J Food Process Preserv. 2019;43:e13981. wileyonlinelibrary.com/journal/jfpp
|
1 of 8 https://doi.org/10.1111/jfpp.13981© 2019 Wiley Periodicals, Inc.
1 | INTRODUCTION
Grape (Vitis vinifera L.) ranks among most important commercial fruit crops being grown and utilized around the globe since long (Al Juhaimi & Özcan, in press; Bail et al., 2008; Hussein & Abdrabba,
2015). The oil from grape seed is now becoming popular as a culi‐ nary oil due to the presence of specialty lipids and it may also serve as a good source of other nutritionally beneficial phytochemicals in‐ cluding phenol compounds, tocopherols, and fatty acids (Bail et al., 2008; Bjelica et al., 2017; Lutterodt, Slavin, Whent, Turner, & Yu,
Received: 25 January 2019
|
Revised: 6 April 2019|
Accepted: 16 April 2019 DOI: 10.1111/jfpp.13981O R I G I N A L A R T I C L E
Effect of varieties on bioactive compounds, fatty acids,
and mineral contents in different grape seed and oils from
Bosnia and Herzegovina
Tijana Banjanin
1| Mehmet Musa Özcan
2| Fahad Al Juhaimi
3| Zorica Ranković‐Vasić
4|
Nurhan Uslu
2| Isam A. Mohamed
3| Kashif Ghafoor
3| Elfadıl E. Babiker
3|
Magdi A. Osman
3| Mustafa A. Gassem
3| Hesham A. A. Salih
31Faculty of Agriculture, Department of Horticulture, University of East Sarajevo, East Sarajevo, Bosnia and Herzegovina 2Faculty of Agriculture, Department of Food Engineering, Selcuk University, Konya, Turkey
3Department of Food Science and Nutrition, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
4Faculty of Agriculture, Department of Horticulture, University of Belgrade, Belgrade, Serbia
Correspondence
Mehmet Musa Özcan, Faculty of Agriculture, Department of Food Engineering, Selcuk University, Konya 42031, Turkey. Email: mozcan@selcuk.edu.tr Funding information
King Saud University, Grant/Award Number: RG‐1439‐80
Abstract
This work aimed to evaluate the phytochemical properties of grape seeds and oils of autochthonous variety Blatina, regional variety Vranac and international varieties Merlot, Cabernet, and Muscat cultivated in Herzegovina province. It is estimated that total phenol contents and antioxidant activities of seed extracts ranged between 502.08 (Merlot)–693.33 mgGAE/kg (Blatina) and 86.68 (Muscat)–90.76% (Cabernet), respectively. The values for 1,2‐dihydroxybenzene and (+)‐catechin in seed extracts changed between 42.32 (Blatina)–450.16 mg/100 g (Muscat) and 56.52 (Cabernet)–9 60.00 mg/100 g(Vranac), respectively. Linoleic and oleic acid contents of grape seed oils were between 61.30 (Cabernet)–67.84% (Merlot) and 19.87 (Merlot)–24.53% (Blatina), respectively. γ‐Tocopherol contents of seed oils were in the range of 1.84 (Cabernet and Blatina)–2.04 mg/g(Merlot). The P and K mineral contents of seeds varied from 3,731.0 (Blatina) to 4,309.3 mg/kg (Muscat) and 10,033 (Cabernet) and 16,674 mg/kg (Blatina), respectively.
Practical
applications
In this paper, analysis of the grape seeds and oils are presented. Following grape varieties are grown in Herzegovina are analyzed: autochthonous variety Blatina, re‐ gional variety Vranac and international varieties Merlot, Cabernet, and Muscat. They are analyzed in terms of the total content of phenol, individual phenols, antioxidant activity, and minerals of grape seed extract. Grape seed oil was also examined for the content of tocopherols and fatty acids. The main interest in oil and seed are the high content of phenol, linoleic acid, and tocopherol used in the pharmaceutical. Based on the presented results it will be possible to compare characteristics of the grape seeds and oils of autochthonous, regional and international varieties.
2 of 8
|
BANJANIN etAl.2011; Geçgel et al., 2017; Ovcharova et al., 2015; Özcan, Al Juhaimi, Gülcü, Uslu, & Geçgel, 2017; Radovanovic, Dekic, & Radovanovic, 2011; Vujovic, Pejin, Popovic Djordjevic, Velickovic, & Tesevic, 2016). According to Wijendran and Hayes (2004) and Beveridge, Girard, Kopp, and Drover (2005), oil from grape seeds contain unsaturated fatty acids, particularly oleic, and linoleic acids, which have been es‐ tablished to play vital role in cardiovascular health of humans. Also, grape seed oil contains considerably high quantity of bioactive com‐ pounds including phenolics and tocopherol and these compounds have been established to add to the antioxidant properties of the oil (Lutterodt et al., 2011; Passos, Silva, Silva, Coimbra, & Silva, 2010; Wen et al., 2016). In addition, grape fruit is a good source for natural antioxidants and it contains many biologically active substances such as anthocyanin, catechin, epicatechin, resveratrol, proanthocyanidin, fatty acids, and vitamins (El Gengaihi, Aboul Ella, Hassan, Shalaby, & Abou Baker, 2013; Murthy, Singh, & Jayaprakasha, 2002; Pejin et al., 2016; Popovic‐Djordjevic et al., 2017;2017). However, the phenolics composition of grape oil depends on variety, harvest time, type of soil, applied agro‐technology, realized grape yields, climatic factors, and whether the oil is extracted from whole grape, seeds, pulp, or skin. While total extractable phenolics in grape seeds is about 70%, extractable phenolics in grape skin is about 35%, while only 10% or less is extractable in pulp.
The aim of the present study was to compare and characterize the nutritive quality of grape seeds and oils from five grape varieties grown in Herzegovina Province of Bosnia and Herzegovina. This was probably the first study dealing with the comparison of grape seeds and oils of autochthonous variety Blatina with regional and inter‐ national varieties Vranac, Merlot, Cabernet Sauvignon, and Muscat Hamburg.
