Materials Science in Semiconductor Processing 110 (2020) 104959
Available online 28 January 2020
1369-8001/© 2020 Elsevier Ltd. All rights reserved.
Hydrothermally synthesized UV light active zinc stannate:tin oxide (ZTO:
SnO
2
) nanocomposite photocatalysts for photocatalytic applications
Elif Keles
a, Mehmet Yildirim
a, Teoman €Oztürk
b, Ozlem Altintas Yildirim
a,*aKonya Technical University, Faculty of Engineering and Natural Sciences, Department of Metallurgical and Materials Engineering, Konya, Turkey bSelcuk University, Faculty of Science, Department of Physics, Konya, Turkey
A R T I C L E I N F O Keywords: Zinc stannate Tin oxide Nanocomposite Hydrothermal method Photocatalytic activity Reusable stable photocatalyst
A B S T R A C T
This study was carried out for the simple synthesis of zinc stannate (Zn2SnO4, ZTO): tin oxide (SnO2) nano-composite photocatalyst in order to investigate the photodegradation of Rhodamine B (RhB) dye molecules under the UV light irradiation. For this purpose, nanocomposite photocatalysts were prepared via a low tem-perature hydrothermal method. Pure ZTO nanoparticles were also synthesized for the comparison purpose of the photocatalytic activity. Formation of the ZTO:SnO2 nanocomposites could be easily controlled by tuning of the hydrothermal reaction temperature. The structural properties of ZTO:SnO2 nanocomposites and ZTO nano-particles were schematically characterized by XRD, XPS and FTIR. Morphological investigation was performed via SEM and TEM. Band structure and optical properties were investigated with UV–Vis spectroscopy and PL analyses. It was found that ZTO:SnO2 nanocomposites keep hexagonal ZTO nanoparticles and SnO2 nanorods together and have more surface defects than that of the ZTO nanoparticles. The degradation of RhB dye mole-cules in the presence of ZTO:SnO2 nanocomposites reached to 62.0% degradation after first 60 min and 94.5% after 240 min. The photocatalytic rate constant of the ZTO:SnO2 nanocomposites (k ¼ 0.0111 min 1) was two times higher than that of the pure ZTO nanoparticles (k ¼ 0.00527 min 1). In addition, ZTO:SnO
2 nano-composites exhibited very high stability in the cycling degradation test with 90.1% RhB degradation even after ten times cycling run.
1. Introduction
In the past decade, water pollution originating from industrial and household applications has become an important threat to environment and human health. Thus, toxic and harmful organic compounds present in polluted water must be eliminated [1]. Recent studies demonstrated that binary metal oxide semiconductor mediated photocatalysis such as TiO2, ZnO, SnO2, Fe2O3, CdS and CuO are highly effective catalysts to
remove organic compounds in waste water [2–8]. Among the various semiconductor photocatalysts proposed; ZnO is one of the most commonly used material in the photocatalytic applications due to its high chemical stability, high oxidation power, low-cost and non-toxic properties [3,7,9,10]. SnO2 is also n-type binary semiconductor metal
oxide and has been widely studied for gas sensing applications due to its excellent combustible gas sensitivity. Recently, porous SnO2
nano-structures have been attracted great attention for removal of organic molecules or heavy metal ions from wastewaters [11]. However, the fast recombination of the photogenerated electron-hole (e -hþ) pairs is the
main disadvantage of binary oxides in commercial applications. Therefore, ternary oxides such as normal (AB2O4 general formula) and
inverse (A2BO4 general formula) spinel structured materials have been
started to use as photocatalyst materials. In this spinel structures, A can be usually Zn2þ, Cd2þ or Mg2þ, and B can be generally Sn2þ and
Al3þmetal ions [12–15]. As inverse spinel structure, zinc stannate
(Zn2SnO4) or zinc tin oxide (ZTO) is a promising n-type ternary
semi-conductor which has a wide band gap of 3.6 eV (space group Fd3m) [16]. In the reverse spinel crystal structure, all Sn4þions are octahe-drally coordinated, while the half of the Zn2þions are located at the
octahedral coordination and the other half of the Zn2þions are
posi-tioned at the tetrahedral coordination [17].
Compared with various binary semiconductor materials, ZTO can be used in humidity detectors, combustible gas sensors, anode material for Li-ion batteries, flame retardant and smoke suppressant materials due to its high electron mobility, electrical conductivity and high chemical/ thermal stability giving opportunity for using in extreme conditions [18–21]. ZTO nanostructures can be produced with various methods
* Corresponding author.
E-mail addresses: ozlemaltintas@gmail.com, oayildirim@ktun.edu.tr (O.A. Yildirim).
Contents lists available at ScienceDirect
Materials Science in Semiconductor Processing
journal homepage: http://www.elsevier.com/locate/mssp
https://doi.org/10.1016/j.mssp.2020.104959
including thermal evaporation, sol-gel synthesis, mechano-chemical synthesis, co-precipitation, sonochemistry with high temperature calcination and hydrothermal method [10,22–26]. Among these methods, hydrothermal technique is a promising technique for the production of well-crystallized ZTO nanoparticles [19]. The low tem-perature synthesis of size and morphology controlled phase pure ZTO nanoparticles with environmentally friendly method is the main advantage of hydrothermal method over the other methods.
ZTO nanoparticles have also attracted extensive research attention due to their good photocatalytic performance for the degradation of pollutants [27–30]. However, similar to binary oxides, recombination of electron-hole (e -hþ) pairs still hinders the photocatalytic activity of
ZTO [12]. Although doping or alteration of size and morphology of ZTO nanoparticles can enhance the photocatalytic activity, formation of composite structure with a binary semiconductor having different band structure than that of ZTO is another effective strategy for providing efficient photocatalytic activity. The previous studies showed that the photocatalytic activity, electrical and photovoltaic responses have been improved with ternary-binary composites between ZTO and TiO2, ZnO,
SnO2, Fe2O3, and WO3, etc. [23] since in such composites, e -hþpairs
are efficiently separated and the absorption range is expanded. Among the binary metal oxide semiconductors, SnO2 can be chosen as a good
couple for ZTO because of its wide band gap which is close to ZTO. Also the conduction band position of ZTO is higher than that of SnO2,
elec-trons easily move to the conduction band of the SnO2 which acts as a
sink [31,32]. This transfer increases the e -hþpair separation and thus
enhances the photocatalytic activity.
To date, ZTO:SnO2 nanocomposite structures have been mostly
studied in device applications like gas sensors [33,34] and dye sensitized solar cells [31,35]. Furthermore, ZTO:SnO2 nanocomposites have
recently begun to find place in photocatalytic studies [32,36–38]. Although many different methods have been applied in the literature for the production of these nanocomposites, high temperature is usually required for sintering and calcinations. Hydrothermal technique is particularly distinguished by low temperature advantage. In detail, Wang et al. [38] prepared ZTO:SnO2 nanocomposites via a one-pot
hydrothermal method applying a calcination treatment to their prod-ucts at high temperatures such as 800–1100 �C and then they
investi-gated photocatalytic activity of these composites with degradation of methyl orange and gaseous benzene. As far as we know, there is no study on the hydrothermal synthesis of ZTO:SnO2 nanocomposites at low
temperature and on photocatalytic applications. In this study, we syn-thesized ZTO:SnO2 nanocomposites via a simple low temperature
hy-drothermal technique. The photocatalytic activity of the ZTO:SnO2
nanocomposite was investigated through the degradation of Rhodamine B (RhB) dye aqueous solution under the UV light irradiation. Possible kinetic mechanism for efficient photocatalytic activity was proposed based on detailed examination of crystal structure, surface chemical states of components, morphology, optical property and photo-kinetic behaviors of nanocomposite performed via XRD, FTIR, XPS, SEM, TEM, PL and UV–Vis spectrophotometer. Our results demonstrated that ZTO:SnO2 nanocomposites contained single crystalline SnO2 nanorods
wrapped the surface of ZTO nanoparticles. Constructed heterojunction regions between SnO2 nanorods and ZTO nanoparticles caused to form
the crystal defects or vacancy states. These developments in the defects or vacancy states provided efficient charge separation and enhanced activity of nanocomposites towards the photocatalytic degradation of RhB dye molecules. Hence, the efficient nanocomposite catalyst might lead to significantly enhanced photocatalysis process under the UV-light irradiation.
2. Experimental procedure
2.1. Materials
ZTO:SnO2 nanocomposites were synthesized by using zinc acetate
dihydrate (Zn(Ac)2, C4H6O4Zn⋅2H2O, 99.0%, Sigma-Aldrich), tin
tetra-chloride pentahydrate (SnCl4⋅5H2O 98.0%, Sigma-Aldrich) and sodium
hydroxide (NaOH pellets, 97%, Merck). Deionized water was used as solvent. All the chemical reagents were used without further purification.
