Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropValorization of the antioxidant, enzyme inhibition and phytochemical
propensities of Berberis calliobotrys Bien. ex Koehne: A multifunctional
approach to probe for bioactive natural products
Saima Khan
a, Mamona Nazir
b, Hammad Saleem
c,d, Naheed Raiz
a, Muhammad Saleem
a,⁎,
Syed Muhammad Muneeb Anjum
d, Gokhan Zengin
e, Mahreen Mukhtar
a,
Muhammad Imran Tousif
f, Fawzi M. Mahomoodally
g, Nafees Ahemad
caDepartment of Chemistry, Baghdad-ul-Jadeed Campus, The Islamia University of Bahawalpur, 63100, Bahawalpur, Pakistan bDepartment of Chemistry, Government Sadiq Women College University, Bahawalpur, 63100, Bahawalpur, Pakistan
cSchool of Pharmacy, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia dInstitute of Pharmaceutical Sciences (IPS), University of Veterinary & Animal Sciences (UVAS), Lahore, 54000, Pakistan eSelcuk University, Science Faculty, Department of Biology, Konya, Turkey
fDepartment of Chemistry, Dera Ghazi Khan Campus, University of Education Lahore, 32200-Dera Ghazi Khan, Pakistan gDepartment of Health Sciences, Faculty of Science, University of Mauritius, Mauritius
A R T I C L E I N F O Keywords: UHPLC-MS analysis Antioxidant potential Enzyme inhibition Bioactive agents Extraction A B S T R A C T
Chemical composition and pharmacological effects of different extracts of Berberis calliobotrys Bien. ex Koehne were studied. The chemical profile of the methanol, ethyl acetate, n-butanol and aqueous extracts were estab-lished by determining their total phenolic and flavonoid contents, besides ultra-high performance liquid chro-matography mass spectrometry (UHPLC-MS) of methanol and ethyl acetate extract of Berberis calliobotrys. Antioxidant potential of the extracts was evaluated as inhibition of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid (ABTS) free radicals, by reducing power via ferric reducing antioxidant power (FRAP), by cupric reducing antioxidant capacity (CUPRAC), and by phosphomolybdenum and metal chelation assays. Enzyme inhibitory potential of the extracts was also tested against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), α-glucosidase, α-amylase and tyrosinase. The Bc-W (Berberis calliobotrys aqueous extract) and Bc-M (B. calliobotrys methanol extract) afforded highest phenolic contents with values of 94.34 and 77.70 mgGAE/g extract, respectively, while the Bc-E (Berberis calliobotrys ethyl acetate extract) contained highest flavonoid contents (11.11 mg RE/g extract). UHPLC-MS analysis of Bc-M and Bc-E showed the presence of known secondary metabolites of alkaloid, phenolic, flavonoid, and terpene classes. The Bc-W was the most active in the ABTS, CUPRAC, FRAP and phosphomolybdenum antioxidant assays, whereas for DPPH and metal chelation activities, Bc-M was found to be more potent. The Bc-E and Bc-B (Berberis calliobotrys n-butanol extract) were least active for antioxidant potential. All the extracts showed considerable cholinesterase inhibi-tion, whereas, Bc-E was most potent inhibitor of α-amylase, α-glucosidase and tyrosinase. It is concluded that B.
calliobotrys extracts contained variety of bioactive constituents; antioxidant and enzyme inhibitors thus can be
considered as a potential candidate for relevant industrial applications.
1. Introduction
Plant-based indigenous medicine systems have always been the mainstay treatment to assuage human suffering. Currently, about 70–80% population of developing countries depends on herbals and derived products for their primary health care because they are cheap and easily available with little or no side effects. The utilization of herbal
medicine is functional for curing many chronic, severe infections and non-communicable ailments because of their strong anticancer, anti-diabetic, antimalarial, and anti-inflammatory effects amongst others (Bhardwaj et al., 2018; Mollica et al., 2017a, Mollica et al., 2017b). Other studies revealed a positive relationship between the existence of bioactive phytochemicals in therapeutic herbs with health maintenance. Subsequently, current research on interesting phytochemicals has
https://doi.org/10.1016/j.indcrop.2019.111693
Received 6 July 2019; Received in revised form 4 August 2019; Accepted 16 August 2019
⁎Corresponding author.
E-mail address:m.saleem@iub.edu.pk(M. Saleem).
Available online 04 September 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
accentuated their distinctive biological potential including antioxidant, antidiabetic, antibacterial, and antiviral properties. Among variety of secondary metabolites, phenolic compounds have received appropriate attention and are possibly the most scrutinized class of natural products for pharmaceutical and dietary concerns owing to their antioxidant, anti-allergic, antimicrobial, and anti-inflammatory activities. Phenolic com-pounds possess radical scavenging and metal chelating potential which are the back support for the treatment and management of world-wide spreading diseases like cardiovascular disorders and cancer (Mocan et al., 2016). Therefore, during last couple of decades, globally pharmaceutical companies have intensive focus to increase the quality and standardize the rising business of herbal products and indigenous medicine. More-over energetic interest has been shown for standardization of these bo-tanical medications. Therefore, there is a pressing need to explore the biopharmaceutical potential of the floral biodiversity (Patwardhan et al., 2005). Medicinal plants are being scrutinized for therapeutic agents to control world-wide spreading diseases such as cancer, diabetes mellitus, and Alzheimer's disease (Bahadori et al., 2017).
