New therapeutic system based on hydrogels for vaginal candidiasis
management: formulation-characterization, antibacterial activity,
vaginal irritation and direct contact test
Muhammet Davut Arpa1, A eg l Y l a 2, Ecehan Onay Tarlan3, Cem e ahi e 4, Hande Sipahi4, Ahme A d 4, Ne liha da Ok 5*
1
Istanbul Medipol University, School of Pharmacy, Department of Pharmaceutical Technology, 34085, Istanbul, TURKEY
2Ege University, Faculty of Science, Fundamental and Industrial Microbiology Division,
Department of Biology, 35040, Izmir, Turkey
3Ege University, Faculty of Pharmacy, Department of Pharmaceutical Technology, 35040,
Izmir, Turkey
4Yeditepe University, Faculty of Pharmacy, Department of Pharmaceutical Toxicology,
34755, Istanbul, Turkey
5University of Health Sciences, Faculty of Pharmacy, Department of Pharmaceutical
Technology, 34668, Istanbul, Turkey
*Address Correspondence: Neslihan st nda Okur. University of Health Sciences, Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul, Turkey.
New therapeutic system based on hydrogels for vaginal candidiasis
management: formulation-characterization, antibacterial activity,
vaginal irritation and direct contact test
Abstract
The objective of the present research was to examine the possible usage of terbinafine loaded hydrogels for vaginal application as part of vaginal candidiasis treatment. Vaginal candidiasis belongs to the most frequent gynecological disorders. Various antifungal drugs are used for its treatment, with Terbinafine being one of them. In this study, new gel formulations were prepared for Terbinafine vaginal delivery. Natural polymers such as chitosan, sodium carboxymethylcellulose and Carbopol were used for the development of Terbinafine vaginal gels. The developed gels were examined for their viscosity and spreadability, pH and mechanical properties. The most optimal formulations were further evaluated for their in vitro release behavior and antifungal activities. In further, the cytotoxicity and irritation inducing capacity of optimum gel formulations were evaluated. In vitro drug release studies demonstrated that terbinafine release was prolonged whereas anti-candida activity in several species showed the superiority of the gels compared to the marketed product. G-5 and G-8 gels did not cause lysis, hemorrhage and coagulation therefore classified as non-irritant. The optimal formulations were also studied for their stability, demonstrating that they were stable for 3 months.
Keywords: vaginal delivery; terbinafine; hydrogels; HET-CAM; chitosan; Carbopol;
sodium carboxymethylcellulose
1. INTRODUCTION
In the last two decades, more than one billion people per year have been infected with fungal diseases with an increase in the incidence of fungal infections (Siafaka, st nda Okur, et al. 2016; Park et al. 2017; Sa ant & Khan 2017; st nda Okur, Yo gatl , et al. 2019). Fungal infections can be either local or systemic, but the most common fungal infections are oral and vaginal candidiasis (Voltan et al. 2016; Park et al. 2017). More than twenty different species of Candida have been reported as human pathogens like Candida albicans, Candida glabrata, Candida parapsilosis,
Candida tropicalis, Candida infections(Mendes Giannini et al. 2013; Theill et al.
2016). Cancer chemotherapy, immunosuppressive therapy and transplantation have increased the risk of Candida infections (Siafaka, st nda Okur, et al. 2016; st nda Okur et al. 2018). Candida albicans, which is the most studied of all
Candida species, is the causative fungal agent in 60% 80% of Candida infections
(Theill et al. 2016).