2.2. Experimental methods
ZTO:SnO2 nanocomposites were prepared using common
hydro-thermal synthesis conditions for ZTO [27]. In the synthesis procedure, first required amount of Zn(Ac)2 for the 2:1:8 Zn:Sn:OH molar ratio was
dissolved in 40 mL deionized water. The transparent solution was stirred at room temperature for 2 h under mild stirring conditions. In the meantime, the determined amount of SnCl4⋅5H2O was dissolved in again
40 mL deionized water at room temperature for 2 h; then both solutions were slowly mixed under constant stirring. 2 M NaOH aqueous solution which was used as a mineralizer was added to the prepared Zn:Sn so-lution drop by drop using an automated injection pump at a rate of 2 mL/h until the reaching the pH value of 8.0. After magnetic stirring for 1 h, the slurry solution (100 mL total volume) was transferred into a Teflon-lined stainless steel autoclave with 150 mL capacity. The tem-perature of the hydrothermal reactor was adjusted to 180 �C. After 24 h
reaction time, the reactor was naturally cooled down to room temper-ature. The white particles collected from the bottom of the Teflon cru-cible were centrifuged at 10000 rpm for 5 min and washed thoroughly (three times) with deionized water. The powder products were then dried at 100 �C for 6 h, and no further thermal treatment was carried
out. For this experimental conditions, ZTO:SnO2 nanocomposite
prod-ucts yield through Zn(Ac)2 precursor was determined as 94%. This
indicated that the proposed hydrothermal mechanism was highly effective for the synthesis of ZTO:SnO2 nanocomposites. In order to
investigate the effect of the composite structure on the photocatalytic properties of nanocomposites, same experimental procedure was repeated at 160 �C for the synthesis of pure ZTO nanoparticles.
2.3. Characterization
The crystal structures of the obtained ZTO:SnO2 nanocomposites and
ZTO nanostructures were analyzed by Bruker D8 Advanced X-ray diffraction (XRD) system with a Cu-Kα radiation ðλ ¼ 1:5406 �A Þ
be-tween 2θ ¼ 10–80�boundary values. The XRD spectra were recorded
with an energy step size of 2�/min. Fourier transform infrared spectra
(FTIR) were recorded with a Bruker IFS 66/S model (Bruker AXS GmbH) spectrophotometer using KBr pellets to perceive the bonding formation of the ZTO:SnO2 nanocomposites. X-ray photoelectron spectroscopy
(XPS) analyses were performed with a high resolution Thermo Scientific Kα XPS with monochromatic Al-Kα radiation. The morphology of the
both nanostructures were characterized using TESCAN MAIA3 XMU model scanning electron microscope (SEM) and JEOL JEM 2100F model transmission electron microscope (TEM). In order to examine the chemical composition of the ZTO nanoparticles and ZTO:SnO2
nano-composites, absorption spectra of these nanostructures were obtained using a Cary 5000 UV–Vis spectrophotometer. The photoluminescence (PL) spectra were measured with PTI ASOC-10 model fluorescence spectrophotometer with an excitation wavelength of 310, 325 and 333 nm at room temperature.
2.4. Photocatalytic characterization
Photocatalytic activities of the ZTO nanoparticles and ZTO:SnO2
nanocomposites were investigated by performing decolorization ex-periments of RhB dye in a water cooled cylindrical quartz glass reactor under the UV light irradiation. For the experiments, 5 mg/L RhB con-taining dye solution was prepared with 100 mL deionized water. Pre-pared 40 mg photocatalyst nanoparticles were then put into the dye solution and stirred for 1 h under dark conditions to ensure the
adsorption-desorption equilibrium between catalyst and dye molecules. Photodegradation experiments were performed under the 100 W UV-C (254 nm wavelength) light illumination generated by a lamp enclosed in a dark box for a total duration of 240 min. In order to analyze the maximum absorption level, 3 mL of RhB dye solution exposed to UV light was taken at 10 min intervals and centrifuged to remove nano-particles. After absorption measurement, sample solution was poured back to main dye solution after each step. The activity measurement experiments were performed using these centrifuged solution in ambient conditions without any additive for pH control and recording the maximum absorption band at a wavelength of 554 nm using a Cary 5000 UV–Vis spectrophotometer.
3. Results and discussion
3.1. Structural analysis
The crystal structure and phase analysis of the synthesized products were investigated by XRD. Fig. 1 indicates the XRD diffractograms of the samples obtained at 160 and 180 �C hydrothermal reactor temperatures.
At 160 �C synthesis temperature, all of the diffraction lines were
attributed to pure reverse spinel crystal structure of ZTO (Zn2SnO4
JCPDS card no: 74–2184) and no noticeable phase related with any Sn or Zn compounds was detected. The lattice parameters of the samples prepared at 160 �C (a ¼ b ¼ c ¼ 0.865 nm) well matched with the
standard JCPDS card of ZTO, therefore, phase pure ZTO nanoparticles can be synthesized at 160 �C. The reactions for the formation of pure
ZTO in the applied synthesis procedure can be described as follows;
Snþ4þ6ðOHÞ →SnðOHÞ2
6 (1)
Znþ4þ4ðOHÞ →ZnðOHÞ2
4 (2)
SnðOHÞ26 þ2ZnðOHÞ24 → Zn2SnO4þ4H2O þ 6OH (3)
In the beginning of the experimental procedure, first Zn(Ac)2 and
SnCl2 precursor salts were dissolved in water. Then, these ionized
molecules produced SnðOHÞ2
6 and ZnðOHÞ24 ions according to the
re-actions (1) and (2), respectively. After the reaction of these ions in the hydrothermal reactor, ZTO nanoparticles were formed as in the reaction (3).
According to the literature, highly crystalline ZTO nanoparticles can be synthesized at 180 �C, when different mineralizer such as Na
2CO3,
KOH and tert-butyl amine are used [39–41]. However, in the presence of NaOH as a mineralizer, cubic crystalline ZTO nanoparticles without any binary oxide can be synthesized at the same temperature after an additional annealing process [42] or at higher temperatures such as 200
�C [43,44] or 220 �C [26,45] without additional heat treatments. In this
study, in contrast to general hydrothermal reaction using NaOH base mineralizer, highly crystalline ZTO nanoparticles without any SnO2 or
ZnO phases were produced at lower temperature (160 �C) than that used
in other studies [46,47]. This is originating from very slowly addition of NaOH without the formation of concentration gradient during the continuously supplying OH ions.
The formation mechanism of ZTO:SnO2 nanocomposites during the
hydrothermal process is based on the dissolution, recrystallization and growth of ZnðOHÞ2
4 and SnðOHÞ26 ions. Similar mechanism was also
reported by Annamalai et al. [41]. Recrystallization and growth phe-nomena’s in the supersaturated solution media provided by mineralizer are affected by several parameters such as reaction temperature, con-centration of the mineralizer and precursors. A change in the hydro-thermal temperature may affect the formation of the ZTO:SnO2
nanocomposites as the most determinant factor. Therefore, synthesis procedure for ZTO nanoparticles was repeated at 180 �C. XRD data
obtained from the sample synthesized at 180 �C is also shown in Fig. 1.
Apart from the ZTO diffraction lines, a very low intensity peak at 2θ ¼ 26.55�can be indexed with (110) diffraction line of SnO
2 phase (JCPDS
card no: 41–1445). Appearance of this weak peak in the XRD pattern of sample prepared at relatively higher temperature suggests that higher temperature is more proper for the synthesis of ZTO:SnO2
nano-composites. Furthermore, the intensities of the diffraction lines of ZTO phase synthesized at 180 �C are much higher than that of the sample
prepared at 160 �C. This shows that ZTO:SnO
2 nanocomposites have
highly crystalline ZTO phase compared to the ZTO nanoparticles. The formation of co-product of SnO2 as second phase clusters occurs
according to the following reactions;
Snþ4þ3OH →H
2SnO3þHþ (4)
H2SnO3→ SnO2þH2O (5)
At relatively higher hydrothermal temperature, due to the stronger hydrolysis effect of Snþ4, the consuming of OH ions in the solution
becomes more quickly. Thus, some colloidal H2SnO3 forms (reaction
(4)) and these colloids transforms to SnO2 (reaction (5)). According to
these results, although lower temperature is favorable for the synthesis of phase pure ZTO nanoparticles with relatively lower crystallinity, higher reaction temperature promotes to formation of the highly crys-talline ZTO:SnO2 nanocomposites.