The genus Berberis comprises ˜500 species widely growing in Europe, Asia, North-South America and Africa and is well-known in European and Indian traditional medicinal systems (Rahimi-Madiseh et al., 2017). The plants of this genus have been reported to exhibit bacterial, anti-pruritic, anti-arrhythmic, anti-pyretic, anti-inflammatory, anti-choli-nergic, laxative, anti-malarial, anti-leishmaniasis, anti-histaminic activ-ities, besides cures vomiting, nausea, heart diseases, block the action of acetylcholine, diabetes, jaundice, and sore throat. Similarly, the roots of many Berberis species possess strong antipyretic, diaphoretic, hepato-protective, and anti-cancerous effects (Khan et al., 2016; Kolar et al., 2010). Bereberine and berberamine are the main chemical constituents present in this genus which have been used as anti-hyperglycemic and anti-cancer agents (Imanshahidi and Hosseinzadeh, 2008). B. aristata is an important spinous shrub with yellow flowers and red to bluish black fruit found in mountainous region of India and Nepal, where a decoctions made from its leaves has been used against skin diseases, ear, eye urinary tract infection and menorrhagia. Its extracts have been reported to ex-hibit antibacterial, antiviral, antioxidant, antifungal and anti-diabetic activities. History reveals that the root extract of B. aristata is used for the treatment of wounds and ulcer infection, malaria and gastric disorder (Khan et al., 2016). B. lyceum powered root extract is applied for the treatment of fever, eye diseases, liver and kidney disorders, throat pain, and internal wounds (Shabbir et al., 2012). B. calliobotrys is widely dis-tributed in hilly areas of Pakistan, where the local community is using this plant to treat fever, pharyngitis, jaundice and backache. Methanolic extract of its roots exhibit analgesic, anti-inflammatory and antipyretic potential (Alamgeer et al., 2016). Crude methanolic extract of stem and branches possess antimicrobial and anticonvulsant properties. HPLC analysis of ethyl acetate extract highlights the presence of quercitin, gallic acid, trans-4- hydroxyl, 3-methoxy cinnamic acid, caffeic acid, chlorogenic acid, vanillic acid, and p-coumaric acid (Rasool et al., 2015). Literature studies showed the presence of dimeric aporphine benzyl isoquinoline-khyberine, pakistanamine, 1-O-methylpakistanine, pakista-nine, chitraline and kalashine in the root extract of B. calliobotrys (Srivastava et al., 2015). Keeping in view the medicinal importance of this genus, the present work was planned to evaluate the phytochemical composition, antioxidant potential and enzyme inhibition properties of different solvent extracts of B. calliobotrys. Chemical composition of the extracts was determined through the analysis of total bioactive contents and UHPLC-MS secondary metabolite composition. Various assays (DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum and metal chela-tion assays) were performed for evaluachela-tion of antioxidant potential of the extracts. Moreover, inhibitory potential against key enzymes involved in common ailments like diabetes (α-glucosidase and α-amylase), neuro-degenerative disorder (AChE and BChE) and skin problems (tyrosinase) were also estimated. Statistical studies were also performed to highlight possible interactions between the bioactive contents and tested biological assays.
2. Materials and methods
2.1. Plant material and extraction
Stem part of Berberis calliobotrys was collected in October 2013 from Ziarat Valley, Baluchistan, and was authenticated by Dr. R. B. Tareen, botanist in the Botany Department of University of Baluchistan, Quetta, Pakistan, where a voucher specimen number BC-10-BUH was deposited in the University herbarium for future reference. 15 Kg of the shade dried (20 days) plant material was chopped into small pieces and was extracted with methanol (20 L, thrice) at room temperature for one week. The solvent was evaporated using rotary evaporator to get a dark brown extract (105 g), which was then suspended in distilled water (1.0 L) and was extracted with ethyl acetate and n- butanol to get 25 and 20 g fractions, respectively, whereas, the amount of aqueous so-luble part was measured as 60 g.
2.2. Total bioactive contents and UHPLC-MS analysis
The total phenolics contents (TPC) was measured by already es-tablished method, Folin-Ciocalteu, while total flavonoid contents (TFC) were assessed using the AlCl3 assays (Slinkard and Singleton, 1977;
Zengin et al., 2016b). In these analyses, the phenolic and flavonoid contents are presented as equivalents of gallic acid (mgGAEs/g extract) and rutin (mgRE/g extract), respectively.
Secondary metabolic pictures of the extracts were scanned by RP-UHPLC-MS. UHPLC of Agilent 1290 Infinity LC system coupled to Agilent 6520 Accurate-Mass Q-TOF mass spectrometer with dual ESI source was used. Agilent Zorbax Eclipse XDB-C18 column with narrow bore 2.1 x 150 mm, 3.5 μm (P/N: 930990-902). Other Instrument spe-cification and working methodology of RP-UHPLC-MS was same as reported by (Saleem et al., 2018andKhan et al., 2019)
2.3. Antioxidant assays
Already established protocols (Grochowski et al., 2017; Zengin et al., 2018) to measure antioxidant activity of the extracts were fol-lowed. DPPH, ABTS, FRAP, CUPRAC and total antioxidant capacity (phosphomolybdenum assay) were expressed as trolox equivalent while metal chelating effects of the extracts were calculated by ethylenedia-minetetraacetic acid (EDTA) as reference.