Vaginal candidiasis belongs to the most frequent Candida infections in females. It has been reported that almost 75% of women would present this disease at least once during their life (Salehei et al. 2012; van Schalkwyk et al. 2015). The vaginal cavity has significant advantages for the administration of the drug both locally and systemically (Vermani & Garg 2000; Pa eli et al. 2001; Hussain & Ahsan 2005). The main advantages of vaginal drug delivery are its large surface area, rich blood supply, the avoidance of hepatic first pass metabolism, high permeability for low molecular weight drugs and self-insertion (Vermani & Garg 2000; Hussain & Ahsan 2005; Cook & Brown 2018). Several drug delivery systems are used for the treatment of vaginal infections such as semi-solids, tablets, capsules, pessaries, liquid preparations, vaginal films, vaginal rings, foams, tampons and douches. The most widely used semi-solid preparations for vaginal drug administration are creams, ointments, and gels (Vermani & Garg 2000; das Neves & Bahia 2006; Abruzzo et al. 2013; st nda Okur et al. 2020). The main advantages of gel formulations are acceptability, feasibility, and low cost (das Neves & Bahia 2006). Moreover, gels are semisolid formulations, with water base (hydrogels), or organic liquid base (organogels) (Korkmaz et al. 2013; Rakesh et al. 2014; Bassi & Kaur 2015; Langasco et al. 2016; Tunca Tanr erdi et al. 2018). Hydrogels also possess a degree of
flexibility very similar to natural tissue, due to their significant water content (Ibrahim et al. 2013).
Topical delivery of drugs is very challenging since the active molecules should remain in the application site for efficient time to provide the desired effect in the vaginal area (Chang et al. 2002; Özer et al. 2007). Consequently, various polymers have been applied for gels preparation to ensure this desired effect (Korkmaz et al. 2013; Bassi & Kaur 2015; Langasco et al. 2016; Tunca Tanr erdi et al. 2018). In this study, three different polymers were used for the preparation of hydrogels (sodium carboxymethyl cellulose, chitosan and Carbopol). These polymers are hydrophilic, biocompatible and biodegradable (Kadajji & Betageri 2011; Kumar et al. 2014; Filippousi et al. 2015; st nda Okur, H kenek, et al. 2019).
Sodium carboxymethyl cellulose (Na-CMC), a cellulose derivative, is a low-cost, water-soluble polymer (Kumar et al. 2014). This semi-synthetic polymer is often included in topical formulations (Gajdo o et al. 2016). Carbopol is a cross-linked polymer of polyacrylic acid with allyl sucrose or allyl penta erythritol (Asane et al. 2008). This water-soluble high molecular weight polymer has the ability to form good gelation (Mundhe et al. 2015; Russo et al. 2016; st nda Okur, H kenek, et al. 2019). Chitosan, is a natural substance derived from partial deacetylation of chitin; in fact, is a polysaccharide with various molecular weights, biocompatibility, biodegradability, and non-toxicity (Caramella et al. 2010). Given that chitosan can be dissolved only in an aqueous acidic medium, various chitosan derivatives are developed, to improve its hydrophilicity (Filippousi et al. 2015; Siafaka, Zisi, et al. 2016; Siafaka, Mone, et al. 2016).
Terbinafine hydrochloride (poorly water soluble) is a fungicidal allylamine antifungal drug (molecular formula C21H16ClN, molecular weight 327.9 g/mol), applied for
ocular and dermal fungal infections as well as vaginal candidiasis (Kyle & Dahl 2004; Kumar et al. 2008; Özcan et al. 2009; Gianni 2010; Mahmoudabadi et al. 2013). Terbinafine, which is used both orally and topically, has a broad-spectrum antifungal effect against a variety of yeast species, especially Candida (Kyle & Dahl 2004; Ahmed et al. 2007).
The Hen s Egg Test-Chorioallantoic Membrane (HET-CAM) assay is an in vitro alternative test to in vivo Draize Rabbit Eye test that mimics vascular changes in
CAM. HET-CAM assay is recommended by the Interagency Coordinating Committee on the validation of alternative methods (ICCVAM) and preferred for the evaluation of eye injury hazard potential of chemicals (Sipahi et al. 2019). According to the International Organization for Standardization (ISO) on the biological evaluation of medical devices for irritation, any material that is irritating to the skin or eyes should be identified as a potential irritant for vaginal tissue without further testing on the vagina tissue (ISO 10993-10). Therefore, the HET-CAM assay was also preferred for the assessment of vaginal irritation potential of substances (Palmeira-de-Oliveira et al. 2018).