For comparison, the crystallite size of ZTO nanoparticles and ZTO: SnO2 nanocomposites were estimated using the line broading method
with well-known Debye-Scherrer equation. The determined crystallite sizes of ZTO using (311), (511) and (440) diffraction peak are 23.5 � 7.8 nm for ZTO nanoparticles and 20.7 � 4.9 nm for ZTO:SnO2
nano-composites, respectively. It is well known that the smaller crystallite size is obtained if nucleation rate is greater than the growth rate. Thus, at higher temperature, nucleation phenomena is probably more favorable than the growth providing finer ZTO nanoparticles. In addition, the size of the ZTO nanoparticles synthesized via hydrothermal method were usually between 25 and 37 nm, so our results agreed well with the data present in the literature [28].
The chemical nature of the ZTO:SnO2 nanocomposites and ZTO
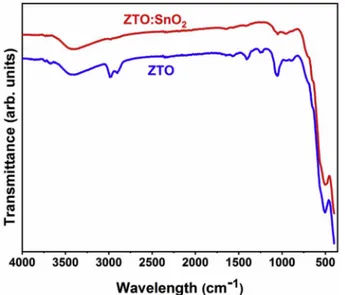
nanoparticles were also investigated with FTIR spectroscopy. Fig. 2
shows FTIR spectra of both samples. According to the spectra, ZTO nanoparticles exhibited three characteristic strong absorption peaks observed at 390, 505 and 1055 cm 1. While the absorption peak observed at 390 cm 1 can be connected with Zn–O bonds from the
tetrahedral site of the inverse spinel structure, the absorption peak observed at 505 cm 1 can be related with Sn–O bonds from the
Fig. 1. XRD patterns of samples synthesized at 160 �C and 180 �C hydrother-mal reactor temperature.
octahedral site and the absorption peak at 1055 cm 1 can be assigned to
Sn–O–Zn bondings in the Zn2SnO4 [28,48]. The absorption peak
observed at 1400 cm 1 can be assigned to the C–H vibration mode
originating from the organic residuals. The absorption peak at 3280 cm 1 can be ascribed to the possible hydrogen bonds. The broad band
located at 3100 to 3600 cm 1 could be related to the hydroxyl groups
[13]. The obtained FTIR spectra can be more resolved to investigate ZTO:SnO2 nanocomposite structure. As seen from the spectra, while the
relative intensity of the absorption peaks related with Zn–O and Sn–O bonds were preserved, the intensity of the peak assigned to Sn–O–Zn bonds drastically decreased. It is worth to mention that the result of FTIR analysis for ZTO:SnO2 nanocomposite was somewhat contradictory with
XRD data showing higher crystallite of ZTO phase. However, this contradiction could be attributed to hindering effect of SnO2
nano-particles wrapped the surface of the ZTO nanonano-particles, which will further discussed with TEM analysis in Section 3.2.
The surface chemical compositions and oxidation states of funda-mental elements of ZTO nanoparticles and ZTO:SnO2 nanocomposites
have been identified by XPS analysis. Fig. 3(a) shows the survey XPS spectra of both samples. The spectrums show that the nanoparticles and nanocomposites exhibited strong Zn, O and Sn lines and relatively weak C line located at 285.0 eV used as the reference for the calibration of the binding energies. The Sn:Zn atomic ratio was determined by the ratio of Sn(3d3/2):Zn(2p3/2) signals of XPS analysis as 0.42 for ZTO
Fig. 2. FTIR spectra of ZTO:SnO2 nanocomposites and ZTO nanoparticles.
Fig. 3. XPS spectra of ZTO:SnO2 nanocomposites and ZTO nanoparticles; (a) survey analyses and high resolution regional XPS spectra of (b) Zn(2p), (c) O(1s) and
nanoparticles and 0.64 for ZTO:SnO2 nanocomposites. The higher
atomic ratio of Sn(3d3/2) signal of nanocomposites also indicates the
formation of the SnO2 phase. The high resolution XPS spectra of Zn, O
and Sn elements are displayed in Fig. 3(b–d), respectively. Fig. 3(b) il-lustrates the Zn(2p) region of the XPS spectrum of both samples. In the spectrum of ZTO nanoparticles prepared at 160 �C, the double peaks
appeared at 1043.39 and 1020.23 eV could be assigned to binding en-ergies of Zn(2p1/2) and Zn(2p3/2) signals which were originated from
strong spin orbit coupling [49]. It has been also found that energy dif-ference of 23.16 eV between doublet binding energies agreed well with the previously reported binding energy value of stoichiometric ZTO [50]. In the spectrum of ZTO:SnO2 nanocomposites prepared at 180 �C,
the locations of Zn(2p3/2) and Zn(2p1/2) signals shifted to relatively
higher binding energies (Zn(2p3/2):1043.51eV and Zn
(2p1/2):1020.35eV). These shifts in the binding energies of Zn(2p)
sig-nals could be ascribed to reduce of electron density due to electron transfer from ZTO to SnO2 and demonstrated the surface modification of
ZTO with SnO2 and so formation of composite structure. Furthermore,
the relative intensity of Zn(2p) XPS signal of ZTO:SnO2 nanocomposites
was higher than that of the pure ZTO nanoparticles. This could be explained with the formation of SnO2 nanoparticles at higher
tempera-ture as identified with XRD data. SnO2 nanoparticles resulted in the
inhabitation of XPS signal generation from the surface of ZTO nanoparticles.
The binding states of O(1s) spectra belonging to ZTO nanoparticles and ZTO:SnO2 nanocomposites are shown in Fig. 3(c). In the spectra of
both nanostructures, XPS peaks of O(1s) exhibited the distinct asym-metry and these peaks could be divided into two different Gaussian peaks by using Origin 2018 software. For ZTO nanoparticles, Gaussian components were centered on 531.00 eV and 529.35 eV. Binding energy values of O(1s) signals of ZTO nanoparticles were also good agreement with that of stoichiometric ZTO [50]. The O(1s) spectra at higher binding energy side can be assigned to oxygen vacancies, whereas the
lower side component could be attributed to lattice O2 ions with
Zn–O–Sn bond structure [51]. A change of the intensity of this oxygen vacancy related components could be ascribed to the change in the concentration of surface oxygen defects. For ZTO:SnO2 nanocomposites,
the intensity of oxygen vacancy related peak was clearly higher than that of the ZTO nanoparticles. The formation of ternary:binary hetero-junctions at the surface of ZTO nanoparticles could be responsible for the enhancement in the number of oxygen vacancies.
The Sn(3d) region of the XPS spectrum of both ZTO nanoparticles and ZTO:SnO2 nanocomposites are presented in Fig. 3(d). In the XPS
spectrum of ZTO nanoparticles, due to spin orbit splitting of the 3d electrons, the double peaks were observed at 493.48 and 485.05 eV assigned to the binding energies of Sn(3d3/2) and Sn(3d5/2), respectively
[52]. These signals were ascribed to the tetravalent oxidation states of Sn (Sn4þ). In the XPS data of ZTO:SnO
2 nanocomposites, binding
en-ergies of Sn(3d3/2) and Sn(5d5/2) shift to higher binding energies
(493.62 eV and 485.19 eV, respectively). These 0.14 eV positively shift compared to that of ZTO nanoparticles suggest that they have different bonding environments. The intensity of these signals was also slightly higher than that of the ZTO nanoparticles which suggested the forma-tion of the surface SnO2 spaces. Furthermore, for both ZTO and ZTO:
SnO2 samples, the energy difference between doublet binding energies
were determined as 8.46 eV, which was in good agreement with the standard value of 8.50 eV [38].
3.2. Morphological analysis
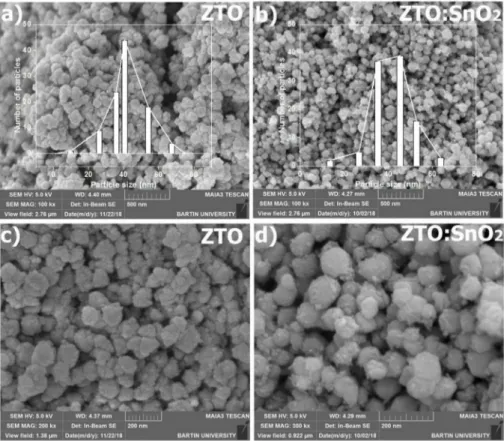
Fig. 4(a) and (b) show the low magnification SEM images ZTO nanoparticles and ZTO:SnO2 nanocomposites. The images indicated the
high-yield growth of both samples. Particle size distributions of the nanostructures are also given as insets in the figures. Average particle size of the nanostructures have been measured using Image J software by selecting 100 particles and found to be 40.6 � 3.7 nm and 45.4 � 4.2
Fig. 4. (a–b) The low and (c–d) high magnification SEM image of ZTO:SnO2 nanocomposites and ZTO nanoparticles. The particle size distributions of the nano-structures were given as inset in (a) and (b).
nm for ZTO nanoparticles and ZTO:SnO2 nanocomposites, respectively.