2.4. Enzyme inhibition assays
For the enzyme inhibition potential of the extracts, different in-vitro assays were performed against AChE (electric ell acetylcholinesterase, Type-VI-S, EC 3.1.1.7), BChE (horse serum butyrylcholinesterase, EC 3.1.1.8), tyrosinase (from mushroom, EC 1.14.18.1), α-amylase (ex-porcine pancreas, EC 3.2.1.1) and α-glucosidase (from Saccharomyces cerevisiae, EC 3.2.1.20) as reported previously (Mollica et al., 2017a, Mollica et al., 2017b), where the enzyme inhibitory effects were eval-uated as equivalents of acarbose for α-amylase and α-glucosidase, ga-lantamine for AChE and BChE, and kojic acid for tyrosinase
2.5. Statistical analysis
All the experiments were performed in triplicates, while the results are presented as mean values. Statistical analysis was performed using one-way ANOVA and SPSS v. 17.0 program. The heat map and Pearson linear correlation was utilized to perceive any link between bioactive contents and studied biological activities. PC analysis was also carried out to observe variability of the tested extracts. The statistical proce-dures were performed by R software v. 3.5.1, and the statistics revealed significant p value i.e. < 0.05.
3. Results and discussion
3.1. Total bioactive contents and secondary metabolites
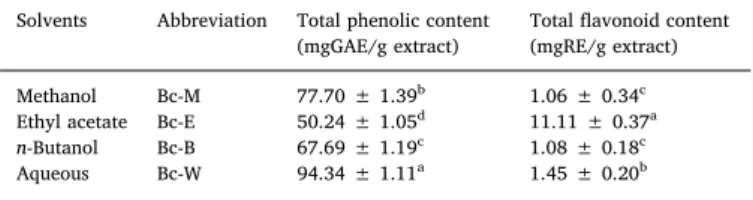
Phenolic and flavonoids are the bioactive phytochemicals having important roles for maintenance of overall human health (Lin et al., 2016). In the present study, the Bc-W showed high phenolic contents (94.34 mgGAE/g extract) followed by Bc-M (77.70 mgGAE/g extract), Bc-B (67.69 mgGAE/g extract), and Bc-E (50.24 mgGAE/g extract) as presented inTable 1. The high contents of flavonoids were found in Bc-E 11.11 mg RBc-E/g extract (Table 1), while for other extracts the contents ranges between 1.06–1.45 mgRE/g extract (Table 1).
UHPLC-MS analysis of Bc-M and Bc-E was carried out in negative ionization mode to have a look into the present secondary metabolite components. Total ion chromatograms of both the extracts with com-plex peak pattern are shown in Fig. 1. This analysis revealed 16 dif-ferent secondary metabolites in Bc-M extract as presented inTable 2; alkaloids were found as main constituents, while lignin, fatty acid and terpenoids were also observed. Identified alkaloids included obromine, 1,9-dimethyluric acid, citbismine C, aromoline, (S)-autumnaline, ber-bamunine, armepavine, aromoline and papaverine, besides iso-bergaptene (coumarin), 5,8,12-trihydroxy-9-octadecenoic acid (fatty acid), acanthoside D and (+)-syringaresinol-O-β-D-glucoside (Lignin) and (6S)-dehydrovomifoliol (terpene) were found in the extrcat. The presence of alkaloids, as major component in B. calliobotrys is in
accordance with other Berberies species (Srivastava et al., 2015). Bc-E analysis showed the existence of 18 different secondary metabolites (Table 3), where phenolics and flavonoids were found as main con-stituents. Among phenolics, four compounds were identified as p-sal-icylic acid, 3,4-dihydroxybenzoic acid, ferulic acid, 2-hydroxy-3,4 di-methoxybenzoic acid, whereas, the flavonoids were found as kaempferide, wightin, demethyltorosaflavone D, phellodensin D, meli-simplexin, agecorynin C, browniine, epigallocatechin 7-O-gallate. In addition to three terpenes (hydroxyisonobilin, neoglabrescin A and betulinic acid), one saponin (corchoroside B), one propiophenone (Kakuol) and one fatty ester (erythrinasinate A) were also identified. As per our knowledge, this is the first comprehensive study on chemical composition of B. calliobotrys extracts.
3.2. Antioxidant assays
An imbalance in production of oxidants and antioxidants causes oxidative stress that leads to improper body functioning. Oxidative stress initiates a series of destructive disease including atherosclerosis, rheumatoid arthritis, cancer, and neurodegenerative malfunctioning. Antioxidants are the compounds that have key role in the defense system against such widely spreading diseases (Zengin and Aktumsek, 2014). Comprehensive assessment of antioxidant potential of B. callio-botrys extract was made through DPPH and ABTS free radical scaven-ging, FRAP and CUPRAC reducing power, phosphomolybdenum and metal chelating assays; the results are presented in Table 4. Bc-M showed highest radical scavenging activity in DPPH (204.73 mgTE/g extract) assay, while the Bc-W exhibited highest antioxidant potential in ABTS (451.90 mg TE/g extract) assay.
CUPRAC and FRAP assays were used to measure reducing power by estimating their electron donating capacity, which is the best approach for evaluation of antioxidant activity of crude plant extracts (Zengin et al., 2016c). In CUPRAC assay cupric ion is reduced to cuprous while FRAP assay involves the reduction of ferric ions to ferrous ions in the presence of radical scavenging agent (Zengin et al., 2016a). Our results are parallel to ABTS assay supporting excellent activity of the Bc-W in both CUPRAC and FRAP assays with values of 778.98 and 718.48 mgTE/g extract, respectively. The total antioxidant capacity of the Table 1
Total phenolic and flavonoid contents of B. calliobotrys extracts*.