2 | MATERIAL AND METHODS
2.1 | Material
Grapes were transferred to the laboratory and were manually sepa‐ rated into seeds and skin. The samples were stored at −18°C until analysis. Grape seeds analyzed for different phytochemical attrib‐ utes were obtained from ripened fruits of Blatina, Cabernet, Merlot, Muscat Hamburg (Black), and Vranac grape varieties cultivated in experimental field located in Trebinje in Herzegovina province of Bosnia and Herzegovina. Blatina is the most important autochtho‐ nous variety of Bosnia and Herzegovina. Their flowers are function‐ ally female, the pestile is normal, but the stamen has been hardened and lowered. Therefore, Blatina must be planted in combination with other varieties to be pollinators. The cluster is medium sized to large, depending on the success of fertilization. The berries are round with a thin and smooth skin. The color of the skin is dark blue to black. The meat is colorless. Vranac is the most important regional variety from Balkan grown in Herzegovina. The flower is a hermaphrodite. The cluster is cylindrically conical, medium sized or large. The weight of the cluster varies from 150 to 300 g. The berries are medium sized or large, round or slightly oval with a thin, dark‐blue skin. Grape juice
is a colorless and neutral scent. Merlot, Cabernet Sauvignon, and Muscat Hamburg are international varieties which are widespread in the Herzegovina province. Grapes were transferred to the labora‐ tory and were manually separated into seeds and skin.
2.2 | METHODS
2.2.1 | Proximate analysis
In preparation for chemical analysis, the grape seeds were ground and sieved using 0.4 screen. Moisture and crude oil content of the grapes were determined using the method of Association of Official Analytical Chemists (AOAC, 1990). Nitrogen combustion method using Leco combustion analyzer was used for crude protein meas‐ urement and 6.25 was used as conversion factor (AACC,1999).
2.2.2 | Soxhlet extraction
Oil extraction was done using Soxhlet extractor. In the process, ground grape seeds were carefully placed in the Soxhlet extrac‐ tor and petroleum ether (50°C) was used, after which the solvent evaporation was carried out under low pressure. The oil extract so obtained were kept at −18°C in glass bottles, properly sealed, before analysis.
2.2.3 | Extraction for grape sample for
phenolics and antioxidant activity determination
Bioactive components of the grape seeds were extracted as de‐ scribed by Talhaoui et al. (2014) with slight modification. About 4 g of ground grape seed sample along with methanol (20 ml) was soni‐ cated for 15 min followed by centrifugation at 5,000 rpm for 10 min. The extraction process was repeated twice, and the supernatants obtained after centrifugation were collected. A rotary evaporator operating under reduced pressure and 37°C temperature was used to obtained concentrated extracts from supernatants followed by making up the volume as 25 ml using methanol.
2.2.4 | Total phenol
The Folin–Ciocalteau reagent method of Madaan, Bansal, Kumar, and Sharma (2011) using spectrophotometer was used to measure the phenolic content of the grape seeds. Total phenolic content was estimated using gallic acid standard curve and the value was expressed using gallic acid equivalent. Approximately 10 mg of gallic acid was mixed with about 100 ml of 50% methanol μg/ml, followed by dilution in concentration of 12.5, 25, 50, or 100 μg/ ml. 0.076 ml was thereafter taking from individual dilution and then diluted further to 0.76 ml with distilled water. A 1 N Folin– Ciocalteu solution (0.12 ml) was dissolved with the extract, al‐ lowed to react for about 5 min at 37°C, followed by the mixing of 0.32 ml of Na2CO3 (20% w/w) solution. The contents of each test tube were made up to 2 ml using distilled water. This was followed
by vortexing of both samples and standards which were then left for about 30 min at room temperature. Optical activity was then taking at 765 nm using UV/VIS spectrophotometer (Schimadzu, Japan) and distilled water was used as blank. Estimation of plant samples in test tube was carefully done using dilute methanolic extracts of about 0.76 ml.
2.2.5 | Antioxidant activity
The Lee et al. (1998) method was employed for antioxidant activity determination in samples. A methanolic solution of 0.1 mM DPPH radical was prepared for antioxidant activity assay. About 2 ml of methanolic solution of DPPH was vigorously mixed with 1 ml of ex‐ tract, followed by incubation for 30 min at room temperature (37°C). Spectrophotometer was thereafter used to measure absorbance at 517 nm wavelength.
2.2.6 | Determination of phenolic compounds
An HPLC system (Shimadzu) connected to PDA detector and equipped with Inertsil ODS‐3 (5 µm; 4.6 × 250 mm) column was employed in the determination of individual phenolic compounds in grape seed extract. The mobile phase consisted of A (0.05% of acetic acid in water) and B (acetonitrile) and the flow rate was set at 1 ml/ min. The sample volume injected to the system was 20 µl at detec‐ tion was carried out at 30°C. Each sample was run for 1 hr and the peak detection was carried out at 330 nm. Pure standards were also run by using the same method and conditions for comparison calcu‐ lation of quantitative results.
2.2.7 | Determination of fatty acids
Analysis for fatty acids consisted of first the conversion of fatty acids in grape seed oils to the corresponding fatty acid methyl esters and then their contents were evaluated through measurement of differences between sample retention time and that of suitable fatty acid methyl esters standards. A gas chromatography system (Varian 5890) consisting of a capillary column (CP‐Sil 88, length 100 m, in‐ ternal diameter of0.25 mm, and film thickness of 0.2 μm) was uti‐ lized for the fatty acid determination according to a previous method (Matthaus & Özcan, 2006).
2.2.8 | Tocopherol content
HPLC system was used for determination of tocopherol content of the grape seed oils. In the process, about 20 μl of oil sample was promptly introduced @1.3 ml/min into a 25‐cm‐long Diol phase HPLC column (Merck, Darmstadt, Germany) having an internal di‐ ameter of 4.6 mm. Tocopherol present in the samples were then quantified using Spika et al. (2015) method. The HPLC system used in this analysis was connected to a PDA detector and was equipped with a LiChroCART Silica 60 (4.6 × 250 mm, 5µ; Merck, Darmstadt, Germany) column. The concentration of standard α‐, β‐, γ‐, and δ‐ tocopherol solutions was maintained between 0 and 100 mg/L and the quantification of their contents in the samples was accomplished through comparison of chromatographic results.
2.2.9 | Mineral content
Approximately 15 ml of pure NHO3 was added to 0.2 g of grape seed samples in burning cup, followed by addition of 2 ml H2O2 (% 30
w/v) solution. Incineration of sample was carried out at 210°C in a MARS 5 microwave oven. The mixture was allowed to digest, after which No 42 Whatman filter paper was used to filter the sample. The filtrate was then carefully collected using 50‐ml flasks, followed by analysis using ICP‐AES. Mineral content of grape seed samples was thereafter analyzed and then compared with that of the standard solutions with known concentration (Skujins, 1998).
