Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=irnf20
Renal Failure
ISSN: 0886-022X (Print) 1525-6049 (Online) Journal homepage: http://www.tandfonline.com/loi/irnf20
Oxidative Stress in Children with Acute
Glomerulonephritis
Mehmet Bülbül, Ayşe Öner, Gülay Demircin & Özlem Erdoğan
To cite this article: Mehmet Bülbül, Ayşe Öner, Gülay Demircin & Özlem Erdoğan (2008) Oxidative Stress in Children with Acute Glomerulonephritis, Renal Failure, 30:2, 209-214, DOI: 10.1080/08860220701813319
To link to this article: https://doi.org/10.1080/08860220701813319
Published online: 07 Jul 2009.
Submit your article to this journal
Article views: 67
ISSN: 0886-022X print / 1525-6049 online DOI: 10.1080/08860220701813319
209
LRNF
CLINICAL STUDY
Oxidative Stress in Children with Acute Glomerulonephritis
Oxidative Stress in AGN
Mehmet Bülbül
Department of Pediatric Nephrology, Dr. Sami Ulus Children’s Hospital, Ankara, Turkey
Ayse Öner
Department of Pediatric Nephrology, Ufuk University, Medical School, Ankara, Turkey
Gülay Demircin and Özlem Erdogan
Department of Pediatric Nephrology, Dr. Sami Ulus Children’s Hospital, Ankara, Turkey
Background. Oxidative stress has not been adequately
investigated in acute glomerulonephritis (AGN); therefore, we aimed to evaluate the oxidative stress (OS) status in children with AGN both at acute and remission stages. Patients and methods. Seventeen children (mean ± SEM, age 9.0 ± 0.5 years)
with AGN and 17 healthy controls were included. In addition to routine laboratory investigations, two blood samples were obtained from patients, at admission and after 6–10 weeks, to measure erythrocyte superoxide dismutase (SOD) activity and plasma malondialdehyde (MDA) level. Results. Significantly
elevated MDA levels (5.11 ± 0.28 vs. 3.15 ± 0.25 nmol/mL; p < 0.001) were found in acute stage of AGN compared with the con-trols; however there was no significant difference in SOD activi-ties (3732 ± 193 vs. 4035 ± 142 U/gHb; p > 0.05) between acute stage-AGN and control subjects. Significantly elevated SOD activities (3985 ± 195 U/gHb, p = 0.034) and decreased MDA levels (4.01 ± 0.38 nmol/mL, p = 0.001) were found at remission stage when compared with the acute stage. MDA levels and SOD activities of remission phase were similar to those of controls (p > 0.05). A significantly positive correlation was found between MDA levels and SOD activities in remission period (r = 0.654, p = 0.004). Patients with and without impaired renal functions had similar MDA levels and SOD activities (p >0.05). No significant correlation was found between glomerular filtration rates (GFR) and MDA levels (p > 0.05) and between GFR and SOD (p > 0.05) activities in acute stage-AGN. Conclusions. Oxidative
stress may play important role in the pathogenesis of AGN and not be correlated with renal functions. Further research is needed
to determine magnitude of OS and indications for antioxidants in other glomerulopathies.