Solvents Abbreviation Total phenolic content
(mgGAE/g extract) Total flavonoid content(mgRE/g extract) Methanol Bc-M 77.70 ± 1.39b 1.06 ± 0.34c Ethyl acetate Bc-E 50.24 ± 1.05d 11.11 ± 0.37a
n-Butanol Bc-B 67.69 ± 1.19c 1.08 ± 0.18c
Aqueous Bc-W 94.34 ± 1.11a 1.45 ± 0.20b
* Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent. Different letters indicate differ-ences in the extracts (p < 0.05).
extracts was elucidated through phosphomolybdenum assay. In this method, the test compound (antioxidant) reduces Mo (IV) to Mo (V) resulting in the formation of Mo (V) green phosphate compounds which showed absorption maxima at 695 nm (Sarikurkcu et al., 2015). The phosphomolybdenum assay also revealed the highest activity for the Bc-W (2.04 mmolTE/g extract) while Bc-M exhibited the lowest po-tential (1.88 mmolTE/g extract). In metal chelating ability to further evaluate antioxidant potential iron–ferrozine complex was formed which showed red color with strong absorption observed at 562 nm.
Ferrous ion (Fe2+) plays important role in production of hydroxide
radical from peroxide like in fenton reaction which enmesh in the human cardiovascular diseases (Ebrahimzadeh et al., 2010). In this assay, the Bc-M was found more active (60.32 mgEDTAE/g extract) than Bc-W (36.44 mgEDTAE/g extract), might be because of non-phe-nolic chelator, antagonistic or synergetic action of phytochemicals (Marini et al., 2018). Our result showed linear correlation between bioactive constituents and antioxidant potential which are also sup-ported by Kouadri et al. (Kouadri et al., 2017) who resup-ported high Table 2
UHPLC-MS analysis of methanol extract of B. calliobotrys.
S. No RT(min) Base peak(m/z) Peak height AUC Proposed compounds Compound class Mol. formula DB
(ppm) Mol. mass
1. 0.62 179.05 292350 1330440 Theobromine Alkaloid C7H8N4O2 0.59 180.06
2. 0.624 215.03 681898 2124628 Isobergaptene Coumarin C12H8O4 8.83 216.04
3. 0.631 165.04 301308 964565 1-Methylxanthine Alkaloid C6H6N4O2 0.45 166.0
4. 0.632 195.05 135445 469761 1,9-Dimethyluric acid Alkaloid C7H8N4O3 0.95 196.05
5. 0.64 683.22 368932 2819487 Citbismine C Alkaloid C37H36N2O11 −4.05 684.23
6. 0.90 191.02 580742 2694188 Citric acid Fatty acid C6H8O7 −4.51 192.02
7. 7.82 593.26 70421 600931 Aromoline Alkaloid C36H38N2O6 −0.12 594.27 8. 7.86 372.18 61503 280744 (S)-Autumnaline Alkaloid C21H27NO5 −0.69 373.18 9. 8.21 595.28 189464 1483642 Berbamunine Alkaloid C36H40N2O6 −0.08 596.28 10. 8.26 312.16 150069 926471 Armepavine Alkaloid C19H23NO3 −2.23 313.16 11. 8.27 741.26 61334 708860 Acanthoside D Lignin C34H46O18 −2.24 742.27 12. 8.31 593.26 324753 2817485 Aromoline Alkaloid C36H38N2O6 −1.44 594.27
13. 9.12 579.20 322569 2143860 (+)-Syringaresinol O-beta-D-glucoside Lignin C28H36O13 −1.58 580.21
14. 9.48 338.14 312848 2398858 Papaverine Alkaloid C20H21NO4 1.75 339.14
15. 11.45 329.23 129225 1018956 5,8,12-trihydroxy-9-octadecenoic acid Fatty acid C18H34O5 −1.71 330.24
16. 12.33 221.11 76148 585072 (6S)-dehydrovomifoliol Terpene C13H18O3 0.04 222.12
RT: retention time; AUC: area under curve. Table 3
UHPLC-MS analysis of ethyl acetate extract of B. calliobotrys.