The average particle size (40.6 � 3.7 nm) determined from SEM and the crystallite size value calculated from the Debye-Scherrer formula (37.7 nm) were consistent for ZTO nanoparticles. However, they (45.4 � 4.2 nm(SEM) and 25.1 nm (XRD)) differed for ZTO:SnO2 nanocomposites.
This difference was probably originated from higher surface roughness of nanocomposites due to SnO2 nanorods covered the surface of ZTO
nanoparticles. The high magnification SEM micrographs of samples are shown in Fig. 4(c) and (d), respectively. While ZTO nanoparticles exhibited a conjoined and clustered morphology, ZTO:SnO2
nano-composites displayed the separation of the conjoined particles. This result could be attributed to the enhancement of the atomic mobility and better grain growth with the increasing temperature [53].
The morphologies and microstructural features of both nano-structures were also examined by using TEM. Fig. 5(a) and (b) indicated low magnification TEM micrograph of the ZTO nanoparticles and nanocomposites, respectively. TEM images revealed that ZTO nano-particles had mostly hexagonal morphology. The high resolution TEM (HRTEM) images of the ZTO nanoparticles in Fig. 5(c) displayed that the surface of ZTO nanoparticles was smooth and flat. However, HRTEM micrograph of ZTO:SnO2 nanocomposites indicated that the presence of
SnO2 nanorods wrapped on the surface of ZTO nanoparticles (Fig. 5(d)).
The dimension of SnO2 nanorods could be estimated as 17.5 � 3.2 nm in
length and 2.5 � 0.6 nm in the width.
The formation of composite structure between ZTO and SnO2 phases
were further confirmed by the clear lattice fringes from HR-TEM image shown in Fig. 5(e). Lattice fringes of ZTO nanoparticles and SnO2
nanorods are observable in the HR-TEM images of individual structures and show single crystal nature. The observed distance between lattice fringes could be assigned to the (311) reflection of ZTO (JCPDS card no:24–1470) and to the (100) reflection of SnO2 (JCPDS cards
no:41–1445).
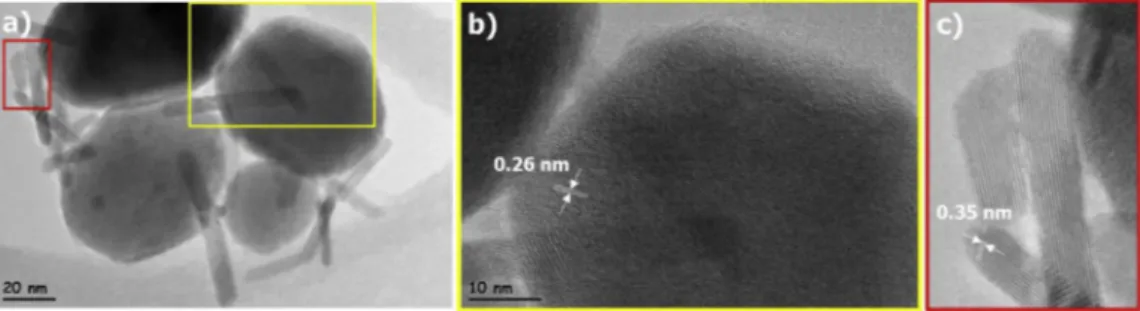
Up to now, the formation of ZTO:SnO2 nanocomposite was proved by
the XRD data exhibiting a peak related with SnO2 phase and XPS results
showing a change of the position and intensity of signals. Unlike XPS data, XRD results couldn’t be totally trusted for the minor amount of existing phase. Therefore, to confirm the coexistence of SnO2 nanorods,
TEM micrographs of ZTO:SnO2 nanocomposites should be investigated
in detail. Fig. 6(a) displayed a representative image of ZTO:SnO2
nanocomposites closer view of selected regions marked with yellow and red rectangles are shown in Fig. 6(b) and (c), respectively. HRTEM image (Fig. 6(b)) indicated both hexagonal nanoparticle and nanorods in the field of view. The distance between lattice fringes of nanoparticles was 0.26 nm corresponding to the (311) plane of ZTO. Fig. 6(c) exhibited atomic resolved HRTEM micrograph of selected nanorods. 0.35 nm distance between lattice fringes of single crystalline nanorods corresponded to (110) d values of SnO2. Therefore, Fig. 6(c) confirmed
that nanorods appeared in the sample synthesized at 180 �C
hydro-thermal temperature were SnO2 and further proved the XRD and XPS
results.
3.3. Optical analysis
Fig. 7(a) shows the room temperature PL spectrum of both samples. The PL measurements were performed with a excitation wavelength of 325 nm. In the spectrum of ZTO nanoparticles, a broad and strong asymmetric emission band was detected. This asymmetric band contains two different emission bands; green emission band positioned at 676 nm and red emission band located at 558 nm. The sharp green band was dominant and has already been reported for various semiconductor materials including ZnO, TiO2 and ZTO nanostructures having different
particle size and morphology [54–56]. Obviously, the green emission was not originated from band-to-band transition due to 3.6 eV band gap of ZTO [57], but it could be assigned to oxygen vacancy. The red emission observed in the spectrum could be indexed to cation vacancy (Zn or Sn cations) [39]. In the spectrum of ZTO:SnO2 nanocomposites,
the intensity of the both green and red emission peaks increased, how-ever, the intensity of the red emission increases nearly two times than that of the ZTO nanoparticles. This increment indicated the formation of the higher amount of cation defect states in the ZTO:SnO2
nano-composites. These results had good agreement with the XPS data.
Fig. 7(b) indicates PL spectrums of ZTO:SnO2 nanocomposites
measured under different excitation wavelengths such as 310, 325 and 333 nm. The intensity of the both green and red emissions significantly decreases with increasing excitation wavelength. This could be explained with defect position. The defect caused to both emissions were probably located at and just below the surface of the ZTO nanoparticles. These surface defects have been reported for non-radiative recombina-tion of photogenerated charge carriers [58]. Furthermore, increasing the wavelength of light also caused a shift of the peak position to the higher wavelengths. The determination of the recombination behaviors of these charge carriers were beyond the scope of this study.
The absorbance spectra of ZTO:SnO2 and ZTO nanoparticles are
shown in Fig. 6(c). As seen from Fig. 7(c), for the composite nano-particles, the intensity of the absorption spectra is higher than that of the ZTO nanoparticles which specifies the better photocatalytic activity and crystalline quality [59]. Although, the absorption edge wavelengths are determined as 282 nm for both nanostructures, there is also a slope change at 293 nm for ZTO:SnO2 nanocomposites. The rise of light
ab-sorption towards to UV region can be assigned to intrinsic band gap absorption [60,61]. In order to determine the optical band gap energy (Eg) of the nanoparticles from the absorption spectra, Tauc’s equation is
used. According to this equation, optical absorption intensity depends on the difference between hν the photon energy and Eg as fallowing
equation; ðαhνÞ1=n¼A hν Eg
�
(6) where α is the absorption coefficient, A is a constant related with the
material and n is a number that characterizes the transition nature of ZTO. Semiconductors such as ZTO are known as direct band gap semi-conductors, hereby n should be taken 1/2. The optical band gap energy was determined by plotting ðαhνÞ2 vs ðhνÞcurve given as inset in Fig. 7 (c). While the band gap energy value of ZTO nanoparticles is determined
as 3.69 eV from the fit of the blue curve, two different band gap energy values of ZTO:SnO2 nanocomposites are determined at 3.45 eV and 3.69
eV from the fit of the red curve. 3.69 eV is the characteristic band gap energy of ZTO which is consistent with the literature [62]. According to literature, the band gap energy of SnO2 is around 3.45 eV [63].
3.4. Photocatalytic properties
In order to investigate the photodegradation of RhB dye in the presence of ZTO nanoparticles and ZTO:SnO2 nanocomposites under the
illumination of UV light, the maximum absorption wavelength (λmax) of
RhB appeared at 554 nm was used.