Keywords acute glomerulonephritis, superoxide dismutase, malondialdehyde, oxidative stress
INTRODUCTION
Growing evidence has indicated that an imbalance between oxidative stress and antioxidant defense mecha-nisms plays an important role in the pathogenesis of various toxic, inflammatory, metabolic, and glomerular diseases.[1–3] Although renal experimental studies on oxidative injury have been carried out in glomerular diseases and vasculitis,[4–9] oxidative stress has not been adequately evaluated in chil-dren with acute glomerulonephritis (AGN), and clinical investigations on this issue are scarce.[10–12]
Acute glomerulonephritis is an inflammatory and proliferative glomerular disease that is generally triggered by an infectious agent, such as group A beta hemolytic streptococci. Histologically, AGN is characterized by marked proliferation of mesangial and endothelial cells in glomerulus, together with infiltration by hematogenous cells, most notably neutrophils. Reactive oxygen species (ROS), including the superoxide anion, hydrogen peroxide, and hydroxyl radical, may be generated by activated neu-trophils, monocytes, and mesangial cells during disease processes. Oxidative stress can occur due to excessive reactive ROS production, an impaired antioxidant system, or a combination of these factors.[1]
High peroxidative potential of erythrocytes results from their large quantity of unsaturated lipid content,
Address correspondence to Dr. Mehmet Bülbül, Dr. Sami Ulus Çocuk Hastanesi, Telsizler, Ankara, Turkey; Tel.: +90-533-552 06 59; Fax: +90- 312- 317 03 53; E-mail: mbbjkank@ hotmail.com, mbbjkank@yahoo.com.tr
210 M. Bülbül et al.
increased oxygen concentration, and the presence of iron in them. Therefore, erythrocytes have often been considered as a useful indicator for susceptibility to oxidative stress.[11,13] Biological membranes are particularly suscep-tible to peroxidative attack by ROS due to their high poly-unsaturated fatty acid content.[14] Lipid peroxidation can alter membrane structure and function in renal tissue.[7]
There are several markers of oxidant/antioxidant balance, including malondialdehyde (MDA), a lipid peroxidation product, and several enzymatic and non-enzymatic antioxidants. MDA is considered to be the main product of auto-oxidation and degradation of polyunsatu-rated fatty acids or their esters.[15]
The biological effects of ROS are controlled in vivo by antioxidative defense mechanisms, including antioxi-dant enzymes such as superoxide dismutase (SOD), which catalyzes the dismutation of the superoxide anion (O2−). SOD is an important antioxidant defense in nearly all cells exposed to oxygen, and therefore serves a key antioxidant role in the body.[3] Some studies have shown decreased antioxidant enzyme activities of erythrocytes and increased plasma MDA levels in acute stage of acute poststreptococ-cal GN compared to healthy controls.[10–12] However, in previous studies, oxidative stress has been measured only at the acute stage of the disease, and an evaluation of the same patients at follow-up period has never been performed. Furthermore, although AGN patients had been included in previous studies for which the study groups consisted of miscellaneous renal diseases,[10,12] their numbers have been few (i.e., seven or eight).
The aim of this study was to assess the oxidant/ antioxidant status in acute and remission phases of chil-dren with AGN in comparison with healthy control sub-jects. Thus, the present study is the first in the English literature with the viewpoint of comparing oxidant/antiox-idant status of acute stage AGN with that of remission period and of healthy subjects.
PATIENTS AND METHODS Patients
Seventeen children (13 boys, 4 girls) suffering from AGN, aged between 5.5 to 13 years, were included in the study group. The diagnosis of AGN was based on the acute disease onset, absence of a previous renal pathology or a family history of kidney disease, and addition of the following criteria: a recent history of pharyngitis, hema-turia, oliguria, edema or hypertension, and decreased serum complement 3 (C3) level. Lupus nephritis was excluded by the negative anti-nuclear antibody (ANA) test. The patients were divided into two subgroups
according to the presence or absence of impaired renal functions at admission. Impaired renal function was defined as serum creatinine level over the normal limits for age and gender standards.[16]
None of the patients were receiving any drug that can interfere with oxidant status at the beginning of the study. Detailed medical histories were obtained; thorough physi-cal examinations and routine laboratory tests were performed in all patients. Sixteen age-matched healthy children (10 boys, 7 girls) were included in the control group. Control subjects were selected from the children that admitted to hospital for routine check-up or elective surgery, including repair of inguinal hernia, hypospadias or undecendent testes, and had no clinical or laboratory signs of a systemic illness. The control subjects had normal physical examinations, blood pressures, serum electro-lytes, urea, and creatinine levels. Informed consents were taken from the parents of participants.