S.No RT(min) Base peak(m/z) Peak height AUC Proposed compounds Compound class Mol. formula DB
(ppm) Mol.mass
1 1.478 153.0192 22487 90608 3,4-Dihydroxybenzoic acid Phenol C7H6O4 1.45 154.0264
2 3.085 137.0245 34358 292148 p-Salicylic acid Phenol C7H6O3 −0.49 138.0318
3 8.095 193.0505 53525 194273 Kakuol Propiophenone C10H10O4 0.82 194.0577
4 8.201 193.0505 109241 438072 Ferulic acid Phenol C10H10O4 −0.16 194.0579
5 8.41 197.0455 48343 197418 2-Hydroxy-3,4-dimethoxybenzoic Acid Phenol C9H10O5 1.02 198.0526
6 9.103 361.1659 62848 241533 Hydroxyisonobilin Terpene C20H26O6 0.12 362.1729 7 9.388 431.1356 44075 187186 Agecorynin C Flavonoid C22H24O9 −1.65 432.1427 8 10.052 385.0937 26135 106111 Melisimplexin Flavonoid C20H18O8 −2.89 386.1013 9 10.205 449.1824 29095 122594 Neoglabrescin A Terpene C23H30O9 −0.46 450.1892 10 10.313 355.0447 27799 126517 Demethyltorosaflavone D Flavonoid C18H12O8 3.5 356.052 11 10.626 299.0565 37211 164743 Kaempferide Flavonoid C16H12O6 −1.48 300.0638 12 11.833 343.0831 182566 829314 Wightin Flavonoid C18H16O7 −2.41 344.0904
13 11.834 457.076 26137 127558 Epigallocatechin 7-O-gallate Flavonoid C22H18O11 3.47 458.0833
14 12.349 355.1196 61220 258942 Phellodensin D Flavonoid C20H20O6 −2.73 356.127
15 16.714 455.3528 47344 336630 Betulinic Acid Terpene C30H48O3 0.57 456.3601
16 18.202 585.4862 54451 397558 Erythrinasinate A Fatty ester C38H66O4 4.81 586.4933
17 19.502 466.2828 36583 239994 Browniine Alkaloid C25H41NO7 −3.92 467.2901
18 19.503 517.2814 869799 5182485 Corchoroside B Saponin C29H42O8 −1.07 518.2885
RT: retention time; AUC: area under curve.
Table 4
Antioxidant properties of B. calliobotrys extracts*.
Plant
extracts Radical scavenging assays Reducing power assays Total antioxidant activity Ferrous ion chelation DPPH (mgTE/g
extract) ABTS (mgTE/g extract) CUPRAC (mgTE/gextract) FRAP (mgTE/gextract) Phosphomolybdenum(mmolTE/g) Metal chelaing(mgEDTAE/g) Bc-M 204.73 ± 2.63a 342.25 ± 17.34b 744.72 ± 18.86a 566.20 ± 3.69b 1.88 ± 0.06b 60.32 ± 1.53a Bc-E 71.54 ± 0.91d 125.24 ± 4.45d 308.47 ± 13.53c 272.56 ± 4.39d 1.93 ± 0.05b 17.03 ± 0.61d Bc-B 95.93 ± 0.41c 177.76 ± 4.46c 523.30 ± 18.67b 410.57 ± 8.20c 2.01 ± 0.13a 27.36 ± 1.78c Bc-W 165.73 ± 9.75b 451.90 ± 26.90a 778.98 ± 8.38a 718.48 ± 9.47a 2.04 ± 0.08a 36.44 ± 0.57b
* Values expressed are means ± S.D. of three parallel measurements. TE: Trolox equivalent; EDTAE: EDTA equivalent. Different letters indicate differences in the extracts (p < 0.05).
amount of phenolic contents (10.23 mgEGA/g) in methanolic extract of B. vulgaris, and high percentage of DPPH inhibition (Boudjelthia et al., 2017). In addition, study of ethyl acetate extract of B. baluchistanica exhibited direct correlation between DPPH (IC50: 15.96 μg/mL) and
FRAP (360 TE μM/mL) with phenolic content (143.51 GAE mg/g) (Abbasi et al., 2013).
3.3. Enzyme inhibition potential
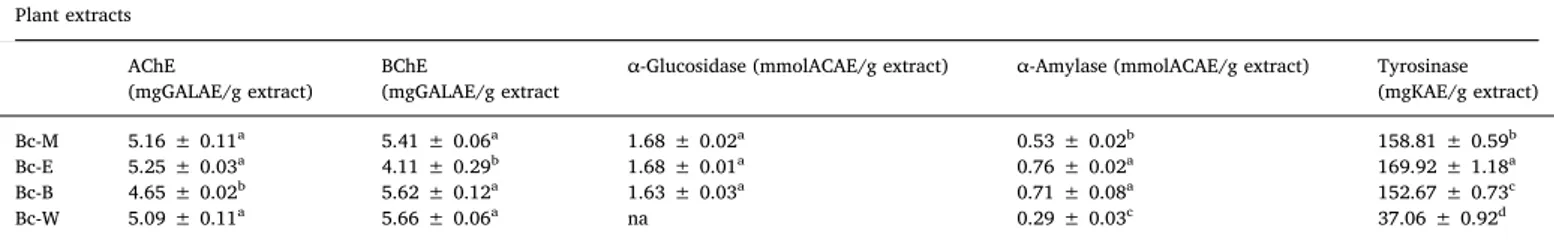
Inhibition of some enzymes that are involved in several body functions, is key strategy to control body improper functioning (Zengin et al., 2019a; Zengin et al., 2019b). The present work describes the enzyme inhibitory activity of B. calliobotrys extracts against AChE, BChE, α-glucosidase, α-amylase, and tyrosinase; the results are pre-sented inTable 5. Cholinesterases are the key enzymes responsible for severe mental and chronic disorders like Alzheimer's disease (AD) be-cause of its acetyl- and butyrylcholine hydrolyzing activity. Cholines-terase inhibitor controls this hydrolysis and increases the level of the acetylcholine and ultimately result into a better neurotransmission (Nisa et al., 2017). All the extracts of B. calliobotrys have shared capable and promising results in both aforesaid assays. Bc-E showed highest activity against AChE (5.25 mgGALAE/g extract) followed by Bc-M
(5.16 mgGALAE/g extract), Bc-W (5.09 mgGALAE/g extract) and Bc-B (4.65 mgGALAE/g extract). In BChE assay, Bc-W exhibited highest (5.66 mgGALAE/g extract) inhibition while Bc-E showed least anti-BChE potential with value of 4.11 mgGALAE/g extract. Literature re-view revealed that crude ethyl acetate bark extract of B. vulgaris was inactive in anti-AChE assay but was active against BChE with IC50value