The purpose of this work was the preparation and characterization of potential terbinafine hydrochloride loaded vaginal hydrogels prepared by Na-CMC, Carbopol and chitosan. Consequently, the physicochemical characterization, in vitro release behavior, stability studies and antimycotic effects of the hydrogels were evaluated. In this study, we also aimed to evaluate the cytotoxicity and irritation inducing potential of optimum gel formulations by testing them in vitro.
2. MATERIALS AND METHODS 2.1. Materials
Terbinafine hydrochloride was kindly gifted from Amino Chemicals Limited (Marsa, Malta). Carbopol 940 and sodium carboxymethyl cellulose (Na-CMC), triethanolamine was purchased from Doga Ilac, Turkey. Chitosan (1048 cP) was kindly gifted from Primex, Iceland. Acetic acid, sodium hydrogen phosphate, potassium hydrogen phosphate, glycerin, methanol, isopropanol, acetonitrile, and phosphoric acid were purchased from Sigma, USA. Triethylamine was purchased from Merck, Germany. The used dialysis membrane presents the following characteristics: Spectra/por, Mw= 12-14 kDa and it was purchased from Spectrum,
USA. Throughout the study, the distilled aqueous medium was applied whereas the other chemical reagents were of analytical grade. DMEM was purchased from Gibco, NY, USA.
For microbiological studies, Mueller-Hinton II Agar (70191) and glucose (G8270) were purchased from Sigma-Aldrich Co. and methylene blue dye was purchased from SSI Diagnostica, Hillerød, Denmark. Type cultures of several Candida species were purchased from ATCC, USA.
2.2. Preparation of vaginal gels
The preparation of vaginal gel formulations was conducted using different concentrations (1%, 3%, 6% w/w) of the three polymers, chitosan (1048 cP), Carbopol 940 and sodium carboxymethyl cellulose (Na-CMC) are utilized. For the preparation of chitosan solution, glacial acetic acid was used as a solvent. Chitosan and glacial acetic acid were dissolved in a part of distilled water. Then, glycerin added to the beaker. In further, benzalkonium chloride was dissolved in distilled water. These mixtures and the remaining water were added to the beaker and mixed on the magnetic stirrer for 24 hours.
Na-CMC formulations were prepared using phosphate buffer. The preparation of phosphate buffer (pH 4) was done using 5.04 g disodium hydrogen phosphate and 3.01 g potassium dihydrogen phosphate. Then they were dissolved in a sufficient amount of distilled water and completed to 1000 mL with distilled water (Mundhey et al. 2015). The phosphate buffer was used instead of water because the appropriate pH for vaginal delivery is 4 5.
To prepare the Carbopol formulations, Carbopol was added to triethanolamine (gelling agent) and pH was adjusted in the last stage. The ingredients and ratios of formulations were shown in Table 1.
2.3. Characterization of vaginal hydrogels
To determine the ideal formulations for vaginal delivery the characteristic properties such as pH, viscosity, spreadability and drug content uniformity were evaluated.
2.3.1 pH of hydrogels
The pHs of the hydrogel formulations were measured by a pH-meter (Mettler Toledo, Switzerland). The electrode was inserted into the hydrogel and constant value was noted. The measurements were repeated three ties at 25 °C (Okur et al. 2019;
st nda Okur, Yo gatl , et al. 2019).
2.3.2 Viscosity
The viscosities of hydrogel formulations were used using Brookfield RV-10 viscometer at 25 °C (Brookfield, USA) (Okur et al. 2019; st nda Okur, Yo gatl , et al. 2019).
2.3.3 Spreadability
The spreadability of gels was evaluated using 1 g of gel which was poured into the central point of a glass plate (20x20 cm2). Then, another glass plate with the same size was put upon the plate for compressing. After one minute the diameter of the spread area was measured. The measurement was performed in triplicate (Okur et al. 2019;
st nda Okur, Yo gatl , et al. 2019).