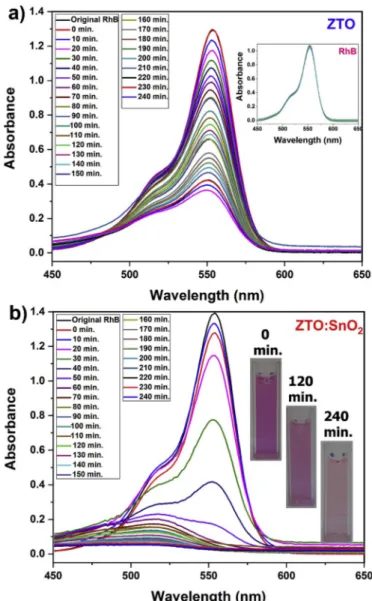
To understand the catalyst effect of ZTO on the degradation behavior of dye, RhB solution in the absence of catalyst was also examined under the UV light. The inset in Fig. 8(a) shows an insensible change in the maximum absorption band of RhB after 240 min UV irradiation. Fig. 7
(a) displays the absorption peaks of RhB solution containing ZTO nanoparticles as catalyst. The dye solutions, analyzed at the beginning and after stirring 1 h in the dark give similar maximum absorption band intensity, which suggests that the amount of adsorption on ZTO nano-particles is neglectable. Therefore, electron transfer ratio from the excited RhB molecules to ZTO nanoparticles is also negligible. After the UV light exposure, declination tendency in the intensity of absorption peaks were observed as a function of time. After 240 min of UV irradi-ation, the remaining dye was 30.5% of its initial amount.
Fig. 8(b) shows the degradation behavior of RhB in the presence of ZTO:SnO2 nanocomposites as catalyst. The degradation of the RhB in the
presence of nanocomposites displayed faster decrease of λmax in first 60
min. UV irradiation which reveals better photocatalytic efficiency of ZTO:SnO2 nanocomposites than pure ZTO nanoparticles. The remaining
dye was determined as 85.7% of its initial amount in the solution after 60 min and 97.4% after 240 min. UV irradiation. Another distinct dif-ference between degradation behavior of RhB in the presence of nano-composites and nanoparticles is the large blue shift in the λmax of RhB
absorption spectrum. Similar observations were also reported by T. Watanabe and coworkers [64] and H.M. Sung-Suh et al. [65]. They found that the blue shift in the λmax of RhB resulted in deethylation of
RhB simultaneously with the degradation of chromophore ring under the UV irradiation in the presence of aqueous CdS or TiO2 particles.
During the deethylation of RhB, N,N,N0,N’-tetraethylated RhB, N,N,
N0-Trietylated RhB, N,N0-diethylated RhB and N-ethylated RhB
mole-cules exhibit λmax at 552, 539, 522 and 510 nm, respectively [64].
Furthermore, although RhB degradation is based on the solution bulk process, RhB deethylation is mainly a reaction taking place on the sur-face of RhB. Therefore, faster decrease in the intensity of λmax and large
blue shift in the position of λmax caused from degradation of
chromo-phore ring under the UV irradiation simultaneously with deethylation of RhB on the surface of ZTO:SnO2 nanocomposites. Real time aspect of the
dye solution photographed at the beginning and after stirring 120 and 240 min under the illumination of UV light are also given as insets in
Fig. 8(b). The color of the RhB dye solution changes from the hot pink (0 min) to very faint pink (240 min). Therefore, RhB dye molecules almost
decolorized completely after UV light irradiation.
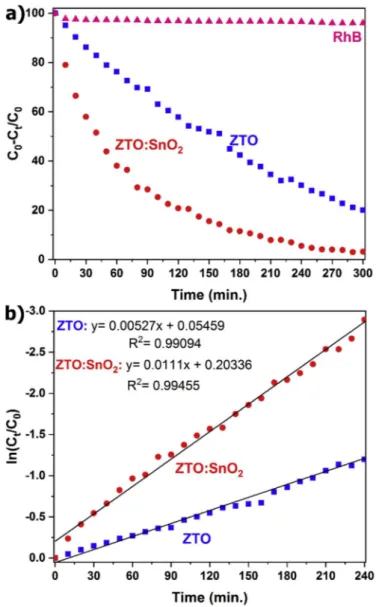
Fig. 9(a) displays the temporal profile of the photocatalytic degra-dation of RhB solution without catalyst addition and RhB solutions with the addition of ZTO nanoparticles and ZTO:SnO2 nanocomposites,
respectively. As expected from the inset figure in Fig. 8(a), RhB behaves decisively under the UV-light and does not degrade even after 240 min without photocatalyst. Photocatalytic degradation of RhB dye molecule under the UV-light showed that ZTO:SnO2 nanocomposites displayed
higher photocatalytic performance compared with ZTO nanoparticles, reaching a degradation value of 94.5% and 69.8% after 240 min light irradiation, respectively. The kinetics of photocatalytic degradation of RhB reveal that the decomposition reactions followed a Langmuir- Hinshelwood apparent first-order kinetics model [66]. Apparent first-order model equation (ln (Ct/C0) ¼ kappt) can be applied to
calcu-late the apparent first-order rate constant (kapp) [67,68]. In here, C0 and
Ct represent the initial concentration and the concentration at any time t
determined by λmax. ln (Ct/C0) for RhB solutions without/with ZTO
nanoparticles and ZTO:SnO2 nanocomposites were plotted as a function
of the irradiation time (Fig. 9(b)). It is clear that both nanostructures revealed a nearly linear behavior. The kapp values for degradation were
determined from the slope of these curves. In contrast to RhB blank
Fig. 7. (a) PL spectra for ZTO:SnO2 nanocomposites and ZTO nanoparticles under 325 nm excitation wavelengths, (b) PL spectra for ZTO:SnO2 nano-composites under different excitation wavelengths and (c) The UV–Vis spectra of both nanostructures. The insets show the plots of (αhυ)2 as a function of
photon energy (eV) for both samples.
Fig. 8. UV–Vis absorption spectra of (a) ZTO nanoparticles and (b) ZTO:SnO2 nanocomposites containing dye solutions. The insets show (a) photo-degradation of RhB without catalyst addition and (b) appearance of dye solu-tions in the presence of ZTO:SnO2 nanocomposites at different UV exposure time.
solution with kapp constant 0.0001342 min 1, kapp constant for RhB
degradation were found as 0.00527 min 1 for ZTO nanoparticles and 0.0111 min 1 for ZTO:SnO2 nanocomposites, respectively. Therefore,
the kinetic of the RhB degradation in the presence of nanocomposites is nearly 2 times faster than that of the ZTO nanoparticles.
Based on the above experimental results, the enhancement of the photocatalytic activity in the presence of ZTO:SnO2 nanocomposites can
depend on the two main factors; (i) the formation of a ternary:binary heterojunction and (ii) producing more number of the cation and oxygen vacancies leading to more efficient photogenerated charge separation. As a first factor; functionalization of ZTO nanoparticles with SnO2
nanorods clearly displayed improved photocatalytic activity. This enhancement could be attributed to the effective charge distribution similar to the other ternary:binary heterostructures [69,70]. To obtain insights about the electronic properties of composite, energy band alignment at the heterojunction interface was examined using core levels of XPS analyses. For this purpose, the valence band (VB) maximum of composite, ΔEV, was determined by using the core level
peak positions of Zn(2p) and Sn(3d) as well as the VB maximum of the bulk ZTO and SnO2 according to fallowing equation;
ΔEV ¼ EZn; 2p EV; ZTO
�ZTO
Bulk ðESn; 3d EV; SnO2Þ
SnO2
Bulk ΔEC;L (7)
where EZn; 2p and ESn; 3d are the binding energies of Zn(2p) and Sn(3d)
peaks determined from Fig. 2(b) and (d), respectively. EV; ZTO and
EV; SnO2 are the VB maximum in the bulk ZTO and SnO2, and taken as
0.23 and 0.26, respectively [16]. In the equation, ΔEC,L represents the
energy difference between Zn(2p) (1020.35 eV) and Sn(3d) (485.19 eV) core levels measured from the heterostructured ZTO:SnO2 and
calcu-lated as 535.19 eV. Finally, the conduction band (CB) maximum, ΔEC,
was determined by the formula;
ΔEC¼ΔEV þEZTOg ESnOg 2 (8)
in here, EZTO
g and ESnOg 2 are the band gap of ZTO (3.69 eV) and SnO2
(3.45 eV), respectively (Fig. 6(c)). From these calculations, ΔEV and ΔEC
were estimated as 0.03and 0.27 eV, respectively. Fig. 10 shows a sche-matic representation for the resulting band energy alignment at the ternary:binary interface providing better photocatalytic activity of the ZTO:SnO2 nanocomposites.
When the catalyst semiconductor material is irradiated with light energy (hѵ) having equal or greater energy than the band gap energy of catalyst, electrons in the CB are excited and leave behind holes in the VB [51]. These photogenerated electrons in CB can react with absorbed O2
on the surface of ZTO or dissolved in water to generate superoxide anion radicals (.O
2). In the meantime, the photogenerated holes can interact with OH or
H2O and convert them hydroxyl radicals (OH.). These very strong
oxidizing OH. radicals are responsible for the decomposition of the
organic dye molecules to harmless CO2 and H2O.