The following laboratory tests were performed to each patient: complete blood count; erythrocyte sedimen-tation rate (ESR); biochemical analyses, including serum electrolytes, blood urea nitrogen (BUN), creatinine, uric acid, total protein, albumin, total cholesterol, and triglyc-erides; prothrombin time; partial thromboplastin time; urinalysis; serum complement factors 3 and 4 (measured at disease onset and after 6–10 weeks in remission); immu-noglobulin A (IgA), IgG, IgM, C-reactive protein (CRP); ANA; anti-dsDNA; hepatitis B surface antigen; hepatitis A antibodies; and bacterial cultures. Chest x-rays and abdom-inal sonographies were also performed. Antistreptolysin O (ASO) tests and throat culture were performed to detect an antecedent streptococcal pharyngitis. Based on the tests carried out before the study, none of the patients had any other systemic disease. Clearance of creatinine was calcu-lated by Schwartz formula[17] to estimate glomerular filtra-tion rate (GFR).
On admission, a water and salt restriction was put into practice in all patients, and furosemide was given when needed. Hypertension could be controlled by captopril in two patients, and a peritoneal dialysis was performed in one who had uremic encephalopathy and convulsions.
Samples
Blood samples were obtained twice from patients, prior to any treatment at presentation and again in the remission phase, when the patients had no clinical and laboratory signs of AGN and did not receive any drug, after 6–10 weeks. Venous blood samples were obtained from patients and controls into heparinized tubes and cen-trifuged at 1200 × g for 10 minutes. Plasma was separated, and buffy coat was discarded by aspiration. Plasma was
stored at −33°C until analysis. Erythrocytes were washed three times with cold physiological saline and stored at −33°C until analysis. All of the measurements were performed twice, and all serum samples were analyzed simultaneously. The serum was analyzed for biochemical analyses using commercial kits with a Hithachi autoana-lyzer (Hithachi Ltd, Tokyo, Japan).
Measurement of Erythrocyte SOD Activity and Plasma MDA Level
To determine the activity of the erythrocyte antioxi-dant enzyme SOD, red blood cell lysates were prepared by freezing and thawing three times in dry ice. The lysates were diluted 1:5 with distilled water and frozen at −4°C until analysis. The suspension of erythrocytes was used for measurements of SOD activity. Erythrocyte SOD was measured by the method of Winterbourn et al.[18] based on the inhibition of the reduction of nitro blue tetrazolium (Sigma Chemical Co., St. Louis, Missouri, USA) by O2 produced via photo reduction of riboflavin (Sigma). Fifty percent inhibition was defined as one unit of SOD activity, and it was expressed as U/gHb.
Plasma lipid peroxidation was measured according to the method of Ohkawa et al.[19] This assay was based on the formation of a red adduct (absorption maximum: 532 nm) between thiobarbituric acid and malondialde-hyde, a colorless end product of lipid peroxidation. Plasma MDA level was expressed as nmol/mL.
Statistical Analysis
The analyses were performed using SPSS (version 12.0). Values were expressed as the mean plus/minus stan-dard error of mean (SEM). Statistical differences between patients and control groups were evaluated using the chi-square and Mann-Whitney U tests. The Wilcoxon signed ranks test was used to compare initial and follow-up mea-surements of the patient group. Spearman’s correlation analysis was performed to determine the magnitude of cor-relations. Differences with a p value of less than 0.05 were accepted as statistically significant.
RESULTS
The mean age (±SEM) of the study group was 9.0 ± 0.5 years (median 9.0 years, range 5.5–13) and that of con-trol group was 7.7 ± 0.5 years (median 8.0 years, range 4– 11). No difference was found between the mean age of the patients and healthy subjects (p > 0.05).
