Transition-Metal Catalyst Free Oxidative Radical Arylation of
N‑Methylpyrrole
Esma Kocaog
̆lu, Muhammed A. Karaman, Hatun Tokgöz, and Oktay Talaz
*
Department of Chemistry, Kamil Özdağ Science Faculty, Karamanoğlu Mehmetbey University, 70100 Karaman, Turkey
*
S Supporting InformationABSTRACT: This study represents an expansion of the application of catalysis in air through conventional coupling and free radical processes. A reactive free aryl radical intermediate was generated via the oxidation of an activated Ar−NH−NH2 bond by air as a simple and readily available oxidant. For this purpose, the usability of phenylhydrazine and phenylhydrazine hydrochloride salt reagents for the direct
arylation of pyrrole with aryl radicals was investigated. The facile coupling of N-methylpyrrole with aryl radicals was easily applied for the convenient direct synthesis of C-2 arylated pyrroles without a transition-metal catalyst.
■
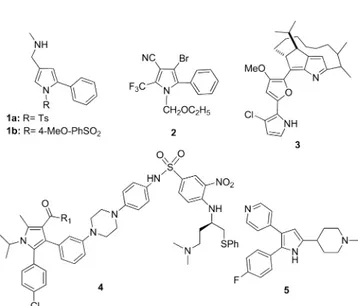
INTRODUCTIONFound in many natural products and used in many pharmaceutically relevant and other functional synthesis,1 pyrrole ring is among the most important heterocyclic structural units. 2-Arylpyrrole derivatives are examples of such pharmaceutically and biologically active compounds; com-pound 1a exhibited selective potent H+, K+-ATPase inhibition activity as a potassium-competitive acid blocker (P-CAB), compound 1b demonstrated potent inhibitory action for histamine-stimulated gastric acid secretion in rats and Heidenhain pouch dogs,2 C-chlorfenapyr compound 2 was thefirst commercialized insecticide-miticide,3roseophilin 3 was isolated from a culture broth of Streptomyces griseoviridis, which displayed cytotoxicity against K562 erythroid leukemia and KB human epidermoid carcinoma cell lines,4 and compound 4 potently inhibited cancer cell growth in the H146 small-cell lung cancer cell line, achieving a nanomolar IC50 value.
5
Furthermore, trisubstituted pyrrole 5 displayed activity against both erythrocytic and sporozoite parasite stages in vitro and in vivo,6respectively (Figure 1).
However, despite the numerous applications of the arylated pyrroles, their synthesis remains a challenge in organic chemistry because pyrroles are very reactive and easily polymerize. Transition-metal-catalyzed (especially Pd, Rh, and Ru complexes) cross-coupling reactions of pyrroles are phenomenally successful for the formation of C sp2−sp2 bonds.7 Palladium catalysts are the most important transition metals among others to perform the arylation of pyrroles. Doucet and co-workers have reported palladium-catalyzed regioselective arylation of pyrroles.8 Recently, a cheap and environmentally friendly iron catalyst was developed.9Despite numerous applications of arylated pyrroles, there is deep need to develop a new methodology because these transition-metals are very expensive and rare metals.
In contrast, metal-free methodologies are rare and challeng-ing. Very recently, considerable progress was made in the
transition-metal-free arylation of pyrroles. Gryko and co-workers reported both the direct arylation of pyrroles with electron-withdrawing groups containing aryl iodides in super-basic media without the external addition of a transition-metal catalyst and the photocatalytic arylation with diazonium salts catalyzed by a porphyrin.10 Yu and co-workers found that pyrroles could be directly arylated with diarylidonium salts.11 Konig, Zhu, and group independently reported the use of perylene diimides as photocatalysts and mediators for the reduction of arylhalides containing electron-withdrawing groups for the arylation of pyrroles with aryl radicals via a Received: July 13, 2017
Accepted: August 16, 2017 Published: August 28, 2017
Figure 1. Pharmaceutical and biologically active compounds containing 2-aryl-pyrrole framework.