Under the UV irradiation, e transfer could occur from ZTO to SnO2
and hþtransfer could occur from SnO
2 to ZTO until the system reaches
equilibrium. The preferentially transfer of electrons through SnO2
nanorods and holes through ZTO nanoparticles result in more effective charge carrier separation, thus, reduced charge carrier recombination increases the carrier lifetime. Similar mechanism was also reported by Uttin et al. for the nanostructured ZnO:SnO2 heterojunction
photo-catalysts with improved photocatalytic activity for the MB degradation [71]. In addition, the photo-excited e moved to the CB of SnO2 can now
react more efficiently with the O2 and produce O2. radicals, in the
meantime, the holes on the VB of ZTO can oxidize H2O or OH into .OH
radicals. Therefore, formation of the ternary:binary composite results in enhanced photocatalytic activities according to pure ZTO nanoparticles. As a second factor; formation of oxygen vacancies also contributes to the enhancement of the photocatalytic efficiency of ZTO nanoparticles. Lee et al. reported an Ab initio simulation study for the effect of oxygen vacancy on the electronic characteristics of ZTO [72]. They simulated two different vacancies called as O-A and O–B. In these vacancies, Zn is always located on the tetrahedral site of ZTO, however, 1Sn– 2Zn and 2Sn - 1Zn are located on the octahedral site for O A and O–B vacancies,
Fig. 9. (a) Temporal course and (b) fitted data of the pseudo first-order kinetics of RhB dye, ZTO:SnO2 nanocomposites and ZTO nanoparticles.
Fig. 10. Photocatalytic pathway for RhB degradation under the UV light in the presence of ZTO:SnO2 nanocomposite photocatalyst.
respectively. They found that formation energy of the O-A vacancy is lower than that of the O–B vacancy. Therefore, the possibility of the formation of O-A type vacancies in the ZTO:SnO2 nanocomposites
should be higher than that of the ZTO nanoparticles as confirmed by XPS and PL results. These vacancies lead to the formation of some new defect levels in ZTO structure. Defect states play an important role in electron traps providing more efficient e -hþ separation and transferring of
photogenerated electrons in the VB of ZTO through the CB or impurity energy levels. Electron transfer to the adsorbed O2 acting as electron
acceptor yields numerous superoxide radicals (.O2
) under the UV light irra-diation. Meanwhile, hydroxyl radical species (.OH) with high oxidability form
because of transferring of the photo-excited hþin the VB of ZTO through
the surface of the ZTO:SnO2 nanocomposites. These transitions also
significantly decrease the recombination rate of e -hþpairs. Therefore,
combining effect of the formation of ternary:binary heterostructures composite and more amount of oxygen vacancies delay the recombi-nation process more effectively and provide better photocatalytic effi-ciency for ZTO:SnO2 nanocomposites than that of the ZTO
nanoparticles.
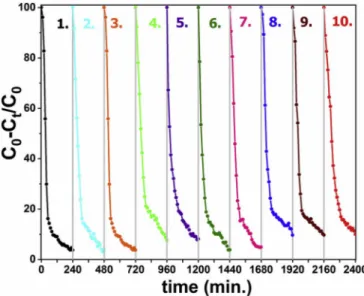
For a possible industrial applications, reusing of the ZTO:SnO2
nanocomposite catalyst was also investigated under the same experi-mental conditions. For this purpose, after each run, centrifuged catalyst particles were added a fresh concentrated RhB solution (40 mg catalyst into 100 mL RhB solution). Fig. 11 shows ten times recycling experiment results. It is clearly observed that the degradation of RhB for all cycling is nearly unchanged which proves the perfect photostability of nano-composites. After 10th cycling run, the degradation of RhB dye is about %90.1 which is higher than those reported. For example Junploy et al. reported 70% methylene blue degradation efficiency of ZTO:SnO2
nanocomposite after 5 time cycling experiments [32]. In summary, the proposed ZTO:SnO2 nanocomposite system is not limited for SnO2 and
can be applied other binary oxide semiconductor materials Therefore, it can provide useful insights for large-scale production of photocatalyst systems with efficiently reusable stable structure.
4. Conclusions
In summary, ZTO:SnO2 nanocomposites and phase pure ZTO
nano-particles were synthesized via hydrothermal method. It was found that the hydrothermal synthesis temperature plays the controlling role for the formation of nanocomposites; relatively higher temperature pro-motes the synthesis of highly crystalline ZTO:SnO2 nanocomposites. The
crystal structure, morphology, chemical and optical properties of both nanostructures were examined by various analysis methods and dis-cussed in detail. The photocatalytic activities of ZTO:SnO2
nano-composites and ZTO nanoparticles were evaluated by degrading RhB dye under UV light irradiation. According to results, the degradation efficiency of nanocomposites (kapp ¼ 0.0111min 1) was improved nearly two times in comparison with pure ZTO nanoparticles (kapp ¼ 0.00527min 1). The improved photocatalytic efficiency could be resulted in the better charge separation and inhibition of the e -hþ
recombination as a result of the formation of the ternary:binary heter-ojunction and producing more number of defect sites. The photostability investigation of the ZTO:SnO2 nanocomposites with repeated cycling
experiments were also performed and exhibited remarkable stability and reusability even after ten cycles. Finally, we expect that this detailed research of ZTO:SnO2 nanocomposites prepared via hydrothermal
method could be useful for designing new class ternary:binary semi-conductor photocatalyst with efficient photocatalytic activities.
Declaration of competing interest
The authors declare no conflict of interest.
CRediT authorship contribution statement
Elif Keles: Conceptualization, Formal analysis, Data curation. Mehmet Yildirim: Writing - original draft, Writing - review & editing. Teoman €Oztürk: Writing - original draft, Writing - review & editing. Ozlem Altintas Yildirim: Writing - original draft, Conceptualization,
Data curation, Formal analysis, Writing - review & editing.
Acknowledgments
The authors would like to thank the European Cooperation in Science and Technology through COST Action CA15114 Anti-Microbial Coating Innovations to prevent infectious diseases and the Scientific and Tech-nological Research Council of Turkey (TÜBITAK Grant number 216M011). The authors thank to Savas¸ S€onmezo�glu and Ayse Culu for their assistance in FTIR, PL and UV–Vis. analyses. Scientific Research Foundation of Selcuk University under Project No:17401142 for the financial support of this research.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi. org/10.1016/j.mssp.2020.104959.
References
[1] M.N. Chong, B. Jin, C.W. Chow, C. Saint, Recent developments in photocatalytic water treatment technology: a review, Water Res. 44 (10) (2010) 2997–3027. [2] E. Mena, A. Rey, F. Beltr�an, TiO2 photocatalytic oxidation of a mixture of emerging
contaminants: a kinetic study independent of radiation absorption based on the direct-indirect model, Chem. Eng. J. 339 (2018) 369–380.
[3] O.A. Yıldırım, H.E. Unalan, C. Durucan, Highly efficient room temperature € synthesis of silver-doped zinc oxide (ZnO: Ag) nanoparticles: structural, optical, and photocatalytic properties, J. Am. Ceram. Soc. 96 (3) (2013) 766–773. [4] S. Dursun, I.C. Kaya, V. Kalem, H. Akyildiz, UV/visible light active CuCrO2
nanoparticle–SnO2 nanofiber p–n heterostructured photocatalysts for photocatalytic applications, Dalton Trans. 47 (41) (2018) 14662–14678. [5] M. Mishra, D.-M. Chun, α-Fe2O3 as a photocatalytic material: a review, Appl.
Catal. Gen. 498 (2015) 126–141.
[6] N. Bao, L. Shen, T. Takata, K. Domen, Self-templated synthesis of nanoporous CdS nanostructures for highly efficient photocatalytic hydrogen production under visible light, Chem. Mater. 20 (1) (2007) 110–117.
[7] E. Baylan, O. Altintas Yildirim, Highly efficient photocatalytic activity of stable manganese-doped zinc oxide (Mn:ZnO) nanofibers via electrospinning method, Mater. Sci. Semicond. Process. 103 (2019) 104621.
[8] S. Safa, R. Azimirad, S. Safalou Moghaddam, M. Rabbani, Investigating on photocatalytic performance of CuO micro and nanostructures prepared by different precursors, Desalination and Water Treatment 57 (15) (2016) 6723–6731. Fig. 11. Ten times recycling experiments of ZTO:SnO2 nanocomposite photo-catalyst for RhB degradation under UV light irradiation.
[9] O.A. Yildirim, Synthesis of Ag-doped ZnO nano fibers using electrospinning method and their photocatalytic activities, Selcuk Univ. J. Eng. Sci. Technol. 6 (4) (2018) 633–642.