A history of previous upper respiratory tract infection was found in 15 (88.2%) and a history of skin infection in 2 (11.8%) patients. At presentation, microscopic hema-turia was detected in all patients, macroscopic hemahema-turia in 12 (70.6%), proteinuria in 13 (76.5%) and edema in 9 (52.9%) patients. Throat cultures grew Group A beta-hemolytic streptococci (GABHS) in 6 (35.3%) patients. Results of blood pressure measurements and some labora-tory analyses including renal function tests are shown in Table 1. Impaired renal functions were found in 5 (29.4%) and hypertension in 6 (35.3%) patients. The mean serum albumin and total protein levels were within normal refer-ence values (see Table 1).
All patients had decreased C3 levels and two patients had decreased C4 levels in the acute stage of the disease. In all patients, C3 and C4 levels returned to normal levels after 6–10 weeks of follow-up. None of the patients had a positive ANA or anti-dsDNA test. As shown in Table 2, white blood cell count (WBC) was found to be elevated in 41.2%, ASO in 41.2%, CRP in 35.3%, ESR in 88.2%, IgA in 35.3%, and IgG in 23.5% of patients. A total of 11 patients (64.7%) either had elevated ASO titer or positive throat culture for GABHS.
All but one patient gave good response to supportive management and recovered within 7–10 days. In that one patient, renal functions were rapidly impaired; oliguria, hypertensive encephalopathy, and convulsion developed. After a temporary peritoneal dialysis therapy, renal functions returned normal values, and the patient recovered.
Children with AGN had significantly higher MDA levels at presentation (MDA1, 5.11 ± 0.28 nmol/mL) com-pared to controls (3.15 ± 0.25 nmol/mL; p < 0.001). At the disease onset, SOD activities (SOD1, 3732 ± 193 U/gHb) were found to be similar to that of control subjects
Table 1
Results of biochemical analyses and blood pressure measurements in patients with acute glomerulonephritis (AGN)
at presentation (mean ± standard error of mean, SEM)
Mean ± SEM Range
Patients over upper limits, n (%) Albumin (g/dL) 4.1 ± 0.2 3.2–5.3 — Total protein (g/dL) 7.1 ± 0.2 5.6–8.9 — BUN (mg/dL) 25 ± 6 8–98 6 (35.3) Creatinine (mg/dL) 0.92 ± 0.13 0.6–2.6 5 (29.4) Uric acid (mg/dL) 5.4 ± 0.6 3.0–11.9 5 (29.4) Systolic BP (mm Hg) 114 ± 5 90–170 6 (35.3) Diastolic BP (mm Hg) 77 ± 5 40–120 5 (29.4)
212 M. Bülbül et al.
(4035±142 U/g Hb; p = 0.119; see Table 3 and Figures 1 and 2). After 6–10 weeks of follow up period, mean SOD activity (SOD2, 3985±195 U/gHb) significantly increased (p = 0.034) and mean MDA level (MDA2, 4.01±0.38 nmol/mL) significantly decreased (p = 0.001) compared with the initial values. The mean MDA2 and SOD2 val-ues were similar to those of control subjects (p = 0.079 and p = 0.734, respectively; see Table 3 and Figures 1 and 2. In patient and control groups, boys and girls had similar SOD activities and MDA levels (data not shown;
p > 0.05).
Patients divided into two subgroups according to impaired (5 patients) and normal (12 patients) renal func-tions. No significant differences were found in MDA1 and SOD1 values between patients with and without renal insufficiency (data not shown; p > 0.05).
Correlations
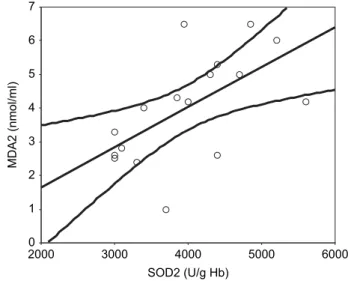
No significant correlation was found between GFR and MDA1 levels (p > 0.05) and between GFR and SOD1 activities (p > 0.05) in acute stage of AGN. In the remis-sion period, a significant positive correlation was found between MDA levels and SOD activities (r = 0.654, p = 0 .004; see Figure 3).