Article http://pubs.acs.org/journal/acsodf copying and redistribution of the article or any adaptations for non-commercial purposes.
photoinduced electron-transfer (PET) mechanism and indirect electroreductive coupling.12The aim of the present work is to a perform metal-free coupling using pyrroles and arylhydrazine salts (Scheme 1).
■
RESULTS AND DISCUSSIONArylhydrazine salts have been widely used for organic synthesis because of their stability, high reactivity, and availability. These compounds have been utilized for the Fischer indolization of various carbonyl compounds13 and transition-metal-mediated cross-coupling reactions.14Recently, considerable progress has been made in the transition-metal-catalyzed arylation of inactive C−H bonds with arylhydrazine salts.15However, new develop-ments are needed for metal-free arylations of inactive C−H bonds.
Arylhydrazine salts can be transformed into aryl radicals via the formation of instable diazene intermediates.16 In 2009, Wang and co-workers reported the arylation of [60] fullerene for the synthesis of 1,4-fullerenols C60ArOH with arylhydrazine
salts in the absence of a transition-metal catalyst.17 Interest-ingly, in 2014, Heinrich and co-workers reported the direct arylation of anilines with arylhydrazine salts in the absence of a Scheme 1. Direct Arylation of Pyrroles
Table 1. Optimization of the Reaction Conditionsa
entry solvent base yield of 8e (%)
1 benzene NaOH trace
2 toluene KOH trace
3 acetonitrile K2CO3 trace 4 dimethyl sulfoxide Cs2CO3 n.d. 5 n.d. 6 K2CO3 14 7 Na2CO3 12 8 NaHCO3 5< 9 TMEDA n.d. 10 DMAP n.d. 11 Et3N n.d. 12 K3PO4 10 13 Cs2CO3 10 14 t-BuOK n.d. 15 NaOH 90
(7e) in a variety of polar and nonpolar solvents was not successful. The pyrrole and phenylhydrazine salt ratio was tested at very different ratios starting from 1:1. Some of these
are shown in Table 1. The best yield and homogeneous
medium for the reaction was determined to be 20: 1 for the pyrrole and phenylhydrazine salt ratio. Therefore, pyrrole was used excessively. The cross coupling occurred in the presence of a base at room temperature (rt) in air without solvent to form the product. Because a long reaction time and low NaOH concentration favor the arylation, our goal was to achieve a high yield. For this reason, different from previous studies,18−20
the addition of a lower NaOH concentration (0.5 M) and 50 h of reaction time increased the yield. Notably, the functionalization
of the pyrrole occurred selectively at the C-2 position to give only one regioisomer of an arylated pyrrole. The best yield (90%) for 8f was achieved for the reaction with pyrrole as the solvent. The optimization of the reaction conditions of pyrrole and 4-chlorophenylhydrazine hydrochloride salt is summarized inTable 1.
Afterward, the influence of various bases in the cross coupling was examined (Table 1, entries 5−16). Several of organic and inorganic bases were screened, and only the use of NaOH and KOH (Table 1, entries 15−16) provided high-yielding products (74−90%).
Unfortunately, the desired cross-coupling product was not obtained without addition of base or any other additives. After screening several different bases and metal additives, 0.5 M
NaOH was determined to be the most efficient additive,
providing a 90% yield of the desired cross-coupling product under solvent-free conditions using the pyrrole as both the solvent and reagent (entry 15). Further, solvent-free reactions focused on the screening of different bases were also studied.
In each case, a single C-2 arylation product from the pyrrole was isolated in excellent yield; neither N-arylation nor C-2 and C-5 diarylation products were detected under the utilized conditions (Table 1). The yields listed in Scheme 2 indicate that the phenylhydrazine hydrochloride salt, with an electron-withdrawing substituent, can provide C-2 aryl products with better results. Excellent yields of≥70% were achieved for the reactions involving chloro, bromo, andfluoro groups (entries 8e, 8f, and 8i,Scheme 2). The phenylhydrazine hydrochloride salt containing an electron-withdrawing nitro group also gave a good yield.16,17
On the basis of the results of previous studies,18−20 a plausible reaction mechanism for this reaction via the possible pathway is shown (Scheme 3) after the formation of the aryl radical from the phenylhydrazine salts in the presence of the base and air. The desired arylated product was produced via an initial electrophilic aryl radical reacting with pyrrole (6) to form an allyl radical (10), which was stabilized by resonance (11) by O2before losing an electron and eliminating a proton to afford
the C-2 arylated pyrrole (8).