2.3 | Statistical analysis
Completely randomized split plot block design was used and data analysis was conducted using analysis of variance (ANOVA; JMP version 9.0 (SAS Inst. Inc., Cary, N.C.U.S.A). Analysis was con‐ ducted in triplicate and the results were expressed as mean ± SD (MSTAT C) of 25 independent grape varieties (Püskülcü & İkiz, 1989).
3 | RESULTS AND DISCUSSION
The chemical and bioactive properties of seeds of five grape vari‐ eties growing in Herzegovina province of Bosnia and Herzegovina
TA B L E 1 Chemical and bioactive properties of different grape seeds
Grape varieties Moisture (%)
Crude protein (%;Nx6.25) Crude oil (%) Total phenolic content (mgGAE/kg) Antioxidant activity (%)
Merlot 9.60 ± 0.27a* 8.0 ± 0.35b 7.46 ± 0.05d 502.08 ± 0.01e 90.04 ± 0.00b
Vranac 9.58 ± 0.45ab** 10.9 ± 0.29a 8.63 ± 0.03cd 685.28 ± 0.03b 90.28 ± 0.00ab
Cabernet 8.66 ± 0.22cd 10.4 ± 0.63a 8.79 ± 0.02c 619.17 ± 0.04d 90.76 ± 0.00a
Blatina 9.26 ± 0.36b 8.0 ± 0.17b 9.83 ± 0.03b 693.33 ± 0.02a 88.79 ± 0.00c
Muscat (Black) 8.70 ± 0.44c 8.0 ± 0.12b 11.85 ± 0.04a 674.44 ± 0.01c 86.68 ± 0.00cd
*Mean ± SD
4 of 8
|
BANJANIN etAl.are presented in Table 1. While moisture contents of grape varieties change between 8.66 (Cabernet) and 9.60% (Merlot), the amount of oil present in the samples changed from 7.46 (Merlot) and 11.85% (Muscat). Crude protein contents of grape seeds changed between 8.0 (Muscat, Cabernet and Blatina) and 10.9% (Vranac). According to Fernandes, Casal, Cruz, Pereira, & Ramalhosa, 2013 and Wen et al., 2016, the oil content of grape seeds varied between 3.95 and 20.71%, depending of the grape variety. Radovanovic et al. (2011) reported 7.2% crude protein for seeds obtained from Vranac grape variety. Grape seed obtained from boiled grape juice production contained 54.61 mgGAE/g total phenol and 7.41% moisture (Selçuk et al., 2011). Additionally, while the total phenolic contents of grape seed extracts quantified varied from 502.08 mgGAE/kg in Merlot and 693.33 mgGAE/kg in Blatina, the antioxidant activity values of seed samples changed from 86.68 (Muscat) to 90.76% (Cabernet). In previous research conducted by Obreque‐Slier, López‐Solís, Castro‐Ulloa, Romero‐Díaz, and Peña‐Neira (2012), the total phe‐ nolic contents of the samples ranged from 10.2 ± 0.2 mgGAE/g seed (Cabernet Sauvignon) to 13.5 ± 0.6 mgGAE/g seed (Merlot). This total phenol content values are similar to the results published by Pardo, Fernańdez, Rubio, Alvarruiz, and Alonso (2009) (10.68–34.43 mgGAE/kg), but much lower than those reported by Lutterodt et al. (2011) (0.16–0.80 mg GAE/kg). Radovanovic et al. (2011) reported that the results of spectrophotometric analysis of Vranac grape seed extracts showed high contents of total phenol (385.21 ± 5.29 mgGAE/kg) and antioxidant activity (99.02 ± 0.11%). Wen et al. (2016), however, reported different values for total phenol contents of various grape seeds extracts; 46.60 mgGAE/kg (Chardonnay) and 98.19 mgGAE/kg (Cabernet Sauvignon). Merlot contained
80.68 mgGAE/kg total phenol (Wen et al., 2016). These values, how‐ ever, were found different than the results reported by Wen et al. (2016) (46.50 mgGAE/kg), 10.68–34.43 mgGAE/kg by Pardo et al. (2009), and 385.21 ± 5.29 mgGAE/kg reported by Radovanovic et al. (2011). These variations may be attributed to varietal differences.