DISCUSSION
Acute glomerulonephritis is characterized by the pres-ence of hematuria, proteinuria, edema, and sometimes hypertension and acute renal failure. Acute post-strepto-coccal glomerulonephritis (APSGN) is the prototypic dis-ease of AGN. It is seen after both streptococcal pharyngeal and skin infections, with a latent period from infection.[20] Based on history, throat culture results, and elevated ASO titers, at least 13 of our patients (76.5%) can be considered as APSGN. In the remaining four patients, AGN may be secondary to skin infections or the patients may be carriers for GABHS with normal ASO titers despite preceding strep-tococcal infection. The most commonly used antibody assays for GABHS are ASO and antideoxyribonuclease B (anti-DNase B). The ASO test is usually obtained first, and if not elevated, an anti-DNase B test may be done. ASO titers rise and fall more rapidly than anti-DNase B. Anti-DNase B titers may remain elevated for several months.[20] We could not use anti-DNase B test in our study due to limited laboratory facilities. On the other hand, the low proportion (35.3%) for growing group A beta hemolytic streptococcus in throat cultures may result from previously used antibiotics at other medical institu-tions before admission to our hospital.
In this study, elevated plasma MDA levels as a marker of increased lipid peroxidation were found in the early stage of AGN. However the difference in SOD activ-ities between acute stage and controls could not reach to a significant level. After 6–10 weeks, during the remission period, plasma MDA levels returned normal control values. Our results indicated a transiently increased oxida-tive stress in the disease process of AGN.
Higher MDA levels of our patients in acute stage AGN can be considered a sign of increased oxidative stress at the beginning of the disease. In the pathogenesis of glomerulonephritis, different stimuli, such as activated complement, deposition of immune complexes, or release of cytokines, induce ROS formation by intrinsic glomeru-lar cells or infiltrated leukocytes.[8] Whether the increased plasma MDA comes directly from the excessive synthesis of ROS by kidneys or by blood cells in patients with glom-erular diseases is not known.[3] In the study of Turi et al., Table 2
Acute phase reactant levels and some immunological parameters in children with acute glomerulonephritis
Mean ± SEM Range
Patients outside the limits, n (%)
WBC (103/mm3) 11.2 ± 1.0 6.3–21.2 7 (41.2) C3 (mg/dL) 40.4 ± 4.9 20–75 17 (100.0) C4 (mg/dL) 24.1 ± 3.1 5–44 2 (11.8) ASO (Todd units) 313 ± 52 200–800 7 (41.2) CRP (ng/mL) 13.1 ± 3.6 6–48 6 (35.3) ESR (mm/h) 43 ± 5 3–80 15 (88.2) IgA (mg/dL) 227 ± 19 110–400 6 (35.3) IgG (mg/dL) 1286 ± 103 570–2260 4 (23.5) IgM (mg/dL) 108 ± 7 73–170 —
Abbreviations: WBC = white blood cell count, C = comple-ment factor, ASO = anti-streptolysin O, CRP = c-reactive pro-tein, ESR = erythrocyte sedimentation rate.
Table 3
Comparison of antioxidant and oxidant markers between acute and remission stages of acute glomerulonephritis (AGN) and
healthy subjects (mean ± SEM) AGN-acute (n = 17) AGN-remission (n = 17) Controls (n = 17) Malondialdehyde (nmol/mL) 5.11 ± 0.28, 4.01 ± 0.38, 3.15 ± 0.25 *p = 0.001, †p = 0.079 † p < 0.001 Superoxide dismutase (U/gHb) 3732 ± 193, 3985 ± 195, 4035 ± 142 *p = 0.034, †p = 0.734 † p = 0.119
* p: acute versus remission stage (with Wilcoxon test). †p: compared with controls (with Mann-Whitney U test).
