nm, which is originated from EY transformed into fluorescein-like molecules [25]. The stability of HER and non-de-gradation of EY may be explained by good charge transport and se-paration efficiency of Laponite D [12]. Also disc-shaped nanoparticles supply electron accumulation on itself and suppressing the backward reaction like photocorrosion or recombination [26]. Disc shaped and having asymmetrical charge array of Laponite D structure supply the well charge separation and transport efficiency. The role of Laponite D is accumulating and switching the excited electrons to H+ions to re-duce into hydrogen gas. In addition, its large surface area provides more active site to take place reactions [11,12]. In contrast to this study, most similar work reported that ErB sensitized ATP clay shows no HER activity in the absence of loading co-catalyst. The stability of dye sensitized and co-catalyst loaded ATP clay was decreased gradually during the HER reaction [10].
The photocatalytic HER by EY sensitized Laponite D could be pro-ceeded by the electron transfer mechanism (Fig. 3). It was speculated that excited electrons from EY were injected to the positively charged edges of Laponite D. EY supplies both molecular link to tie between electron donor and Laponite D, and to sensitize the visible light. The excited electrons were transferred to negatively charged radius surface because protons are adsorbed onto the negatively charged surface [11]. The zwitterionic property of Laponite D was suppressed the backward reaction [12]. Finally, the excited electrons and protons reacted to produce hydrogen and TEOA gave an electron to regenerate of EY. 4. Conclusion
It is found a favorable and cheap route to hydrogen generation by using Laponite D as the catalytically active surfaced nanostructure thanks to the its high surface area. Because of the low-cost fabrication, abundance of the raw materials and notable stability for HER, Laponite D has great potential in HER compared with modified clays. Laponite D shows great stability due to the zwitterionic properties. This study shows that Laponite D can be used as the catalytically active electron transfer mediator for different energy conversion applications. Fig. 1. (a) XRD, (b) TEM, (c) Raman and (d) XRF spectra of Laponite D.
E. Aslan Journal of Photochemistry & Photobiology A: Chemistry 390 (2020) 112335
Author statement
The whole article is realized by one author, which is Dr. Emre Aslan. Declaration of Competing Interest
The author declares that they have no known competingfinancial interests or personal relationships that could have appeared to in flu-ence the work reported in this paper.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jphotochem.2019. 112335.
References
[1] Y. Ishida, T. Shimada, D. Masui, H. Tachibana, H. Inoue, S. Takagi, Efficient excited energy transfer reaction in Clay/Porphyrin complex toward an artificial light-har-vesting system, J. Am. Chem. Soc. 133 (2011) 14280–14286.
[2] C. Ley, J. Brendlé, A. Walter, P. Jacques, A. Ibrahim, X. Allonas, On the interaction of triarylmethane dye crystal violet with LAPONITE® clay: using mineral nano-particles to control the dye photophysics, J. Chem. Soc. Faraday Trans. 17 (2015) 16677–16681.
[3] Q. Li, Z. Jin, Z. Peng, Y. Li, S. Li, G. Lu, High-efficient photocatalytic hydrogen evolution on eosin Y-Sensitized Ti−MCM41 zeolite under visible-light irradiation, J. Phys. Chem. C 111 (2007) 8237–8241.
[4] J. Zhang, R. He, X. Liu, Efficient visible light driven photocatalytic hydrogen pro-duction from water using attapulgite clay sensitized by CdS nanoparticles, Nanotechnology 24 (2013) 505401.
[5] J. Zhang, X. Liu, Photocatalytic hydrogen production from water under visible light
Fig. 2. (a) Influence of pH on photocatalytic HER over 1 h, (b) time courses of the photocatalytic HER, (c) UV–vis absorption spectra of the EY before and after photocatalytic HER.
Fig. 3. Schematic representation of mechanism for photocatalytic HER.
E. Aslan Journal of Photochemistry & Photobiology A: Chemistry 390 (2020) 112335
irradiation using a dye-sensitized attapulgite nanocrystal photocatalyst, J. Chem. Soc. Faraday Trans. 16 (2014) 8655–8660.
[6] Z. Liu, J. Wang, H. Ma, L. Cheng, S. Ar, J. Yang, Q. Zhang, A new natural layered clay mineral applicable to photocatalytic hydrogen production and/or degradation of dye pollutant, Environ. Prog. Sustainable Energy 37 (2018) 1003–1010. [7] J. Ryu, Y.J. Jang, S. Choi, H.J. Kang, H. Park, J.S. Lee, S. Park, All-in-one synthesis
of mesoporous silicon nanosheets from natural clay and their applicability to hy-drogen evolution, NPG Asia Mater. 8 (2016) e248.
[8] G. Kenne Dedzo, E. Pameté Yambou, M.R. Topet Saheu, G. Ngnie, C.P. Nanseu-Njiki, C. Detellier, E. Ngameni, Hydrogen evolution reaction at PdNPs decorated 1:1 clay minerals and application to the electrocatalytic determination of p-ni-trophenol, J. Electroanal. Chem. 801 (2017) 49–56.