Table 2 depicts the effect of grape varieties on phenolic com‐ pounds of various grape seeds obtained from Bosnia and Herzegovina. Phenolic compounds of grape seed extracts varied significantly de‐ pending on grape varieties. Predominant phenolics in seed samples were 1,2‐dihydroxybenzene, (+)‐catechin, 3,4‐dihydroxybenzoic acid, gallic acid, caffeic acid, rutin trihydrate, trans‐ferulic acid, and syringic acids. According to Table 2 1,2‐dihydroxybenzene contents of grape seed extracts change between 42.32 mg/100 g (Blatina) and 986.37 mg/100 g (Muscat), (+)‐catechin contents of seed samples varied between 56.52 mg/100 g (Cabernet) and 1,196.00 mg/100 g (Vranac). Also, gallic acid contents of seeds varied between 7.42 mg/100 g (Merlot) and 167.94 mg/100 g (Muscat), 3,4‐dihy‐ droxybenzoic acid contents of seed samples were found to vary between 10.95 mg/100g (Blatina) and 607.43 mg/100 g (Muscat). In addition, caffeic acid contents of ranged from 9.56 mg/100 g (Blatina) to 155.73 mg/100g (Muscat). Interestingly, the highest sy‐ ringic acid, rutin trihydrate, trans‐ferulic acid, apigenin‐7‐glucoside, and quercetin were found in Muscat grape seed extract. Also, na‐ ringenin was found in Muscat and Merlot seed samples. However, statistical differences were not observed among resveratrol, trans‐ cinnamic acid, and isorhamnetin contents of the extracts, while sta‐ tistical differences (p < 0.05) were observed among other phenolic compounds present in grape seed extracts. According to Godevac, Tesevic, Velickovic, Vujisic, and Milosavljevic (2010), the gallic acid
TA B L E 2 Phenolic contents of different grape seed (mg/100 g)
Phenolic compounds Muscat (Black) Vranac Merlot Cabernet Blatina
Gallic acid 167.94 ± 15.20a* 53.65 ± 2.56b 7.42 ± 1.80d 10.73 ± 1.56c 7.49 ± 9.92d
3,4‐Dihydroxybenzoic acid 607.43 ± 4.70a** 434.50 ± 3.32b 44.88 ± 13.95c 11.16 ± 3.95d 10.95 ± 2.29d
(+)‐Catechin 986.37 ± 31.56b 960.00 ± 13.31a 61.32 ± 10.05d 56.52 ± 12.93e 68.21 ± 0.78c
1,2‐Dihydroxybenzene 450.16 ± 78.84a 218.91 ± 9.33b 109.91 ± 43.74c 100.16 ± 13.38d 42.32 ± 7.38e
Syringic acid 136.27 ± 3.38b 204.16 ± 7.93a 24.17 ± 12.41c 5.29 ± 5.43d 15.50 ± 6.31cd
Caffeic acid 155.73 ± 1.91a 93.22 ± 9.02b 10.43 ± 6.28cd 11.22 ± 6.18c 9.56 ± 5.51d
Rutin trihydrate 150.17 ± 2.28b 327.84 ± 18.48a 10.60 ± 2.67c 10.65 ± 6.19c 9.56 ± 0.72cd
p‐Coumaric Acid 18.38 ± 0.68b 28.06 ± 1.58a 1.45 ± 0.96c 0.72 ± 0.59d 0.78 ± 0.26d
trans‐Ferulic acid 136.67 ± 1.12a 84.77 ± 1.47b 12.32 ± 0.96c 4.96 ± 0.83e 10.34 ± 2.87d
Apigenin 7 glucoside 113.87 ± 0.71a 57.43 ± 8.49b 11.96 ± 6.83c 4.44 ± 1.95d 4.48 ± 4.05d
Resveratrol 18.44 ± 0.11a 14.40 ± 1.10b 1.18 ± 0.65c 1.40 ± 1.26c 1.07 ± 0.17c
Quercetin 61.27 ± 1.40a 52.90 ± 9.48b 3.99 ± 2.79cd 4.52 ± 0.50c 3.05 ± 1.94d
trans‐Cinnamic acid 4.36 ± 0.08a 4.39 ± 0.39a 0.36 ± 0.27b 0.28 ± 0.15b 0.22 ± 0.44b
Naringenin –*** 12.16 ± 0.35a – 0.77 ± 0.15b 0.75 ± 0.15b
Kaempferol 10.88 ± 0.06b 19.30 ± 2.31a 1.20 ± 0.96c – 0.82 ± 1.43d
Isorhamnetin 27.35 ± 0.87a 20.52 ± 0.79b 1.34 ± 0.36c 1.39 ± 0.87c 1.38 ± 0.31c
*Mean ± SD
**Values in each row with different letters are significantly different (p < 0.05) ***Nonidentified.
contents of grape cultivars grown in some part of Serbia ranged from 4.30 to 22.48 mg/100 g, while 0.78 to 2.44 mg/100 g was re‐ ported for protocatechuic acid, 0.81 to 7.04 mg/100 g for caftaric acid, and 0.24 to 1.43 mg/100 g for p‐hydroxybenzoic acids. In addi‐ tion, Hussein and Abdrabba, (2015) reported 1.45 mg/100 g vanillic acid, 779.57 mg/100 g catechin, 8,729.55 mg/100 g protocatechuic, 11.89 mg/100 g coumarin, 889.20 mg/100 g gallic, 13.0 mg/100 g ferulic, 5,533.14 mg/100 g catechol, 4,039.26 mg/100 g chloro‐ genic, 440.30 mg/100 g synergic, 58.68 mg/100 g pyrogallol, and 7.25 mg/100 g caffeic acids for grape seeds, while Samavardhana, Supawititpattana, Jittrepotch, Rojsuntornkitti, and Kongbangkerd (2015) reported 0.22 and 0.28 mg/g and 5.65 and 5.91 mg/g, to 0.22 and 0.23, and 5.57 and 5.67 mg/g, respectively for catechin and epicatechin in grape seeds obtained from wine process and juice process. Catechin and epicatechin are the predominant flavonols in grape seeds and it has been reported that catechin content of grape varieties are usually similar (Chedea, Braicu, & Socaciu, 2010). These variations may be due to differences in varieties, environmental fac‐ tors, ripenin, and cultural factors. The results obtained in this pres‐ ent study differ from the findings of Godevac et al. (2010), Hussain and Abdrabba (2015) and Samavardhana et al. (2015). White grape cultivars (Italian Riesling, Traminer, and Smederevka) had higher gallic acid contents (over 17 mg per 100 g), compared to 4–23 mg per 100 g free gallic acid obtainable in other grape seed varieties. Generally, colored grape cultivars cultivars had significantly lower gallic acid contents (below 10 mg per 100 g) (Godevac et al., 2010). This is similar to the findings of Rodriguez et al. (2006) for some white and red grape varieties grown in Spain.
The fatty acid composition and tocopherol contents of dif‐ ferent grape seed oils grown in Herzegovina province of Bosnia and Herzegovina are presented in Table 3. As presented, stearic,
palmitic, oleic, and linoleic acids were the predominant fatty acids in grape seed oils. The highest fatty acid in all seed oil samples was lin‐ oleic acid, and then by oleic, palmitic, and stearic acids. Linoleic acid contents of grape seed oils varied from 61.30 (Cabernet) to 67.84% (Merlot) and oleic acid from 19.87 (Merlot) to 24.53% (Blatina). Also, palmitic acid contents of different grape seed oils were varied from 7.08% in Vranac cultivar to 10.60% in Cabernet cultivar. Additionally, stearic acid contents of the oils changed from 3.58% in Merlot vari‐ ety to 5.05% in Vranac cultivar. The remaining fatty acids present in grape seed oils were below 0.34%. On the other hand, the tocopherol contents of the oil were significantly affected by varieties (Table 3). The highest tocopherol was γ‐tocopherol, followed by β‐tocopher‐ ols, DL‐α‐tocopherol, and δ‐tocopherols. While γ‐tocopherol con‐ tents of grape seed oils change between 1.84 (Cabernet and Blatina) and 2.04 mg/g (Merlot), β‐tocopherol contents of oil samples varied between 0.60 mg/g (Merlot) and 0.74 mg/g (Blatina). The highest DL‐α‐tocopherol was found in Vranac grape seed oil (0.15 mg/g). According to Bjelica et al. (2017), grape seed oils had 73.60% (Hamburg)–85.5% linoleic (Sila), 9.40% (Sila)–16.25% (Hamburg), 1.50% (Sila)–3.39% stearic (Hamburg), and 3.12% (Sila)–6.34% pal‐ mitic acids (Hamburg). Beveridge et al. (2005) found between 66.8 and 73.6% linoleic acids in the seed oils of seven different varieties of grapes. The fatty acid results obtained in this present study com‐ mensurate with what is available in literature(Beveridge et al., 2005; Crews et al., 2006; Lutterodt et al., 2011; Bjelica et al. 2017). Also, Crews et al. (2006) reported that variations exist among the fatty acids composition of grape seed oils from Spain, Italy, and France. In addition, Wen et al. (2016) found out that grape seed oils from differ‐ ent varieties contained 63.52–76.77% linoleic, 13.63–22.03% oleic, 6.56–8.55% palmitic, and 2.06–4.59% stearic acids. These results were similar to those reported by Beveridge et al., 2005; Crews et al.
