ELSEVIER
Journal of
Journal of Molecular Structure 349 (1995) 243-246
MOLECULAR STRUCTURE A PHOTOELECTRON SPECTROSCOPIC INVESTIGATION OF
CONDUCTING POLYPYRROLE-POLYAMIDE COMPOSITE FILM
Sefrk Suzer, Bilkent University, Dept. of Chemistry, 06533 Ankara, Turkey, Levent Toppare, Middle East Technical University, Dept. of Chemistry, 06531, Ankara, Turkey
and
Geoffrey C. Allen and Keith R. Hallam, University of Bristol, Interface Analysis Center, Bristol BS2 8BS, England
Abstract
X-ray photoelectron spectrum of the electrochemically prepared polypyrrole and polypyrrole-polyamide composite films exhibit an additional strong high binding energy peak at 402.0 eV corresponding to N+ moieties. Intensity of this peak is significantly reduced upon electrochemical reduction. Atomic concentrations derived from the observed N+ and F (stemming from the dopant BF4-) peaks reveal a slightly higher cation/anion ratio for this composite and suggest that the composite has a different chemical composition than the corresponding polymers.
Introduction
Of the many conducting polymers polypyrrole is the most studied one’. Use of electron spectroscopic methods for various structural determinations of conducting polymers has been reviewed recently2g3. Chemically or electrochemically prepared polypyrrole has poor mechanical properties’, and various physical and/or chemical methods have been utilized to improve these properties. Blending or making composites with other polymers having better mechanical properties have been successful for many applications415. Polyamide (PA) has proved to be especially successful. In our previous studies we have shown that electrochemically prepared blends of polypyrrole-polyamide composite films have better mechanical and/or electrical properties 6. In this contribution we report an x-ray photoelectron spectroscopic investigation of these films.
0022-2860/95/$09.50 Q 1995 Elsevier Science B.V. All rights reserved SSDI 0022-2860(95)08754-O
244
Experimental
PPy/PA composites were prepared by electrochemical polymerization of pyrrole onto a polyamide coated electrode at a constant predetermined potential. The polyamide films were dipcoated from chloroform solution of a concentrated polyamide resin (Aldrich Co. 19, 101-9). The potentiostatic polymerization was carried out in a three compartment ceil equipped with Pt foils (1 .O cm* each) as working (at +I .I V) and counter electrodes and a capillary reference electrode (Ag/Ag+). The solvent-electrolyte couple was acetonitrile -tetrabutylammonium tetrafluoroborate. Films having only the polypyrrole and only polyamide have also been prepared using the same procedure for comparison. Electrochemical reduction was achieved by reversing the polarity and discharging the prepared composite in the cell. X-ray photoelectron spectra of the various films are recorded after washing and drying the films while intact on their platinum electrodes. The spectrometer used is a Kratos ES300 using AlKa source.
Results and Discussion
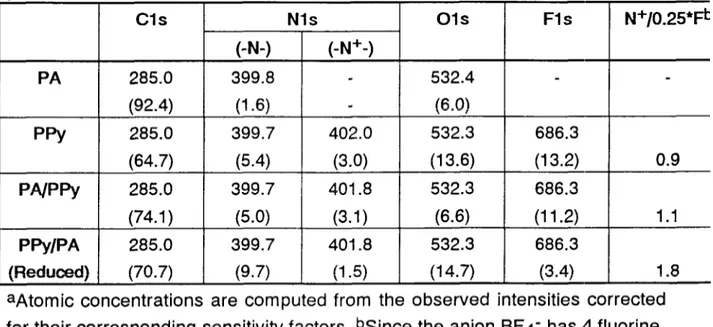
Figure 1 depicts the spectrum of the polyamide (PA), polypyrrole (PPy), and polyamide-polypyrrole composite film as prepared (PA/PPy) and after electrochemical reduction (PA/PPy reduced). The main features are due to F Is peak of the dopant anion (tetrafluoroborate ion), 0 Is, N Is and C Is. On the side of the figure detailed N Is regions of the spectra are also included. Except for the polyamide this region displays more than one component which are deconvoluted into two main peaks. The peak at 399.7 eV is assigned to the neutral polypyrrole and/or polyamide nitrogen and the higher binding energy peak at ca. 402.0 eV is assigned to nitrogen carrying positive charge, in agreement with reported data 11~37-9. Besides the binding energies, atomic concentrations have also been computed using’ peak intensities corrected for the corresponding sensitivity factors and are given in Table I.
The high binding energy component assigned to N+ moieties have been reported to correlate with electronic conductivity of the polypyrrole9, hence it is logical to expect a linear correlation of the intensity of this peak with the corresponding dopant peaks i.e. F Is. The last column of the table gives the
245
N+ to F atomic ratios divided by 4 to account for the stoichiometry (there are 4
F atoms for every anion, BF4-). This ratio is 0.9 for polypyrrole and 1 .l for the
composite, however it increases to 1.8 after electrochemical reduction of the
composite. The oxygen concentration of the composite is also considerably
less which may be an indication of the enhanced stability of the film in air. It is
clear,
however,
that the composite
is chemically
different
from
its
components as was postulated in our earlier study’ where the difference was
confirmed using pyrolysis FTIR and mass spectrometric results.
PA/PPY
3ooo. (Reduced&
c1’
moo. Fls
INls
looo~-
PAIPPY
-z
OI b4Oco 3QOO~PA
01 700 600 500 400 3Binding Energy (eV)
-+Qb!i_
i
f
5.. 405 400i
..*
A
4d5 400 L.. Figure 1.Al Ka x-ray
photoelectron spectra of
polyamide-only (PA),
polypyrrole-only (PPY),
polypyrrole-polyamide
(PA/PPY) composite film as
prepared (PA/PPY), and after
electrochemical reduction
(PA/PPY Reduced). The N Is
region for each polyrner is
given on the side. For
polypyrrole and the
composite, N Is region
displays more than one
component which is
deconvoluted into two
gaussian peaks. The first one
at 399.7 eV is assigned to
nitrogen in neutral polypyrrole
and the second one at ca.
402.0 eV is assigned to
nitrogen carrying positive
charge.
246
Acknowledgments: This
work is partially supported by TUBITAK, through the
project TBAG-U/15-7. The authors are thankful to Fatma Selamplnar for the
help in preparation of the samples.
References
1-
G.B.Street,
Handbook of Con. PO&. 1, Chapter8(1984) 1281.
2-
W.R.Salaneck, Rep.
Prog. Pbys.54(1991) 1215.
3- E.T. Kang, K.G. Neoh and K.L. Tan, A&
PO&m. ScilOS(1993) 135.
4- M. De Paoli, R.J. Waltmann, A.F. Diaz and J. Bargon, J.
Chem. Sac.,Chem. Commun. 1015(1984).
5-
H.L. Wang, L. Toppare, J.E. Fernandez,
Macromo/ecu/es23(1990)1053.
6- F. Selamplnar, U. Akbulut, T. Yalcin, S. Suzer and L. Toppare,
Syntb. Met.62 (1994) 201.
7-
P. Pfluger and G.B. Street, J.
Chem. Phys. 80(1984) 544.8-