■
CONCLUSIONSIn conclusion, this paper reports the arylation of N-methylpyrrole via a radical addition reaction by air oxidation of phenylhydrazine salts. This novel and convenient method for the arylation of pyrrole offers several advantages, such as high regioselectivity, operational simplicity, and mild reaction Scheme 2. Reaction of Arylhydrazine Hydrochloride Salts
withN-Methylpyrrole
conditions, and utilizes phenylhydrazine salts, pyrrole, NaOH, H2O, and air at room temperature. This arylation protocol was
used to prepare C-2 arylation products of pyrrole and phenylhydrazine hydrochloride salt, reacting near room temperature and providing easy product isolation.
■
EXPERIMENTAL SECTIONGeneral Procedure for C-2 Arylation of Pyrrole with Phenylhydrazine Hydrochloride Salts. A two-necked 10 mLflask equipped with a magnetic stir bar (2 × 7 mm2) was charged with pyrrole (10 mmol, 670 mg) and phenylhydrazine hydrochloride salt (0.5 mmol, 72 mg). Aqueous sodium hydroxide (0.5 M, 0.5 mL) was then added dropwise over a period of 0.5 h. The resulting mixture was stirred in a two-neckedflask at room temperature for 50−60 h without closing the necks. The excess of pyrrole and water was evaporated at room temperature, and the remaining solid was purified by flash column chromatography (EtOAc/25% hexane).
■
ASSOCIATED CONTENT*
S Supporting InformationThe Supporting Information is available free of charge on the
ACS Publications websiteat DOI:10.1021/acsomega.7b00988. Experimental details and characterization data (PDF)
■
AUTHOR INFORMATION Corresponding Author *E-mail:otalaz@kmu.edu.tr. ORCID Oktay Talaz:0000-0003-2209-5402 NotesThe authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSFinancial support from the Scientific and Technological Research Council of Turkey (TUBITAK, Grant No. KBAG-114Z196) is gratefully acknowledged.
■
REFERENCES(1) (a) Gossauer, A. In Methoden der Organischen Chemie (Houben-Weyl); Kreher, R. P., Ed.; Georg Thieme Verlag: Stuttgart, 1994; Vol. E6a, pp 556−798. (b) Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213−7256. (c) Guizzardi, B.; Mella, M.; Fagnomi, M.; Albini, A. Easy Photochemical Preparation of 2-Dimethylaminophenylfurans, -Pyr-roles and −Thiophenes. Tetrahedron 2000, 56, 9383−9389. (d) Tarasova, O. A.; Nedolya, N. A.; Vvedensky, V. Y.; Brandsma,
(5) Aguilar, A.; Zhou, H.; Chen, J.; Liu, L.; Bai, L.; McEachern, D.; Yang, C.-Y.; Meagher, J.; Stuckey, J.; Wang, S. A Potent and Highly Efficacious Bcl-2/Bcl-xL Inhibitor. J. Med. Chem. 2013, 56, 3048− 3067.
(6) Towle, T.; Chang, I.; Krens, R. J.; Bhanot, P. Chemical probes of a trisubstituted pyrrole to identify its protein target(s) in Plasmodium sporozoites. Bioorg. Med. Chem. Lett. 2013, 23, 1874−1877.