of 64 μg/mL (Kolar et al., 2010), which also supports our results. Alpha-glucosidase is responsible for the disintegration of complex dietary carbohydrates and starch into monosaccharides resulting into increased blood sugar. Inhibition of α-glucosidase and α-amylase to control blood sugar is one of the challenging approach to prevent re-lated health problems (Pettit et al., 2005;Rasouli et al., 2017). Bc-E and Bc-M exhibited equal potential for α-glucosidase (1.68 mmolACAE/g extract) inhibitory activity while Bc-B showed comparable inhibition (1.63 mmolACAE/g extract), however, the Bc-W was inactive. In α-amylase inhibition assay, the pattern was observed as E > Bc-B > Bc-Bc-M > Bc-Bc-W with inhibition values of 0.76, 0.71, 0.53, 0.29 mmolACAE/g extract, respectively. Highest inhibition potential was recorded for Bc-E in both the α-amylase and α-glucosidase assays. B. aristata in powder form has also been reported potent antidiabetic agent in vivo studies that supported our results. Methanol extract of B. vulgaris also reported to be active in α-amylase assay with IC50 value of
Table 5
Enzyme inhibition activities of B. calliobotrys extracts*.
Plant extracts AChE
(mgGALAE/g extract) BChE(mgGALAE/g extract α-Glucosidase (mmolACAE/g extract) α-Amylase (mmolACAE/g extract) Tyrosinase(mgKAE/g extract)
Bc-M 5.16 ± 0.11a 5.41 ± 0.06a 1.68 ± 0.02a 0.53 ± 0.02b 158.81 ± 0.59b
Bc-E 5.25 ± 0.03a 4.11 ± 0.29b 1.68 ± 0.01a 0.76 ± 0.02a 169.92 ± 1.18a
Bc-B 4.65 ± 0.02b 5.62 ± 0.12a 1.63 ± 0.03a 0.71 ± 0.08a 152.67 ± 0.73c
Bc-W 5.09 ± 0.11a 5.66 ± 0.06a na 0.29 ± 0.03c 37.06 ± 0.92d
AChE: Acetylcholinesterase; BChE: Butyrylcholinesterase; GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active. Different letters indicate differences in the extracts (p < 0.05).
* Values expressed are means ± S.D. of three parallel measurements.
Fig. 2. Statistical evaluations, A: Correlation coefficients between total bioactive compounds and biological activities (Pearson Correlation Coefficient (R),
p < 0.05); B and D: Distribution of the tested extracts on the factorial plan and representation of biological activities on the correlation circle based on PCA; C: Heat
0.68 mg/mL (Boudjelthia et al., 2017).
Skin pigmentation disorder such as age spots and melasma are caused by accumulation of melanin. Biosynthesis of melanin is cata-lyzed by tyrosinase which is a copper-containing enzyme. Because of the adverse effects of already marketed synthetic anti-tyrosinase agents, there is a dire need to search for some safer tyrosinase inhibitors of natural origin (Sarikurkcu et al., 2015). The results of tyrosinase in-hibition activity of B. calliobotrys extracts showed that Bc-E exhibited highest inhibition i.e. 169.92 mgKAE/g extract. Bc-M and Bc-B dis-played anti-tyrosinase potential with values of 158.81 and 152.67 mgKAE/g extract, respectively while the Bc-W was found least active (37.06 mgKAE/g extract) in the above assay. Berberine isolated from B. vulgaris is the major constituent of the ‘genus Berberies, found to be active agent in tyrosinase inhibition assay (Zaidi et al., 2017). 3.4. Statistical evaluation
Two principal components as ˜82.1% of the total variability in PCA were found (Fig. 2); the variables pertaining to antioxidant and enzyme inhibition assays contribute mainly to the formation of axis 1 (61.8%) and axis 2 (20.3%) respectively. In under debate studies TPC (R = 0.77-0.99) and DPPH, ABTS, FRAP, CUPRAC offered a valuable relationship, which are in accordance with previous studies showing strong positive correlation between phenolics, radical scavenging and reducing po-tential assays (Llorent-Martínez et al., 2017). On the other hand, a moderate relationship was recorded between TPC (R = 0.33-0.58), metal chelating and phosphomolybdenum activities which may be justified with the antagonistic or synergetic effect of phytochemicals or presence of some non-phenolic chelators (Marini et al., 2018). Likewise, correlation between TPC (R = -0.07 to -0.94) in enzyme inhibitory assays was observed to be negative, except in BChE inhibitory assay, which exhibited positive correlation with TPC (R = 0.81). However, TFC exhibited positive correlation with enzyme inhibitions except AChE which suggests the presence of non-phenolic compounds mod-ulating the inhibitory activities; it is also substantiated the published reports (Zengin et al., 2019a;Zengin et al., 2019b).