2.3.4 Texture Profile Analysis (TPA) of Hydrogels
The mechanical properties of hydrogels formulations were evaluated via a software-controlled penetrometer (TA.XT.Plus C, Haslemere, Surry, UK) equipped with a 5 kg load cell. Each formulation (50 g) was transferred to a 100 mL plastic beaker and left in an ultrasonic bath for about one hour to remove air bubbles. In the study using Perspex probe of 10 mm diameter (SNSP/10, θ: 10 mm); the test was carried out at pretest speed of 2 mm/s, posttest peed of 2 mm/s, test speed of 2 mm/s, trigger force of 0.001 N, compression depth to the gel in each operation of 10 cm, delay period between two compression compressions of 10 s. Hardness, compressibility, adhesiveness, cohesiveness, elasticity etc. mechanical properties of all hydrogels were calculated using the Texture Exponent Connect 7.0.6.0 software package (Cevher et al. 2008; en i it et al. 2014). All experiments were repeated three times at 25 ± 0.5°C.
Hardness is defined, as the force required to ensure the deformation of the gel and is the first peak of the curve. Compressibility is expressed as the work required deforming the gel and is calculated from the area under the curve in the first compression process. Adhesiveness is defined as the work required to get over the attractive forces between the surface of the gel and the surface of the probe. Cohesiveness is defined as the ratio of the area under the curve value in the second compression to the area under the curve value in the first compression. Elasticity is expressed as the rate at which the deformed gel returns to its initial state after removal of the deformation force (Ce her et al. 2008; en i it et al. 2014; Chandra &
2.3.5 FT-IR spectroscopy
ATR FT-IR Spectrometry was used to examine the spectrum of polymers, terbinafine HCl and ideal formulations, to show that substances are compatible with each other. The spectrums were analyzed at 4 cm-1 spectral resolution in the frequency range of 4.000 400 cm-1 using ATR-FTIR Spectrometer (Perkin Elmer, Spectrum 100 FT-IR Spectrometer, USA). The peak position was determined using Perkin Elmer Spectrum Version 6.0.2 Software.
2.3.6 Drug content uniformity
Terbinafine hydrochloride (1% w/w) was loaded to the gel formulations. Firstly, the homogeneity of hydrogel formulations was investigated visually. The sample was taken from different points of the hydrogels. 0.250 g of the hydrogel was accurately weighed and placed in a 100 mL volumetric flask. The hydrogels were dissolved in 50 mL methanol and diluted to 100 mL with methanol (diluted with 100 mL methanol or diluted to 100 mL?). Then the sample filtered through a 0.45 m membrane filter. The UV spectrum of terbinafine hydrochloride in methanol was screened between 190 and 800 nm. The max was determined at 224 nm (Shimadzu UV 1800, Japan). Then
0.5 mL sample was analyzed at 224 nm by using the HPLC system (Agilent 1100, USA). Studies were performed in triplicate for all the hydrogels.
2.4. HPLC assay
High performance liquid chromatography (HPLC) system comprised of the thermostable column department, a gradient pump, and a UV detector provided by Agilent 1100. The used column as the C18 column (5 m, 150 4.6mm; C18, GL. Sciences, Japan). The column compartment was temperature controlled and UV detector was employed throughout the analysis.
The mobile phase consists of methanol and acetonitrile (v/v 60:40) with 0.15% phosphoric acid and 0.15% triethylamine. The flow rate was used at 0.4 mL/min and the volume of injection as adjusted 10 L (Alberti et al. 2001; Vejnovic et al. 2010). A stock solution of terbinafine hydrochloride was prepared by dissolving 10 mg of drug in 100 mL methanol. Drug concentrations in the working solution chosen for the
calibration curves were 1 30 g/mL. Qualit control (QC) samples at 17, 20 and 30 ppm were prepared in the same ways as the calibration standards.
The method was fully validated according to the International Conference on Harmonization guidelines by determination of linearity, precision, accuracy, limit of detection (LOD) and limit of quantification (LOQ). The linearity of the method was tested in the range of 1-30 g/mL terbinafine h drochloride solution.