TA B L E 3 Fatty acid and tocopherol contents of different grape seed oils
Fatty acids (%) Muscat (Black) Vranac Merlot Cabernet Blatina
Myristic – – 0.05 ± 0.00b 0.13 ± 0.02a –
Palmitic 7.37 ± 0.07*c 7.08 ± 0.01c 7.28 ± 0.16c 10.60 ± 0.74a 8.33 ± 0.66b
Stearic 4.57 ± 0.03b** 5.05 ± 0.01a 3.58 ± 0.01c 4.68 ± 0.09b 4.34 ± 0.08b
Oleic 22.07 ± 0.15b 20.28 ± 0.02d 19.87 ± 0.08e 21.77 ± 0.20c 24.53 ± 0.25a
Linoleic 64.74 ± 0.12c 66.24 ± 0.03b 67.84 ± 0.08a 61.30 ± 0.43d 61.37 ± 0.32d
Arachidic 0.25 ± 0.01b 0.21 ± 0.00c 0.20 ± 0.00cd 0.28 ± 0.02a 0.19 ± 0.01d
Linolenic 0.24 ± 0.00e 0.26 ± 0.00d 0.27 ± 0.00c 0.34 ± 0.00a 0.33 ± 0.00b
Behenic –*** 0.07 ± 0.00b 0.05 ± 0.00b 0.17 ± 0.01a –
Arachidonic 0.10 ± 0.00a 0.08 ± 0.00c 0.09 ± 0.00b – –
Tocopherols (mg/g) Muscat Vranac Merlot Cabernet Blatina
DL‐α‐ Tocopherol 0.14 ± 0.00b 0.15 ± 0.04a 0.11 ± 0.04d 0.14 ± 0.04b 0.13 ± 0.04c
β‐Tocopherol 0.66 ± 0.05b 0.61 ± 0.02c 0.60 ± 0.00cd 0.65 ± 0.07bc 0.74 ± 0.07a
γ‐Tocopherol 1.94 ± 0.04b 1.99 ± 0.06b 2.04 ± 0.04a 1.84 ± 0.02c 1.84 ± 0.03c
δ‐Tocopherol – – – 0.01 ± 0.04a 0.01 ± 0.01a
*Mean ± SD
**Values in each row with different letters are significantly different (p < 0.05) ***Nonidentified.
6 of 8
|
BANJANIN etAl.2006; Pardo et al., 2009; Rubio, Alvarez‐Orti, Alvarruiz, Fernandez, & Pardo, 2009 and Fernandes et al., 2013. According to Yousafi, Nataghi, and Gholamian (2013), grape seed oil is a good source of linoleic (65–72%), stearic (8.5–15%), oleic (12–23%), and palmitic (4– 11%) acids. Meanwhile, variations in oil composition due to varietal differences in grape varieties grown in Canada have been reported (Beveridge et al., 2005).Wen et al. (2016) reported that α‐tocopherol contents of different grape variety seeds ranged from 50.80 (Vitis da‐
vidii) to 131.34 mg/kg (Cabernet Sauvignon), α‐tocotrienol contents
of seed oils varied between 177.77 (Chardonnay) and 521.11 mg/kg (Vitis amurensis). Merlot seed oil contained 90 mg/kg α‐tocopherol, 239.95 mg/kg α‐tocotrienol, 156 mg/kg 29 γ‐tocotrienol mg/kg, and 1.75 mg/kg β‐tocopherol (Wen et al., 2016). The tocopherol contents shown partly differences to those reported for Wen et al. (2016). Variations in grape seed oil composition may be attributed to differ‐ ences in grape cultivars (Wen et al., 2016). Differences in tocopherol contents of cold‐pressed grape seed oils have been reported to vary between 27.81 (Hamburg) and 57.52 mg/100 g (Merlot) (Bjelica et al., 2017). Vinification process also produced grape seed oil containing 3.595–20.56 mg/kg α‐tocopherol, 1.947–14.57 mg/kg ɣ‐tocopherol, 8.627–38.39 mg/kg α‐tocotrienol, 29.24–74.99 mg/kg ɣ‐tocotrienol, and 0.319 to 1.257 mg/kg δ‐tocotrienol (Lachman et al., 2013). Also, research conducted by Choi and Lee (2009) revealed grape seed oils predominantly contained α‐tocotrienol (40 mg/kg), ɣ‐tocotrienol (70 mg/kg), and approximately 120 mg/kg total tocopherol. In an‐ other research, Tangolar, Ozogul, Tangolar, and Torun (2009) found about 15.43 mg/kg α‐tocopherol and 1.85 mg/kg ɣ‐tocopherol in grape seed oil. Tocopherols are essential antioxidant compounds which exist majorly in oils. Grape seed oils were a good source of ɣ‐ tocopherol, α‐tocopherol, and α‐tocotrienol (Fernandes et al., 2013). Slight variations could be observed between values obtained in this present study and literature and this could link to differences in vari‐ ety, harvest time, climatic factor, and growing conditions.