[9] J. Zhang, A. Chen, L. Wang, X. Li, W. Huang, Striving toward visible light photo-catalytic water splitting based on natural silicate clay mineral: the interface mod-ification of attapulgite at the atomic-molecular level, ACS Sustainable Chem. Eng. 4 (2016) 4601–4607.
[10] X. Liu, Y. Xue, Y. Lei, F. Wang, S. Min, Cobalt-activated amorphous MoSx nanodots grown in situ on natural attapulgite nanofibers for efficient visible-light-Driven dye-sensitized H2 evolution, Acs Appl. Nano Mater. 1 (2018) 6493–6501.
[11] T. Nakato, T. Fujita, E. Mouri, Synergistic photocatalytic hydrogen evolution over oxide nanosheets combined with photochemically inert additives, J. Chem. Soc. Faraday Trans. 17 (2015) 5547–5550.
[12] T. Nakato, S. Terada, T. Ishiku, S. Abe, S. Kamimura, E. Mouri, T. Ohno, Photoinduced electron transfer in semiconductor–clay binary nanosheet colloids controlled by clay particles as a turnout switch, Appl. Catal. B 241 (2019) 499–505. [13] V.R.R. Cunha, F.C.D.A. Lima, V.Y. Sakai, L.M.C. Véras, J.R.S.A. Leite, H.M. Petrilli,
V.R.L. Constantino, LAPONITE®-pilocarpine hybrid material: experimental and theoretical evaluation of pilocarpine conformation, RSC Adv. 7 (2017) 27290–27298.
[14] J.M. Fraile, J.I. Garcı́a, J. Massam, J.A. Mayoral, Clay-supported non-chiral and chiral Mn(salen) complexes as catalysts for olefin epoxidation, J. Mol. Catal. A Chem. 136 (1998) 47–57.
[15] R.F.A. Teixeira, H.S. McKenzie, A.A. Boyd, S.A.F. Bon, Pickering emulsion poly-merization using laponite clay as stabilizer to prepare armored“Soft” polymer
latexes, Macromolecules 44 (2011) 7415–7422.
[16] D.W. Thompson, J.T. Butterworth, The nature of laponite and its aqueous disper-sions, J. Colloid Interface Sci. 151 (1992) 236–243.
[17] S. Jatav, Y.M. Joshi, Phase behavior of aqueous suspension of laponite: new insights with microscopic evidence, Langmuir 33 (2017) 2370–2377.
[18] M. Fatnassi, C.H. Solterbeck, M. Es-Souni, Clay nanomaterial thinfilm electrodes for electrochemical energy storage applications, RSC Adv. 4 (2014) 46976–46979. [19] J.M. Saunders, J.W. Goodwin, R.M. Richardson, B. Vincent, A small-angle X-ray
scattering study of the structure of aqueous laponite dispersions, J. Phys. Chem. B 103 (1999) 9211–9218.
[20] S.A. Khan, J.K. Lal, A.A. Al-Ghamdi, Thermal annealing effect of on optical con-stants of vacuum evaporated Se75S25-xCdx chalcogenide thinfilms, Opt. Laser Technol. 42 (2010) 839–844.
[21] D. Souri, A.R. Khezripour, M. Molaei, M. Karimipour, ZnSe and copper-doped ZnSe nanocrystals (NCs): optical absorbance and precise determination of energy band gap beside their exact optical transition type and Urbach energy, Curr. Appl. Phys. 17 (2017) 41–46.
[22] M.K. Gonce, E. Aslan, F. Ozel, I. Hatay Patir, Dye-sensitized Cu2 XSnS4 (X=Zn, Ni, Fe, Co, and Mn) nanofibers for efficient photocatalytic hydrogen evolution, ChemSusChem 9 (2016) 600–605.
[23] F. Ozel, E. Aslan, B. Istanbullu, O. Akay, I. Hatay Patir, Photocatalytic hydrogen evolution based on Cu2ZnSnS4, Cu2NiSnS4 and Cu2CoSnS4 nanocrystals, Appl. Catal. B 198 (2016) 67–73.
[24] G. Yanalak, A. Aljabour, E. Aslan, F. Ozel, I.H. Patir, A systematic comparative study of the efficient co-catalyst-free photocatalytic hydrogen evolution by transi-tion metal oxide nanofibers, Int. J. Hydrogen Energy 43 (2018) 17185–17194. [25] R. Abe, K. Hara, K. Sayama, K. Domen, H. Arakawa, Steady hydrogen evolution
from water on Eosin Y-fixed TiO2 photocatalyst using a silane-coupling reagent under visible light irradiation, J. Photochem. Photobiol. A: Chem. 137 (2000) 63–69.
[26] C.-T. Dinh, M.-H. Pham, F. Kleitz, T.-O. Do, Design of water-soluble
CdS–titanate–nickel nanocomposites for photocatalytic hydrogen production under sunlight, J. Mater. Chem. A 1 (2013) 13308–13313.
E. Aslan Journal of Photochemistry & Photobiology A: Chemistry 390 (2020) 112335