2.5. Evaluation of in vitro release and release kinetics
For the in vitro release experiments, 5 g gel formulation added into the dialysis membrane. As the release medium, the phosphate buffer (pH=4) was chosen. Sink conditions were maintained. The system was held at 37±2 °C and stirred continuously with a stirrer at 100 rpm (IKA RT15 power, Germany). Afterward, 0.5 mL of the media was retracted at prearranged periods (0.5 to 8 h and 24 h) and the volume was replaced with fresh buffer. Then, the analysis of the samples was performed with the HPLC system at 224 nm. The % drug release was calculated using the calibration curve of the drug. The experiment was carried out three times. In further, the in vitro release data were analyzed using a computer based kinetic program to understand the release profile and mechanism. Various kinetic models such as zero order, first order, Higuchi and Hixson Crowell were used for evaluation and determination of the release mechanism. In addition, to confirm the kinetics of drug release, data were also analyzed using the Korsemeyers-Peppas equation ( st nda -Okur et al. 2014).
2.6. Stability of vaginal gels
The gel formulations (G5, G7, G8) were added in bottles and put in storage at 5 ± 1°C in the refrigerator and 25±2°C for three months. After their storage for three months, the gels were assessed for their physical appearance. Moreover, pH and drug content were measured. The experiment was performed triplicate. The gels, which showed desirable stability, were chosen for further analysis (Kyle & Dahl 2004; Kumar et al. 2008; Özcan et al. 2009; Gianni 2010; Mahmoudabadi et al. 2013).
2.7. Antifungal activity of vaginal gels
The anti-candidal activity of vaginal gels was determined against Candida albicans ATCC 10231, Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019 and
Candida glabrata ATCC 90030 by well diffusion method ( en i it et al. 2014;
Siafaka, st nda Okur, et al. 2016; st nda Okur et al. 2018; st nda Okur,
Hökenek, et al. 2019). The study was completed based on the standardized protocol CLSI.
Mueller-Hinton II Agar (Sigma Aldrich) supplemented with 2% glucose (Sigma Aldrich) and 0.5 g/mL meth lene blue d e (SSI Diagnostica, Hiller d, Denmark) was used as suggested in CLSI document M44-A for yeasts. Fresh cultures of the microorganisms were suspended to get an inoculum with a 0.5 McFarland standard. 100 L of each suspension as spread evenly onto Mueller-Hinton II Agar supplemented with glucose and methylene dye using a spatula and allowed to dry. Wells (diameter=9 mm) drilled with a sterile corkborer and these wells were filled with the vaginal gels G5, G8 and a commercially sold terbinafine cream (Lamisil, Novartis) to compare the efficacy of the gels with a commercial product. The plates were incubated at 35 °C for 20 24 h and inhibition zone (IZs, in millimeters) diameters were read by using a digital ruler at 24 h. Minor trailing growth in the inhibition zones was ignored. Each test was completed in triplicate and the mean zone size was detected.
2.8. Vaginal irritation, in vitro
To evaluate the potential vaginal irritancy of formulations, the HET-CAM test method recommended by ICCVAM was applied (Palmeira-de-Oliveira et al. 2018) (HET-CAM 2010). According to the test method, fresh, clean, and fertile White Leghorn chicken eggs weighing between 50 to 60 grams were used and those that are nonviable or defective were discarded. Eggs were incubated at 38.3 ± 0.2°C and 58 ± 2% relative humidity in an incubator with a rotating tray. Eggs were removed from the incubator on day 9 and any nonviable and defective eggs were discarded. The air cell of the egg was marked, and the marked section was cut via a rotating saw blade and then pared off. The inner membrane was moistened with 0.9% NaCl and eggs were placed into the incubator for a maximum of 30 minutes. Eggs were removed before application and NaCl solution (0.9%) was decanted from the eggs. The inner membrane was carefully removed with forceps. 0.3 mL of test substances, 0.9% (w/v) NaCl as a negative control (NC) and 0.1 N NaOH as a positive control (PC) were applied directly onto the CAM and reactions were observed over for 300 seconds. The appearance of each of the noted endpoints was monitored and recorded, in seconds. The ICCVAM recommended irritation score (IS) analysis method was used for the evaluation which is based on the development of each of the three HET-CAM
endpoints at fixed time intervals of 0.5, 2 and 5 minutes. A single numerical value is obtained on a scale with a maximum value of 21 by summing up the numerical time-dependent scores for lysis, hemorrhage, and coagulation (Table 2).