[10] K. Mohammadi, S. Safa, S. Safalou Moghaddam, M. Rabbani, R. Azimirad, Photocatalytic activity of one-pot synthesized reduced graphene oxide – zinc oxide nanocomposites, J Nano Res-Sw 37 (2016) 74–84.
[11] B. Jia, W. Jia, X. Wu, F. Qu, Hierarchical porous SnO2 microflowers photocatalyst, Sci. Adv. Mater. 4 (11) (2012) 1127–1133.
[12] S. Dinesh, M. Anandan, V. Premkumar, S. Barathan, G. Sivakumar, N. Anandhan, Photocatalytic and electrochemical performance of hydrothermally synthesized cubic Cd2SnO4 nanoparticles, Mater. Sci. Eng., B 214 (2016) 37–45. [13] E.L. Foletto, J.M. Sim~oes, M.A. Mazutti, S.L. Jahn, E.I. Muller, L.S.F. Pereira, E.
M. de Moraes Flores, Application of Zn2SnO4 photocatalyst prepared by microwave-assisted hydrothermal route in the degradation of organic pollutant under sunlight, Ceram. Int. 39 (4) (2013) 4569–4574.
[14] F.-t. Li, Y. Zhao, Y. Liu, Y.-j. Hao, R.-h. Liu, D.-s. Zhao, Solution combustion synthesis and visible light-induced photocatalytic activity of mixed amorphous and crystalline MgAl2O4 nanopowders, Chem. Eng. J. 173 (3) (2011) 750–759. [15] S. Battiston, C. Rigo, E.d.C. Severo, M.A. Mazutti, R.C. Kuhn, A. Gündel, E. L. Foletto, Synthesis of zinc aluminate (ZnAl2O4) spinel and its application as photocatalyst, Mater. Res. 17 (3) (2014) 734–738.
[16] Y. Sato, J. Kiyohara, A. Hasegawa, T. Hattori, M. Ishida, N. Hamada, N. Oka, Y. Shigesato, Study on inverse spinel zinc stannate, Zn2SnO4, as transparent conductive films deposited by rf magnetron sputtering, Thin Solid Films 518 (4) (2009) 1304–1308.
[17] D. Young, D. Williamson, T. Coutts, Structural characterization of zinc stannate thin films, J. Appl. Phys. 91 (3) (2002) 1464–1471.
[18] Y. Zhang, M. Guo, M. Zhang, C. Yang, T. Ma, X. Wang, Hydrothermal synthesis and characterization of single-crystalline zinc hydroxystannate microcubes, J. Cryst. Growth 308 (1) (2007) 99–104.
[19] S. Baruah, J. Dutta, Zinc stannate nanostructures: hydrothermal synthesis, Sci. Technol. Adv. Mater. 12 (1) (2011), 013004.
[20] T. Tharsika, A. Haseeb, S. Akbar, M.F.M. Sabri, Y.H. Wong, Gas sensing properties of zinc stannate (Zn2SnO4) nanowires prepared by carbon assisted thermal evaporation process, J. Alloys Compd. 618 (2015) 455–462.
[21] Q. Zhao, X. Deng, M. Ding, J. Huang, D. Ju, X. Xu, Synthesis of hollow cubic Zn2SnO4 sub-microstructures with enhanced photocatalytic performance, J. Alloys Compd. 671 (2016) 328–333.
[22] I. Tuncolu, C. Aciksari, E. Suvaci, E. Ozel, S. Rembeza, E. Rembeza, E. Y. Plotnikova, N. Kosheleva, T. Svistova, Synthesis of Zn2SnO4 powders via hydrothermal method for ceramic targets, J. Eur. Ceram. Soc. 35 (14) (2015) 3885–3892.
[23] X.-Y. Liu, H.-W. Zheng, Z.-L. Zhang, X.-S. Liu, R.-Q. Wan, W.-F. Zhang, Effect of energy level matching on the enhancement of photovoltaic response about oxide/ Zn2SnO4 composites, J. Mater. Chem. 21 (12) (2011) 4108–4116.
[24] N. Nikolic, Z. Marinkovic, T. Sreckovic, The influence of grinding conditions on the mechanochemical synthesis of zinc stannate, J. Mater. Sci. 39 (16–17) (2004) 5239–5242.
[25] S. Wang, Z. Yang, M. Lu, Y. Zhou, G. Zhou, Z. Qiu, S. Wang, H. Zhang, A. Zhang, Coprecipitation synthesis of hollow Zn2SnO4 spheres, Mater. Lett. 61 (14–15) (2007) 3005–3008.
[26] J. Fang, A. Huang, P. Zhu, N. Xu, J. Xie, J. Chi, S. Feng, R. Xu, M. Wu, Hydrothermal preparation and characterization of Zn2SnO4 particles, Mater. Res. Bull. 36 (7–8) (2001) 1391–1397.
[27] M.B. Ali, F. Barka-Bouaifel, H. Elhouichet, B. Sieber, A. Addad, L. Boussekey, M. F�erid, R. Boukherroub, Hydrothermal synthesis, phase structure, optical and photocatalytic properties of Zn2SnO4 nanoparticles, J. Colloid Interface Sci. 457 (2015) 360–369.
[28] J. Zeng, M. Xin, K. Li, H. Wang, H. Yan, W. Zhang, Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals, J. Phys. Chem. C 112 (11) (2008) 4159–4167.
[29] X. Fu, X. Wang, J. Long, Z. Ding, T. Yan, G. Zhang, Z. Zhang, H. Lin, X. Fu, Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4, J. Solid State Chem. 182 (3) (2009) 517–524.
[30] E. Baylan, A. Culu, M. Yildirim, T. €Oztürk, S. S€onmezoglu, O. Altintas Yildirim, Hidrotermal Y€ontemle Sentezlenen Çinko Stanat (Zn2SnO4) Nanoparçaciklarin Fotokatalitik Performanslarinin _Incelenmesi Konya, J. Eng. Sci. 7 (3) (2019) 645–653.
[31] B. Li, L. Luo, T. Xiao, X. Hu, L. Lu, J. Wang, Y. Tang, Zn2SnO4–SnO2
heterojunction nanocomposites for dye-sensitized solar cells, J. Alloys Compd. 509 (5) (2011) 2186–2191.
[32] P. Junploy, S. Thongtem, T. Thongtem, A. Phuruangrat, Photocatalytic activity of Zn2SnO4–SnO2 nanocomposites produced by sonochemistry in combination with high temperature calcination, Superlattice. Microst. 74 (2014) 173–183. [33] J.H. Yu, G.M. Choi, Current–voltage characteristics and selective CO detection of
Zn2SnO4 and ZnO/Zn2SnO4, SnO2/Zn2SnO4 layered-type sensors, Sensor. Actuator. B Chem. 72 (2) (2001) 141–148.
[34] Z. Lu, Y. Tang, Two-step synthesis and ethanol sensing properties of Zn2SnO4SnO2 nanocomposites, Mater. Chem. Phys. 92 (1) (2005) 5–9.
[35] M. Liu, J. Yang, S. Feng, H. Zhu, J. Zhang, G. Li, J. Peng, Composite photoanodes of Zn2SnO4 nanoparticles modified SnO2 hierarchical microspheres for dye- sensitized solar cells, Mater. Lett. 76 (2012) 215–218.
[36] H. Ullah, A. Khatoon, Z. Akhtar, Synthesis and photocatalytic study of SnO2/ Zn2SnO4nanocomposite prepared by sol–gel method using single source molecular precursor, Mater. Res. Express 1 (4) (2014), 045001.
[37] P. Junploy, A. Phuruangrat, N. Plubphon, S. Thongtem, T. Thongtem,
Photocatalytic degradation of methylene blue by Zn2SnO4-SnO2 system under UV visible radiation, Mater. Sci. Semicond. Process. 66 (2017) 56–61.
[38] J. Wang, H. Li, S. Meng, L. Zhang, X. Fu, S. Chen, One-pot hydrothermal synthesis of highly efficient SnOx/Zn2SnO4 composite photocatalyst for the degradation of methyl orange and gaseous benzene, Appl. Catal., B 200 (2017) 19–30. [39] A. Annamalai, D. Carvalho, K. Wilson, M.-J. Lee, Properties of hydrothermally
synthesized Zn2SnO4 nanoparticles using Na2CO3 as a novel mineralizer, Mater. Char. 61 (9) (2010) 873–881.
[40] S. Dinesh, S. Barathan, V. Premkumar, G. Sivakumar, N. Anandan, Hydrothermal synthesis of zinc stannate (Zn 2 SnO 4) nanoparticles and its application towards photocatalytic and antibacterial activity, J. Mater. Sci. Mater. Electron. 27 (9) (2016) 9668–9675.