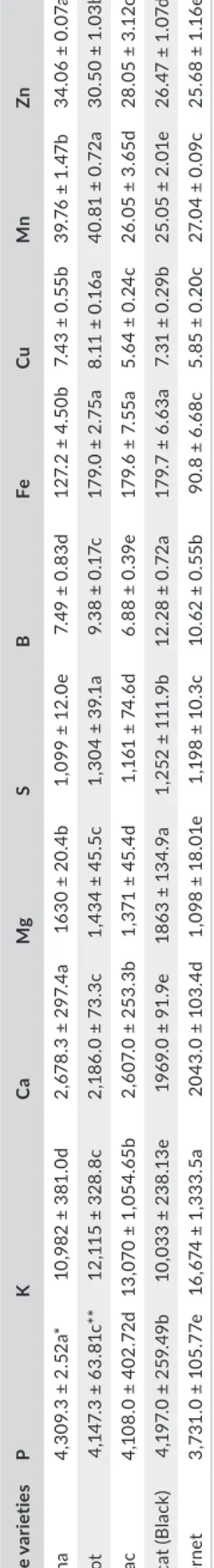
Table 4 showed the various minerals present in grape seeds. The major minerals of different grape seeds were K, P, Ca, Mg, and S, and they mainly depend on the type of soil, grape variety, and maturity along with climatic conditions (Stanimirovic, Djordjević et al. 2018; Stanimirovic, Vujovic et al., 2018). While P contents of seed samples vary between 3,731.0 (Blatina) and 4,309.3 mg/ kg (Muscat), K contents of seed samples changed between 10,033 (Cabernet) and 16,674 mg/kg (Blatina). In addition, while Ca con‐ tents of grape seeds ranged from 1969.0 (Cabernet) to 2,678.3 mg/ kg (Muscat), Mg contents from 1,098 (Blatina) to 1863 mg/kg (Cabernet), S contents of grape seeds changed between 1,099 (Muscat) and 1,304 mg/kg (Vranac), Fe contents of grape seeds changed between 90.8 (Blatina) and 179.7 mg/kg (Cabernet), while Mn contents of seed samples varied between 25.05 (Cabernet) and 40.81 mg/kg (Vranac). The highest Cu (8.11 mg/kg) and Zn (34.06 mg/kg) were found in Vranac and Muscat seeds, respec‐ tively. No statistical difference exists amid Fe contents of grape seed extracts. Radovanovic et al. (2011) reported that Vranac grape seed contained 20.0 mg/100 g P, 10 mg/100 g Ca, 7 mg/100g Mg, 0.36 mg/100 g Fe, and 0.07 mg/100 g Mn. According to Lachman TA B LE 4 M in er al c on te nt s o f d iff er en t g ra pe s ee ds ( m g/ kg ) G ra pe v ar ie tie s P K Ca Mg S B Fe Cu Mn Zn B la tina 4, 30 9. 3 ± 2. 52 a* 10 ,9 82 ± 3 81 .0 d 2, 678 .3 ± 2 97 .4 a 16 30 ± 2 0. 4b 1, 09 9 ± 12 .0 e 7. 49 ± 0 .8 3d 12 7. 2 ± 4. 50 b 7. 43 ± 0 .5 5b 39 .7 6 ± 1. 47 b 34 .0 6 ± 0. 07 a Mer lo t 4, 14 7. 3 ± 63 .8 1c ** 12 ,1 15 ± 3 28 .8 c 2, 18 6. 0 ± 73 .3 c 1, 43 4 ± 45 .5 c 1, 30 4 ± 39 .1 a 9. 38 ± 0 .1 7c 17 9. 0 ± 2. 75 a 8. 11 ± 0 .1 6a 40 .8 1 ± 0. 72 a 30. 50 ± 1 .0 3b Vr an ac 4, 10 8. 0 ± 40 2. 72d 13 ,0 70 ± 1 ,0 54 .6 5b 2, 60 7. 0 ± 25 3. 3b 1, 37 1 ± 45 .4 d 1, 16 1 ± 74 .6 d 6. 88 ± 0 .3 9e 17 9. 6 ± 7. 55 a 5. 64 ± 0 .2 4c 26 .0 5 ± 3. 65 d 28 .0 5 ± 3. 12 c M us ca t ( B la ck ) 4, 19 7. 0 ± 25 9. 49b 10 ,0 33 ± 2 38 .1 3e 19 69 .0 ± 9 1. 9e 18 63 ± 1 34 .9 a 1, 25 2 ± 11 1. 9b 12 .2 8 ± 0. 72 a 17 9. 7 ± 6. 63 a 7. 31 ± 0 .2 9b 25 .05 ± 2 .0 1e 26 .4 7 ± 1. 07 d C ab er ne t 3, 73 1. 0 ± 10 5. 77 e 16 ,6 74 ± 1 ,3 33 .5 a 20 43 .0 ± 1 03 .4 d 1, 09 8 ± 18 .0 1e 1, 19 8 ± 10 .3 c 10 .6 2 ± 0. 55 b 90 .8 ± 6 .6 8c 5. 85 ± 0 .2 0c 27 .0 4 ± 0.0 9c 25 .6 8 ± 1. 16 e *M ea n ± SD ** V al ue s i n e ac h c ol um n w ith d iff er en t l et te rs a re s ig ni fic an tly d iff er en t ( p < 0. 05 ).
et al. (2013), high levels of macrometals in the grape seeds is strongly affected by the solubility of inorganic compounds of the soil. Also, it could be influenced by factors such as climatic changes and the vinification process. In this study, grape seeds content was 2.355–5.030 mg/kg P, 5.511–10.14 mg/kg Cu, 3.562–9.524 mg/ kg K, 25.382–88.532 mg/kg Fe, 0.721–1.714 mg/kg Mg, 7.001– 23.236 mg/kg Mn, 5.502–14.175 mg/kg Zn, 0.038–0.335 mg/kg Na, and 3.246–6.162 mg/kg Ca. Also, Mironeasa, Leahu, Codină, Stroe, and Mironeas (2010) revealed that grape seeds contained 23.051–27.403% K, 15.346–21.676% P, 1.759–2.247% S, 0.173– 0.314% Mn, 0.070–0.149% Zn, and 52.153–5.764% Ca. Our find‐ ings are similar to what had been reported in literature by some authors (Lachman et al., 2013; Mironeasa et al., 2010; Özcan, 2010).
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through re‐ search group no (RG‐1439‐80).