significantly increased plasma MDA and decreased eryth-rocyte SOD activity was found in eight children in acute stage of post-streptococcal glomerulonephritis compared with the healthy controls.[10] Templar et al.[21] also have reported higher plasma MDA levels in patients with chronic renal failure resulting from chronic glomerulopa-thies than those of resulting from non-glomerular diseases, indicating the role of oxidative stress in the pathogenesis of glomerular injury.[21] In another study, the concentra-tion of MDA has been found to be increased four-fold and antioxidant enzyme catalase activity decreased 2.5-fold in
seven children with glomerulonephritis in comparison to 13 healthy subjects.[12] However, neither of the above studies had investigated oxidative stress in remission phase of AGN, as we did in the present study.
The superoxide radical, which released from activated neutrophils, plays a major role in the neutrophil-mediated acute inflammatory response, resulting in extensive tissue damage.[1] Superoxide dismutase has been implicated as an anti-inflammatory protective compound against super-oxide.[22] In our study, the similar activity of antioxidant enzyme SOD was found at disease onset, at remission period, and in the control subjects. If the oxidative damage is excessive, SOD will also be expected to increase for the compensation and protection of tissue injury.[23] Thus, the lack of increase in acute stage SOD activity may be due to some depletion resulting from oxidative stress as detected by higher MDA levels. Sustained SOD activity in acute stage of AGN suggested that antioxidant defense mecha-nisms work well in these patients; antioxidant enzymes were not totally depleted and remained at a steady state as a compensation for protection from hazardous effects of increased ROS. This constancy in enzyme activity, together with the normalized MDA level in the remission period, suggests that complete improvement of these pathological events takes place within 6–10 weeks from onset of the AGN.
The presence of a significant positive correlation between SOD activity and MDA level in remission period also indicates the oxidant/antioxidant balance of recovery phase, like a physiological state.
In present study, a lack of correlation between MDA and GFR and between SOD and GFR, together with similar Figure 1. Erythrocyte superoxide dismutase (SOD) levels at
disease onset and remission stage in comparison with healthy controls in children with acute glomerulonephritis.
Controls AGN-Remission AGN-Onset SOD (U/g Hb) 6000 5000 4000 3000 2000
Figure 2. Plasma malondialdehyde (MDA) levels in patients with acute glomerulonephritis and healthy subjects.
Controls AGN-Remission AGN-Onset MDA (nmol/ml) 8 7 6 5 4 3 2 1 0
Figure 3. The relationship between superoxide dismutase (SOD2) and malondialdehyde (MDA2) in remission stage (r=0.654, p = 0.004). SOD2 (U/g Hb) 6000 5000 4000 3000 2000 MDA2 (nmol/ml) 7 6 5 4 3 2 1 0
214 M. Bülbül et al.
MDA levels and SOD activities in patients with and without impaired renal functions, indicated that oxidative stress was not the primarily result from renal insufficiency in AGN.
More recent evidence implicates ROS as intracellular signaling molecules in mitogenic pathways involved in the injury. In one study, it has been suggested that oxidative stress can cause glomerular injury, not only by infiltrating direct damage on the glomerular structures but also by inducing cellular proliferation in mitogenic pathways.[8] Therefore, the suppression of oxidative stress may result in amelioration of glomerular injury.
Based on our findings, we can state that in acute stage of AGN, antioxidant defense weakens, and these children suffer from increased oxidative stress with an increased susceptibility to lipid peroxidation. Therefore antioxidant supplementation (vitamins E and C, etc.) in the acute stage of AGN may be beneficial in the amelioration of the disease.
In conclusion, oxidative stress may play an important role in the pathogenesis of AGN, and increased lipid per-oxidation seems not related to renal functions. Further research is needed to determine the magnitude of oxidative stress and indications for therapeutic antioxidant interven-tion in other glomerulopathies.