[41] A. Annamalai, Y.D. Eo, C. Im, M.-J. Lee, Surface properties and dye loading behavior of Zn2SnO4 nanoparticles hydrothermally synthesized using different mineralizers, Mater. Char. 62 (10) (2011) 1007–1015.
[42] Q. Zhao, D. Ju, X. Song, X. Deng, M. Ding, X. Xu, H. Zeng, Polyhedral Zn2SnO4: synthesis, enhanced gas sensing and photocatalytic performance, Sensor. Actuator. B Chem. 229 (2016) 627–634.
[43] X. Ji, X. Huang, J. Liu, J. Jiang, X. Li, R. Ding, Y. Hu, F. Wu, Q. Li, Hydrothermal synthesis of novel Zn2SnO4 octahedron microstructures assembled with hexagon nanoplates, J. Alloys Compd. 503 (2) (2010) L21–L25.
[44] J.E. Jeronsia, L.A. Joseph, M.M. Jaculine, P.A. Vinosha, S.J. Das, Hydrothermal synthesis of zinc stannate nanoparticles for antibacterial applications, J. Taibah Univ. Sci. 10 (4) (2016) 601–606.
[45] X. Zhu, L. Geng, F. Zhang, Y. Liu, L. Cheng, Synthesis and performance of Zn2SnO4 as anode materials for lithium ion batteries by hydrothermal method, J. Power Sources 189 (1) (2009) 828–831.
[46] J. Zeng, M. Xin, K. Li, H. Wang, H. Yan, W. Zhang, Transformation process and photocatalytic activities of hydrothermally synthesized Zn2SnO4 nanocrystals, J. Phys. Chem. C 112 (11) (2008) 4159–4167.
[47] W. Wang, H. Chai, X. Wang, X. Hu, X. Li, Ethanol gas sensing performance of Zn2SnO4 nanopowder prepared via a hydrothermal route with different solution pH values, Appl. Surf. Sci. 341 (2015) 43–47.
[48] C.G. Anchieta, D. Sallet, E.L. Foletto, S.S. da Silva, O. Chiavone-Filho, C.A.O. do Nascimento, Synthesis of ternary zinc spinel oxides and their application in the photodegradation of organic pollutant, Ceram. Int. 40 (3) (2014) 4173–4178. [49] O. Altıntas¸ Yıldırım, C. Durucan, Room temperature synthesis of Cu incorporated €
ZnO nanoparticles with room temperature ferromagnetic activity: structural, optical and magnetic characterization, Ceram. Int. 42 (2, Part B) (2016) 3229–3238.
[50] H. Yang, S. Ma, G. Yang, Q. Chen, Q. Zeng, Q. Ge, L. Ma, Y. Tie, Synthesis of La2O3 doped Zn2SnO4 hollow fibers by electrospinning method and application in detecting of acetone, Appl. Surf. Sci. 425 (2017) 585–593.
[51] P.P. Das, A. Roy, M. Tathavadekar, P.S. Devi, Photovoltaic and photocatalytic performance of electrospun Zn2SnO4 hollow fibers, Appl. Catal., B 203 (2017) 692–703.
[52] J. Yin, S. Huang, Z. Jian, Z. Wang, Y. Zhang, Fabrication of heterojunction SnO2/ BiVO4 composites having enhanced visible light photocatalystic activity, Mater. Sci. Semicond. Process. 34 (2015) 198–204.
[53] M. Mary Jaculine, C. Justin Raj, S. Jerome Das, Hydrothermal synthesis of highly crystalline Zn2SnO4 nanoflowers and their optical properties, J. Alloys Compd. 577 (2013) 131–137.
[54] J. Wang, S. Xie, Y. Gao, X. Yan, D. Liu, H. Yuan, Z. Zhou, L. Song, L. Liu, W. Zhou, Growth and characterization of axially periodic Zn2SnO4 (ZTO) nanostructures, J. Cryst. Growth 267 (1–2) (2004) 177–183.
[55] O. Altintas Yildirim, Efficient vapor-liquid-solid synthesis of copper doped zinc oxide (Cu:ZnO) nanonails with highly homogeneous dopant distribution, Mater. Sci. Semicond. Process. 101 (2019) 238–246.
[56] L. Shi, H. Shen, L. Jiang, X. Li, Co-emission of UV, violet and green
photoluminescence of ZnO/TiO2 thin film, Mater. Lett. 61 (25) (2007) 4735–4737. [57] M.A. Alpuche-Aviles, Y. Wu, Photoelectrochemical study of the band structure of Zn2SnO4 prepared by the hydrothermal method, J. Am. Chem. Soc. 131 (9) (2009) 3216–3224.
[58] J.I. Pankove, Optical Processes in Semiconductors, Courier Corporation, 1975. [59] M.J. Height, S.E. Pratsinis, O. Mekasuwandumrong, P. Praserthdam, Ag-ZnO
catalysts for UV-photodegradation of methylene blue, Appl. Catal., B 63 (3–4) (2006) 305–312.
[60] S. Dinesh, S. Barathan, V.K. Premkumar, G. Sivakumar, N. Anandan, Hydrothermal synthesis of zinc stannate (Zn2SnO4) nanoparticles and its application towards photocatalytic and antibacterial activity, J. Mater. Sci. Mater. Electron. 27 (9) (2016) 9668–9675.
[61] L. Shi, Y. Dai, Synthesis and photocatalytic activity of Zn2SnO4 nanotube arrays, J. Mater. Chem. A 1 (41) (2013) 12981–12986.
[62] M. Ben Ali, F. Barka-Bouaifel, H. Elhouichet, B. Sieber, A. Addad, L. Boussekey, M. F�erid, R. Boukherroub, Hydrothermal synthesis, phase structure, optical and photocatalytic properties of Zn2SnO4 nanoparticles, J. Colloid Interface Sci. 457 (2015) 360–369.
[63] M.M. Bagheri-Mohagheghi, N. Shahtahmasebi, M.R. Alinejad, A. Youssefi, M. Shokooh-Saremi, The effect of the post-annealing temperature on the nano- structure and energy band gap of SnO2 semiconducting oxide nano-particles synthesized by polymerizing–complexing sol–gel method, Phys. B Condens. Matter 403 (13) (2008) 2431–2437.
[64] T. Watanabe, T. Takizawa, K. Honda, Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide, J. Phys. Chem. 81 (19) (1977) 1845–1851.
[65] H.M. Sung-Suh, J.R. Choi, H.J. Hah, S.M. Koo, Y.C. Bae, Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO2 under visible and UV light irradiation, J. Photochem. Photobiol. Chem. 163 (1) (2004) 37–44.
[66] A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard, J.-M. Herrmann, Photocatalytic degradation pathway of methylene blue in water, Appl. Catal., B 31 (2) (2001) 145–157.
[67] Z. Zhang, C. Shao, X. Li, L. Zhang, H. Xue, C. Wang, Y. Liu, Electrospun nanofibers of ZnO SnO2 heterojunction with high photocatalytic activity, J. Phys. Chem. C 114 (17) (2010) 7920–7925.
[68] P. Zhang, L. Wang, X. Zhang, J. Hu, G. Shao, Three-dimensional porous networks of ultra-long electrospun SnO 2 nanotubes with high photocatalytic performance, Nano-Micro Lett. 7 (1) (2015) 86–95.
[69] S. Joshi, R.K. Canjeevaram Balasubramanyam, S.J. Ippolito, Y.M. Sabri, A. E. Kandjani, S.K. Bhargava, M.V. Sunkara, Straddled band Aligned CuO/BaTiO3
heterostructures: role of energetics at nanointerface in improving photocatalytic and CO2 sensing performance, ACS Appl. Nano Mater. 1 (7) (2018) 3375–3388. [70] Y. Li, X. Wu, W. Ho, K. Lv, Q. Li, M. Li, S.C. Lee, Graphene-induced formation of
visible-light-responsive SnO2-Zn2SnO4 Z-scheme photocatalyst with surface vacancy for the enhanced photoreactivity towards NO and acetone oxidation, Chem. Eng. J. 336 (2018) 200–210.
[71] M.T. Uddin, Y. Nicolas, C. Olivier, T. Toupance, L. Servant, M.M. Müller, H.- J. Kleebe, J. Ziegler, W. Jaegermann, Nanostructured SnO2–ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes, lnorg. Chem. 51 (14) (2012) 7764–7773.
[72] J. Lee, Y. Kang, C.S. Hwang, S. Han, S.-C. Lee, J.-H. Choi, Effect of oxygen vacancy on the structural and electronic characteristics of crystalline Zn 2 SnO 4, J. Mater. Chem. C 2 (39) (2014) 8381–8387.