CONFLIC T OF INTEREST
The authors have declared no conflicts of interest for this article.
ORCID
Fahad Al Juhaimi https://orcid.org/0000‐0001‐5617‐6476
Isam A. Mohamed https://orcid.org/0000‐0002‐6578‐0795
Kashif Ghafoor https://orcid.org/0000‐0003‐1240‐5358
Elfadıl E. Babiker https://orcid.org/0000‐0001‐6220‐084X
REFERENCES
AACC International. (1999). Method 46‐30.01. Crude protein— Combustion method. In Approved methods of analysis 11th AACC in‐
ternational. St. Paul, MN.
Al Juhaimi, F., & Özcan, M. M. (in press). Effect of cold pres and Soxhlet extraction systems on fatty acid, tocopherol contents, and phenolic compounds of various grape seed oils. Journal of Food Processing and
Preservation, 42(1), 1–7.
Association of Official Analytical Chemists. (1990). Official methods of
analysis (15th ed.). Washington, DC: Author.
Bail, S., Stuebiger, G., Unterweger, H., & Buchbauer, G. (2008). Characterisation of various grape seed oils by volatile com‐ pounds, triacyglycerol composition, total phenols. Food Chem, 108, 1122–1132.
Beveridge, T. H. J., Girard, B., Kopp, T., & Drover, J. C. G. (2005). Yield and composition of grape seed oils extracted by supercritical carbon dioxide and petroleum ether: Varietal effects. Journal of Agriculture
and Food Chemistry, 53, 1799–1804.
Bjelica, M., Vujasinovic, V., Corbo, S., Dimic, S., & Pastor, K. (2017) Fatty acid composition and bioactive compounds of cold pressed‐ grape seed oils from red and white grape cultivars grown in Vojvo‐ dina. In 28th international scientific‐expert conference of agriculture
and food industry, 27–29 September, 2017. Sarajevo, Bosnia and
Herzegovina.
Chedea, V. S., Braicu, C., & Socaciu, C. (2010). Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chemistry, 121, 132–139.
Choi, Y., & Lee, J. (2009). Antioxidant and antiproliferative properties of ‐tococtrienol‐rich fraction. Food Chemistry, 114, 1386–1390. Crews, C., Hough, P., Godeard, J., Brereton, P., Lees, M., Guiet, S., &
Winkelmann, W. (2006). Quantitation of the main constituents of some authentic grape‐seed oils of different origin. J Agric Food Chem,
54, 6261–6265.
El Gengaihi, S., Aboul Ella, F. M., Hassan, E. M., Shalaby, E. A., & Abou Baker, D. H. (2013). Phytochemical investigation and radical scaveng‐ ing activity of wastes of some grape varieties grown in Egypt. Global
Journal of Pharmacology, 7(4), 465–473. https ://doi.org/10.5829/
idosi.gjp.2013.7.4.1115
Fernandes, L., Casal, S., Cruz, R., Pereira, J. A., & Ramalhosa, E. (2013). Seed oils of ten traditional Portuguese grape varieties with interest‐ ing chemical and antioxidant properties. Food Research International,
50, 161–166.
Gecgel, U., Gülcü, M., Aljuhaimi, F., Hamurcu, M., & Özcan, M. M. (2017). Bioactive properties, fatty acid composition and mineral contents of grape seed and oils. South African Journal of Enology and Viticulture,
38, 103–108.
Godevac, D., Tesevic, V., Velickovic, M., Vujisic, L., & Milosavljevic, S. (2010). Polyphenolic compounds in seeds from some grape cul‐ tivarsgrown in Serbia. Journal of Serbian Chemical Society, 75(12), 1641–1652.
Hussein, S., & Abdrabba, S. (2015). Physico‐chemical characteristics, fatty acid, composition of grape seed oil and phenolic compounds of whole seeds, seeds and leaves of red grape in Libya. International
Journal of Applied Science and Mathematics, 2, 175–181.
Lachman, J., Hejtmánková, A., Hejtmánková, K., Hornícková, S., Pivec, V., Skala, O., … Pribyl, J. (2013). Towards complex utilisation of wine‐ makingresidues: Characterisation of grape seeds by total phenols, tocols and essentialelements content as a by‐product of winemaking.
Industrial Crops and Products, 49, 445–453.
Lee, S. K., Mbwambo, Z. H., Chung, H. S., Luyengi, L., Games, E. J. C., & Mehta, R. G. (1998). Evaluation of the antioxidant potential of natu‐ ral products. Combinational Chemistry & High Throughput Screening,
1, 35–46.
Lutterodt, H., Slavin, M., Whent, M., Turner, E., & Yu, L. (2011). Fattyacid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold‐pressed grape seed oils and flours. Food
Chemistry, 128(2), 391–399.
Madaan, R., Bansal, G., Kumar, S., & Sharma, A. (2011). Estimation of total phenolsand flavonoids in extracts of Actaeaspicata roots and antiox‐ idant activity studies. Indian Journal of Pharmaceutical Sciences, 73(6), 666–669.
Matthäus, B., & Özcan, M. M. (2006). Quantitation of fatty acids, ste‐ rols, and tocopherols in turpentine (Pistaciaterebinthus Chia) grow‐ ing wild in Turkey. Journal of Agriculture and Food Chemistry, 54(20), 7667–7671.
Mironeasa, S., Leahu, A., Codină, G.‐G., Stroe, S.‐G., & Mironeas, C. (2010). Grape seed: Physico‐chemical, structural characteristics and oil content. Journal of Agroalimentary Processes and Technologies,
16(1), 1–6.
Murthy, K. N. C., Singh, R. P., & Jayaprakasha, G. K. (2002). Antioxidant activity of grape (Vitis vinifera) pomace extracts. Journal of Agriculture
and Food Chemistry, 50, 5909–5914.
Obreque‐Slier, E., López‐Solís, R., Castro‐Ulloa, L., Romero‐Díaz, C., & Peña‐Neira, Á. (2012). Phenolic composition and physicochem‐ ical parameters of Carménère, Cabernet Sauvignon, Merlot and Cabernet Franc grape seeds (Vitis vinifera L.) during ripening.
LWT ‐ Food Science and Technology, 48(1), 134–141.
Ovcharova, T., Zlatanov, M., & Ivanov, A. (2015). Composition of bulgar‐ ian varieties of grape fruit seeds and the changes in their composition
8 of 8
|
BANJANIN etAl. during development. SSRG International Journal of Agriculture &Environmental Science (SSRG‐IJAES), 2(1), 1–6.