REFERENCES
1. McCord JM. Human disease, free radicals, and the oxidant/ antioxidant balance. Clin Biochem. 1993;26:351–357. 2. Halliwell B. Free radicals, antioxidants, and human disease:
Curiosity, cause, or consequence? Lancet. 1994;344:721– 724.
3. Chen HC, Tomino H, Yaguchi Y, et al. Oxidative metabo-lism of polymorphonuclear leukocytes and superoxide dismutase activity in IgA nephropathy. J Clin Lab Anal. 1992;19:57–60.
4. Shah SV. Evidence suggesting a role for hydroxyl radical in passive Heymann nephritis in rats. Am J Physiol. 1988;254:F337–F344.
5. Rahman MA, Emancipator SS, Sedor J. Hydroxyl radical scavengers ameliorate proteinuria in rat immune complex glomerulonephritis. J Lab Clin Med. 1988;112:619–626. 6. Birtwistle RJ, Michael J, Howie AJ, Adu D. Reactive oxygen
products in heterologous anti-glomerular basement mem-brane nephritis in rats. Br J Exp Pathol. 1989;70:207–213. 7. Kuo HT, Kuo MC, Chiu YW, Chang JM, Guh JY, Chen HC.
Increased glomerular and extracellular malondialdehyde levels in patients and rats with focal segmental glomerulo-sclerosis. Eur J Clin Invest. 2005;35:245–250.
8. Budisavljevic MN, Hodge L, Barber K, et al. Oxidative stress in the pathogenesis of experimental mesangial prolif-erative glomerulonephritis. Am J Physiol Renal Physiol. 2003; 285:1138–1148.
9. Gaertner SA, Janssen U, Ostendorf T, Koch KM, Floege J, Gwinner W. Glomerular oxidative and antioxidative systems in experimental mesangioproliferative glomerulonephritis.
J Am Soc Nephrol. 2002;13:2930–2937.
10. Turi S, Nemeth I, Torkos A, et al. Oxidative stress and anti-oxidant defense mechanism in glomerular diseases. Free
Radical Biol Med. 1997;22:161–168.
11. Devasena T, Lalitha S, Padma K. Lipid peroxidation, osmotic fragility and antioxidant status in children with acute post-streptococcal glomerulonephritis. Clin Chim Acta. 2001; 308:155–161.
12. Pavlova EL, Lilova MI, Savov VM. Oxidative stress in children with kidney disease. Pediatr Nephrol. 2005;20:1599–1604. 13. Marcin J, Maciej J, Michael J, Szcypka M, Gajewska J,
Laskowska-Kilta T. Antioxidant defence of red blood cells and plasma in stored human blood. Clin Chim Acta. 1997;267:129–142.
14. Freeman BA, Crapo JD. Free radicals and tissue injury. Lab
Invest. 1982;47:412–426.
15. Nielsen F, Mikkelsen BB, Nielsen JB, et al. Plasma malondi-aldehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214.
16. Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: Normal values for age and sex. J Pediatr. 1976;88:828–830.
17. Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263.
18. Winterbourn CC, Hawkins KE, Brian M, Carrell RW. The estimation of red cell superoxide dismutase activity. J Lab
Clin Med. 1975;85:337–345.
19. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
20. Pichichero ME. Group A beta-hemolytic streptococcal infec-tions. Pediatr Rev. 1998;19:291–302.
21. Templar J, Kon SP, Milligan TP, Newman DJ, Raftery MJ. Increased plasma malondialdehyde levels in glomerular disease as determined by a fully validated HPLC method.
Nephrol Dial Transplant. 1999;14:946–951.
22. Koninsberger JC, Asbeck BS, Iassen E. Copper zinc-super-oxide dismutase and hydrogen perzinc-super-oxide: A hydroxyl radical generating system. Clin Chim Acta. 1994;23:51–61. 23. Demircin G, Öner A, Ünver Y, Bülbül M, Erdogan Ö.
Eryth-rocyte superoxide dismutase activity and plasma malondial-dehyde levels in children with Henoch-Schönlein purpura.