4. Conclusion
This research work is the foremost detailed investigation into the phytochemical composition, antioxidant and enzyme inhibitory activ-ities of the extracts of B. calliobotrys plant. It is concluded that Bc-M is rich in phenolics, whereas Bc-E comprises the highest flavonoid con-tents. UHPLC-MS analysis of Bc-M and Bc-E revealed the presence of well-known secondary metabolites derived from various alkaloids, phenolics, flavonoids and terpenes. The plant extracts exhibited sig-nificant antioxidant key enzyme inhibitory potentials. Moreover, the Bc-M and Bc-W showed interesting antioxidant properties. This re-search presents meaningful baseline findings for further investigation geared towards the discovery of lead phyto-pharmaceuticals to manage human health and wellness.
Acknowledgments
Corresponding author acknowledges literature support from Mrs. Topsy Smalley.
References
Abbasi, M.A., Naqvi, S.S.H., Rehman, Ur.A., Tareen, R.B., Abbasi, M.A., 2013. Berberis
baluchistanica: assessment of natural antioxidants to reprieve from oxidative stress.
Int. Res. J. Pharm. 4, 101–105.
Alamgeer, N.H., Rasool, S., Raza, S.A., Ahmad, T., Ahsan, H., Mushtaq, M.N., Asif, H., Khan, Z., Noor, N., Utra, A., 2016. Anti-inflammatory, analgesic and antipyretic ac-tivities of the aqueous methanolic extract of Berberis calliobotrys in albino mice. Acta Pol. Pharm. 73, 717–723.
Bahadori, M.B., Asghari, B., Dinparast, L., Zengin, G., Sarikurkcu, C., Abbas-Mohammadi,
M., Bahadori, S., 2017. Salvia nemorosa L.: a novel source of bioactive agents with functional connections. LWT-Food Sci. Technol. 75, 42–50.
Bhardwaj, S., Verma, R., Gupta, J., 2018. Challenges and future prospects of herbal medicine. Int. Res. Med. Health Sci. 1, 12–15.
Boudjelthia, K., Hammadi, K., Kouidri, M., Djebli, N., 2017. Evaluation of antidiabetic activity of two plants Berberis vulgaris and Zygophyllum geslini. J. Phys. Chem. Biophys. 7 2161-0398.
Ebrahimzadeh, M.A., Nabavi, S.M., Nabavi, S.F., Bahramian, F., Bekhradnia, A.R., 2010. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius,
V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 23, 29–34.
Grochowski, D.M., Uysal, S., Aktumsek, A., Granica, S., Zengin, G., Ceylan, R., Locatelli, M., Tomczyk, M., 2017. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 20, 365–372.
Imanshahidi, M., Hosseinzadeh, H., 2008. Pharmacological and therapeutic effects of
Berberis vulgaris and its active constituent, berberine. Phytother. Res. 22, 999–1012.
Khan, I., Najeebullah, S., Ali, M., Shinwari, Z.K., 2016. Phytopharmacological and eth-nomedicinal uses of the genusBerberis (Berberidaceae): a review. Trop. J. Pharm. Res. 15, 2047–2057.
Khan, S., Nazir, M., Raiz, N., Saleem, M., Zengin, G., Fazal, G., Saleem, M., Mukhtar, M., Tousif, M.I., Tareen, R.B., Abdallah, H.H., Mahomoodally, F.M., 2019. Phytochemical profiling, in vitro biological properties and in silico studies onCaragana ambigua stocks (Fabaceae): a comprehensive approach. Ind. Crops Prod. 131, 117–124.
Kolar, D., Wimmerova, L., Kadek, R., 2010. Acetylcholinesterase and
butyr-ylcholinesterase inhibitory activities of Berberis vulgaris. Phytopharmacol. 1, 7–11.
Lin, D., Xiao, M., Zhao, J., Li, Z., Xing, B., Li, X., Kong, M., Li, L., Zhang, Q., Liu, Y., 2016. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21, 1374.
Llorent-Martínez, E., Ortega-Barrales, P., Zengin, G., Mocan, A., Simirgiotis, M., Ceylan, R., Uysal, S., Aktumsek, A., 2017. Evaluation of antioxidant potential, enzyme in-hibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: po-tential sources of bioactive compounds for the food industry. Food Chem. Toxicol. 107, 609–619.
Marini, G., Graikou, K., Zengin, G., Karikas, G.A., Gupta, M.P., Chinou, I., 2018. Phytochemical analysis and biological evaluation of three selected Cordiaspecies from Panama. Ind. Crops Prod. 120, 84–89.
Mocan, A., Zengin, G., Uysal, A., Gunes, E., Mollica, A., Degirmenci, N.S., Alpsoy, L., Aktumsek, A., 2016. Biological and chemical insights of Morina persica L.: a source of bioactive compounds with multifunctional properties. J. Funct. Foods 25, 94–109.
Mollica, A., Zengin, G., Locatelli, M., Stefanucci, A., Mocan, A., Macedonio, G., Carradori, S., Onaolapo, O., Onaolapo, A., Adegoke, J., 2017a. Anti-diabetic and anti-hyperli-pidemic properties of Capparis spinosa L.: in vivo and in vitro evaluation of its nu-traceutical potential. J. Funct. Foods 35, 32–42.
Mollica, A., Zengin, G., Locatelli, M., Stefanucci, A., Macedonio, G., Bellagamba, G., Onaolapo, O., Onaolapo, A., Azeez, F., Ayileka, A., 2017b. An assessment of the nutraceutical potential ofJuglans regia L. leaf powder in diabetic rats. Food Chem. Toxicol. 107, 554–564.