Özcan, M. M. (2010). Mineral contents of several grape seeds. Asian
Journal of Chemistry, 22, 6480–6488.
Özcan, M. M., Al Juhaimi, F., Gülcü, M., Uslu, N., & Geçgel, Ü. (2017). Determination of bioactive compounds and mineral contents of seedless parts and seeds of grapes. African Journal of Enology and
Viticulture, 38, 212–220.
Pardo, J. E., Fernández, E., Rubio, M., Alvarruiz, A., & Alonso, G. L. (2009). Characterization of grape seed oil from different grape varieties (Vitis vinifera). European Journal of Lipid Science and Technology, 111(2), 188–193.
Passos, C. P., Silva, R. M., Da Silva, F. A., Coimbra, M. A., & Silva, C. M. (2010). Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil. Effect of the operating conditions upon oil composition and anti‐ oxidant capacity. Chemical Engineering Journal, 160(2), 634–640. Pejin, B., Stanimirovic, B., Vujovic, D., Popovic Djordjevic, J., Velickovic,
M., & Tesevic, V. (2016). The natural product content of the selected Cabernet Franc wine samples originating from Serbia: A case study of phenolics. Natural Product Research, 30(15), 1762–1765.
Popovic‐Djordjevic, J., Pejin, B., Dramicanin, A., Jovic, S., Vujovic, D., Zunic, D., & Ristic, R. (2017). Wine chemical composition and rad‐ ical scavenging activity of some Cabernet Franc clones. Current
Pharmaceutical Biotechnology, 18(4), 343–350.
Püskülcü, H. & İkiz, F. (1989). Introduction to statistic (p. 333). Bornova. İzmir, Turkey: Bilgehan Press (in Turkish).
Radovanovic, V., Dekic, S., & Radovanovic, B. (2011). Economic poten‐ tial of appling grape seed extract as a natural antioxidant. Journal of
Processing and Energy in Agriculture, 15, 263–266.
Rodríguez‐Montealegre, R., Romero‐Peces, R., Chacón‐Vozmediano, J. L., Martínez‐Gascueña, J., & García‐Romero, E. (2006). Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. Journal of Food Composition and Analysis,
19, 687–693.
Rubio, M., Alvarez‐Orti, M., Alvarruiz, A., Fernandez, E., & Pardo, J. E. (2009). Characterization of oil obtained from grape seeds collected during berry development. Journal of Agriculture and Food Chemistry,
57(7), 2812–2815.
Samavardhana, K., Supawititpattana, P., Jittrepotch, N., Rojsuntornkitti, K., & Kongbangkerd, T. (2015). Effects of extracting conditions on phenolic compounds and antioxidant activity from different grape processing byproducts. International of Food Research Journal, 22(3), 1169–1179.
Selcuk, A. R., Demiray, E., & Yılmaz, Y. (2011). Antioxidant activity of grape seeds obtainedfrom molasses (Pekmez) and winery produc‐ tion. Akademik Gıda, 9(5), 39–43.
Skujins, S. (1998) Handbook for ICP‐AES (Varıan‐Vista). A short guide to
vista series ICP‐AES operation. VarianInt. AGşZug. Version 1.0 (p. 29).
Switzerland.
Spika, M. J., Kraljic, K., Koprivnjak, O., Skevin, D., Zanetic, M., & Katalinic, M. (2015). Effect of agronomical factors and storage conditions on the tocopherol content of Oblica and Leccino virgin olive oil. Journal
of the American Oil Chemists Society, 92, 1293–1301.
Stanimirović, B., Djordjević, J. P., Pejin, B., Maletić, R., Vujović, D., Raičević, P., & Tešić, Ž. (2018). Impact of clonal selection on Cabernet Franc grape and wine elemental profiles. Scientia Horticulturae, 237, 74–80.
Stanimirovic, B., Vujovic, D., Pejin, B., Popovic Djordjevic, J., Maletic, R., Raicevic, P., & Tesic, Z. (2018). A contribution to the elemental pro‐ file of the leaf samples of newly developed Cabernet Franc varieties.
Natural Product Research, 30, 1–5.
Talhaoui, N., Gomez‐Caravana, A. M., Leon, L., De la Rosa, R., Segura‐ Carreter, A., & Fernandez‐Gutierrez, A. (2014). Determination of phenolic compounds of “Sikitita” olive leaves by HPLC‐DAD‐TOF‐ Ms. Comparison with its parents “Arbequina” and “Picual” olive leaves. LWT ‐ Food Science and Technology, 58, 28–34.
Tangolar, S. G., Ozogul, Y., Tangolar, S., & Torun, A. (2009). Evaluation of fatty acid profiles and mineral content of grape seed oil of some grape genotypes. International Journal of Food Sciences and Nutrition,
60(1), 32–39.
Vujović, D., Maletić, R., Popović‐Đorđević, J., Pejin, B., & Ristić, R. (2017). Viticultural and chemical characteristics of Muscat Hamburg prese‐ lected clones grown for table grapes. Journal of the Science of Food
and Agriculture, 97(2), 587–594.
Vujovic, D., Pejin, B., Popovic Djordjevic, J., Velickovic, M., & Tesevic, V. (2016). Phenolic natural products of the wines obtained from three new Merlot clone candidates. Natural Product Research, 30(8), 987–990.
Wen, X., Zhu, M., Hu, R., Zhao, J., Chen, Z., Li, J., & Ni, Y. (2016). Characterisation of seed oils from different grape cultivars grown in China. Journal of Food Science and Technology, 53(7), 3129–3136.
Wijendran, V., & Hayes, K. C. (2004). Dietary n‐6 and n‐3 fatty acid balance and cardiovascular health. Annual Reviews of Nutrition, 24, 597–615.
Yousafi, M., Nataghi, L., & Gholamian, M. (2013). Physicochemical prop‐ erties of two type οfshahrodi grape seed oil (Lal and Khalili). European
Journal of Experimental Biology, 3(5), 115–118.
How to cite this article: Banjanin T, Özcan MM, Al Juhaimi F,
et al. Effect of varieties on bioactive compounds, fatty acids, and mineral contents in different grape seed and oils from Bosnia and Herzegovina. J Food Process Preserv.