(7) (a) Rieth, R. D.; Mankad, N. P.; Calimano, E.; Sadighi, J. P. Palladium-Catalyzed Cross-Coupling of Pyrrole Anions with Aryl Chlorides, Bromides, and Iodides. Org. Lett. 2004, 6, 3981−3983. (b) Yang, S.-D.; Sun, C.-L.; Fang, Z.; Li, B.-J.; Li, Y.-Z.; Shi, Z.-J. Palladium-Catalyzed Direct Arylation of (Hetero)Arenes with Aryl Boronic Acids. Angew. Chem., Int. Ed. 2008, 47, 1473−1476. (c) Jafarpour, F.; Rahiminejadan, S.; Hazrati, H. Triethanolamine-Mediated Palladium-Catalyzed Regioselective C-2 Direct Arylation of Free NH-Pyrroles. J. Org. Chem. 2010, 75, 3109−3112. (d) Ueda, K.; Amaike, K.; Maceiczyk, M. R.; Itami, K.; Yamaguchi.β-Selective C−H Arylation of Pyrroles Leading to Concise Syntheses of Lamellarins C and I. J. Am. Chem. Soc. 2014, 136, 13226−13232. (e) Sollert, C.; Devaraj, K.; Orthaber, A.; Gates, P. J.; Pilarski, L. T. Ru-Catalysed C H Arylation of Indoles and Pyrroles with Boronic Acids: Scope and Mechanistic Studies. Chem.− Eur. J. 2015, 21, 5380−5386.
(8) (a) Zhao, L.; Bruneau, C.; Doucet, H. Palladium-Catalysed Direct Polyarylation of Pyrrole Derivatives. ChemCatChem 2013, 5, 255−262. (b) Roy, D.; Mom, S.; Beauprein, M.; Doucet, H.; Hierso, J.-C. A Versatile Palladium/Triphosphane System for Direct Arylation of Heteroarenes with Chloroarenes at Low Catalyst Loading. Angew. Chem., Int. Ed. 2010, 49, 6650−6654. (c) Jin, R.; Yuan, K.; Chatelain, E.; Soule, J.-F.; Doucet, H. Palladium-Catalysed Direct Desulfitative Arylation of Pyrroles using Benzenesulfonyl Chlorides as Alternative Coupling Partners. Adv. Synth. Catal. 2014, 356, 3831−3841. (d) Roger, J.; Doucet, H. Regioselective C-2 or C-5 Direct Arylation of Pyrroles with Aryl Bromides using a Ligand-Free Palladium Catalyst. Adv. Synth. Catal. 2009, 351, 1977−1990. (e) Fall, Y.; Doucet, H.; Santelli, M. Palladium-Catalysed Direct 3- or 4-Arylation of 2,5-Disubstituted Pyrrole Derivatives: An Economically and Environmentally Attractive Procedure. ChemSusChem 2009, 2, 153− 157.
(9) Wen, J.; Qin, S.; Ma, L.-F.; Dong, L.; Zhang, S.; Liu, S.-S.; Duan, Y.-S.; Chen, S.-Y.; Hu, C.-W.; Yu, X.-Q. Iron-Mediated Direct Suzuki-Miyaura Reaction: A New Method for the ortho-Arylation of Pyrrole and Pyridine. Org. Lett. 2010, 12, 2694−2697.
(10) (a) Vakuliuk, O.; Koszarna, B.; Gryko, D. T. Direct Arylation of Pyrrole Derivatives in Superbasic Media. Synthesis 2011, 17, 2833− 2837. (b) Vakuliuk, O.; Koszarna, B.; Gryko, D. T. Base-Mediated Direct Arylation of Pyrrole Derivatives. Adv. Synth. Catal. 2011, 353, 925−930. (c) Rybicka-Jasińska, K.; König, B.; Gryko, D. Porphyrin-Catalyzed Photochemical C−H Arylation of Heteroarenes. Eur. J. Org. Chem. 2017, 2017, 2104−2107.
(11) Wen, J.; Zhang, R.-Y.; Chen, S.-Y.; Zhang, J.; Yu, X.-Q. Direct Arylation of Arene and N-Heteroarenes with Diaryliodonium Salts without the Use of Transition Metal Catalyst. J. Org. Chem. 2012, 77, 766−771.