Nisa, M., Munawar, M.A., Iqbal, A., Ahmed, A., Ashraf, M., Qurra-tul-Ann, A.G., Khan, M.A., 2017. Synthesis of novel 5-(aroylhydrazinocarbonyl) escitalopram as choli-nesterase inhibitors. Eur. J. Med. Chem. 138, 396–406.
Patwardhan, B., Warude, D., Pushpangadan, P., Bhatt, N., 2005. Ayurveda and traditional Chinese medicine: a comparative overview. Evid. Based Complement. Altern. Med. 2, 465–473.
Pettit, R.K., Weber, C.A., Kean, M.J., Hoffmann, H., Pettit, G.R., Tan, R., Franks, K.S., Horton, M.L., 2005. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 49, 2612–2617.
Rahimi-Madiseh, M., Lorigoini, Z., Zamani-Gharaghoshi, H., Rafieian-Kopaei, M., 2017.
Berberis vulgaris: specifications and traditional uses. Iran. J. Basic Med. Sci. 20, 569.
Rasool, S., Khan, F.Z., ul Hassan, S., Ahmed, M., Ahmed, M., Tareen, R.B., 2015. Anticonvulsant, antimicrobial and cytotoxic activities of Berberis calliobotrys Aitch ex Koehne (Berberidaceae). Trop. J. Pharm. Res. 14, 2031–2039.
Rasouli, H., Hosseini-Ghazvini, S.M.-B., Adibi, H., Khodarahmi, R., 2017. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 8, 1942–1954.
Saleem, H., Htar, T.T., Naidu, R., Nawawi, N.S., Ahmad, I., Ashraf, M., Ahemad, N., 2018. Biological, chemical and toxicological perspectives on aerial and roots of Filago
ger-manica(L.) huds: Functional approaches for novel phyto-pharmaceuticals. Food
Chem. Toxicol. 123, 363–373.
Sarikurkcu, C., Zengin, G., Oskay, M., Uysal, S., Ceylan, R., Aktumsek, A., 2015. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two
Origanum vulgare subspecies (subsp. Vulgare and subsp. hirtum) essential oils. Ind.
Crops Prod. 70, 178–184.
Shabbir, A., Shahzad, M., Arfat, Y., Ali, L., Aziz, R.S., Murtaza, G., Waqar, S.A., 2012.
Berberis lycium Royle: A review of its traditional uses, phytochemistry and
pharma-cology. Afr. J. Pharm. Pharm. 6, 2346–2353.
Slinkard, K., Singleton, V.L., 1977. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28, 49–55.
Srivastava, S., Srivastava, M., Misra, A., Pandey, G., Rawat, A., 2015. A review on bio-logical and chemical diversity in Berberis (Berberidaceae). EXCLI J. 14, 247.
Zaidi, K., Ali, S., Ali, A., Thawani, V., 2017. Natural melanogenesis stimulator a potential tool for the treatment of hypopigmentation disease. Int J Mol Biol. 2 00012.
Zengin, G., Aktumsek, A., 2014. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: an endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 11, 481–488.
Zengin, G., Atasagun, B., Aumeeruddy, M.Z., Saleem, H., Mollica, A., Bahadori, M.B., Mahomoodally, M.F., 2019a. Phenolic profiling and in vitro biological properties of
two Lamiaceae species (Salvia modesta and Thymus argaeus): a comprehensive eva-luation. Ind. Crops Prod. 128, 308–314.
Zengin, G., Locatelli, M., Ceylan, R., Aktumsek, A., 2016a. Anthraquinone profile, anti-oxidant and enzyme inhibitory effect of root extracts of eight Asphodeline taxa from Turkey: can Asphodeline roots be considered as a new source of natural compounds? J. Enzyme Inhib. Med. Chem. 31, 754–759.
Zengin, G., Senkardes, I., Mollica, A., Picot-Allain, C.M.N., Bulut, G., Dogan, A., Mahomoodally, M.F., 2018. New insights into the in vitro biological effects, in silico docking and chemical profile of clary sage–Salvia sclarea L. Comput. Biol. Chem. 75, 111–119.
Zengin, G., Nithiyanantham, S., Locatelli, M., Ceylan, R., Uysal, S., Aktumsek, A., Selvi, P.K., Maskovic, P., 2016b. Screening of in vitro antioxidant and enzyme inhibitory
activities of different extracts from two uninvestigated wild plants: centranthus
long-iflorus subsp. Longlong-iflorus and Cerintheminor subsp. Auriculata. Eur. J. Integr. Med. 8,
286–292.
Zengin, G., Sarıkürkçü, C., Aktümsek, A., Ceylan, R., 2016c. Antioxidant potential and inhibition of key enzymes linked to Alzheimer’s diseases and diabetes mellitus by monoterpene-rich essential oil from Sideritis galaticaBornm. Endemic to Turkey. Rec. Nat. Prod. 10, 195–206.
Zengin, G., Stefanucci, A., Rodrigues, M.J., Mollica, A., Custodio, L., Aumeeruddy, M.Z., Mahomoodally, M.F., 2019b. Scrophularia lucida L. As a valuable source of bioactive compounds for pharmaceutical applications: in vitro antioxidant, anti-inflammatory, enzyme inhibitory properties, in silico studies, and HPLC profiles. J. Pharm. Biomed. Anal. 162, 225–233.