(14) (a) Chauhan, P.; Ravi, M.; Singh, S.; Raju, K. S. R.; Bajpai, V.; Kumar, B.; Yadav, W.; Yadav, P. P. Palladium and copper-catalyzed ligand-free coupling of phenylhydrazines in water. RSC Adv. 2014, 4, 43336−43340. (b) Demir, A. S.; Reis, Ö; Özgül-Karaaslan, E. Manganese(III) acetate-mediated oxidative coupling of phenyl-hydrazines with benzene: a novel method for biaryl coupling. J. Chem. Soc., Perkin Trans. 1 2001, 22, 3042−3045. (c) Demir, A. S.; Reis, Ö.; Emrullahoğlu, M. Manganese(III) acetate-mediated oxidative coupling of phenylhydrazines with furan and thiophene: a novel method for hetero biaryl coupling. Tetrahedron 2002, 58, 8055−8058. (d) Demir, A. S.; Findik, H. Potassium permanganate/carboxylic acid/ organic solvent: a powerful reagent for enone oxidation and aryl coupling reactions. Tetrahedron 2008, 64, 6196−6201.
(15) (a) Zhang, J.-Q.; Cao, J.; Li, W.; Li, S.-M.; Li, Y.-K.; Wang, J.-T.; Tang, L. Palladium/Copper-Catalyzed Arylation of Alkenes with N′-Acyl Arylhydrazines. New J. Chem. 2017, 41, 437−441. (b) Taniguchi, T.; Zaimoku, H.; Ishibashi, H. A Mild Oxidative Aryl Radical Addition into Alkenes by Aerobic Oxidation of Arylhydrazines. Chem.− Eur. J. 2011, 17, 4307−4312. (c) Jasch, H.; Scheumann, J.; Heinrich, M. R. Regioselective Radical Arylation of Anilines with Arylhydrazines. J. Org. Chem. 2012, 77, 10699−10706.
(16) (a) Hofmann, J.; Jasch, H.; Heinrich, M. R. Oxidative Radical Arylation of Anilines with Arylhydrazines and Dioxygen from Air. J. Org. Chem. 2014, 79, 2314−2320. (b) Li, Y.; Liu, W.; Kuang, C. Direct arylation of pyridines without the use of a transition metal catalyst. Chem. Commun. 2014, 50, 7124−7127. (c) Fehler, S. K.; Pratsch, G.; Heinrich, M. R. The Trapping of Phenyldiazenes in Cycloaddition Reactions. Angew. Chem., Int. Ed. 2014, 53, 11361−11365. (d) Huang, P. C.; Kosower, E. M. Properties of Phenyldiimide. J. Am. Chem. Soc. 1967, 89, 3910−3911. (e) Huang, P. C.; Kosower, E. M. Diazenes. 111. Properties of Phenyldiazene. J. Am. Chem. Soc. 1968, 90, 2367− 2376. (f) Kosower, E. M.; Huang, P.-K. C.; Tsuji, T. Diazenes. V. Aryldiazenes. J. Am. Chem. Soc. 1969, 91, 2325−2329. (g) Myers, A. G.; Movassaghi, M.; Zheng, B. Mechanistic Studies of the Free-Radical Fragmentation of Monoalkyl Diazenes. Tetrahedron Lett. 1997, 38, 6569−6572.
(17) Wang, G.-W.; Lu, Y.-M.; Chen, Z.-X. 1,4-Fullerenols C60ArOH: Synthesis and Functionalization. Org. Lett. 2009, 11, 1507−1510.
(18) Hofmann, J.; Jasch, H.; Heinrich, M. R. 1,4-Fullerenols C60ArOH: Synthesis and Functionalization. J. Org. Chem. 2014, 79, 2314−2320.
(19) Li, Y.; Liu, W.; Kaung, C. Direct arylation of pyridines without the use of a transition metal catalyst. Chem. Commun. 2014, 50, 7124− 7127.
(20) (a) Chauhan, P.; Ravi, M.; Singh, S.; Prajapati, P.; Yadav, P. P. Regioselective α-arylation of coumarins and 2-pyridones with phenylhydrazines under transition-metal-free condition. RSC Adv. 2016, 6, 109−118. (b) Ravi, M.; Chauhan, P.; Kant, R.; Shukla, S. K.; Yadav, P. P. Transition-metal-free C-3 arylation of quinoline-4-ones with arylhydrazines. J. Org. Chem. 2015, 80, 5369−5376.