The irritation test is considered acceptable if the NC and PC each induce a response that falls within nonirritating and severely irritating classifications, respectively. According to historical control studies IS the value of negative control (0.9% NaCl) was 0.0 and the IS value of positive controls (1% SDS and 0.1 NaOH) ranged between 10 and 19, respectively. The severe irritancy classification for a test substance is assigned when using the IS analysis method when the value is greater than nine (HET-CAM 2010).
2.9. Cytotoxicity measurement by direct contact assay
Direct contact test that allows measuring quantitative evaluation of cytotoxicity was performed according to ISO recommended protocol (ISO 10993-5). L929 healthy mouse fibroblast cell was obtained from American Type Culture Collection (ATCC, USA) and was cultured in DMEM supplemented with 10% FBS and 1% penicillin (10.000 units/mL) and streptomycin (10.000 µg/ mL) at 37°C under a humidified atmosphere of 5% CO2. The L929 cells were plated in a 24-well culture dish and
incubated until it forms a semi-confluent layer for 24 hours. Then, for direct contact, the G5 and G8 gels are applied to the surface of a 0.45 m filter paper, and G5 and G8 gels are placed onto surface of the cells in the wells. The filter papers were prepared corresponding to the 1:10 ratio of the well surface area and sterilized. Positive (4 % SDS) and negative control (PBS) were also analyzed at the same time. After 24 h, cell medium and filter paper were discarded. MTT solution (0.5 mg/mL) was added to wells and cells were incubated for an additional 2 h at 37°C. After 2 hours of incubation, the cell culture medium as discarded and 100 L of isopropanol was added to wells to dissolve formazan. The absorbance was measured at 570 nm wavelength by an ELISA microplate reader (BioTek, USA). The reduction in viability compared to negative control is calculated using the equation below.
Cell viability (%) = OD570 (sample) × 100/OD570 (negative control)
2.10 Statistical data analysis
Statistical data analysis was performed using the Student t-test with p < 0.05 as the minimal level of significance.
3. RESULTS & DISCUSSION 3.1. Preparation of vaginal gels
The preparation of vaginal gels was performed after the selection of three biocompatible polymers (Carbopol, carboxymethyl cellulose sodium and chitosan) that were used in different proportions (1%, 3%, 6%). The preparation of the vaginal gels was successful. The aforementioned polymers were selected for their water soluble, biocompatible and biodegradable properties (Andrews et al. 2009; Mansuri et al. 2016). Throughout literature, various concentrations of the polymers are used for the preparation of vaginal gels; Na-CMC was used at 1-7% (Ahmad et al. 2008; Sangeetha et al. 2012; Tasdighi et al. 2012; Hani & Shivakumar 2013), Carbopol at 1-2% (Tasdighi et al. 2012; Mundhey et al. 2015; Singh et al. 2017; de Freitas et al. 2018) and Chitosan at 0.5-3% (Tu cu-Demir et al. 2013; Tu cu-Demiröz 2017). Herein, the polymers were used at similar concentrations (1%, 3%, 6%) to compare the effects of the gels on the characteristics. Triethanolamine was added to Carbopol gel formulations to ensure pH adjusting and good consistency (Bachhav & Patravale 2009; Ramchandani & Sangameswaran 2013; Okur et al. 2017). Also, benzalkonium chloride (0.01%) was added to the formulation as antimicrobial preservative (Rowe et al. 2004). In further, the pH of vaginal formulations was around 4, since the vaginal pH of healthy women is pH 4 5 (Vermani & Garg 2000; Singh et al. 2017). Subsequently, for the preparation of Na-CMC hydrogels, phosphate buffer (pH=4) was used whereas glacial acetic acid was added to chitosan formulations to ensure pH to be around 4.
3.2. Characterization of vaginal hydrogels
The physicochemical characterization is very significant as preliminary studies. Thus, for each gel formulations, pH, viscosity and spreadability values were measured. Table 3 lists the physicochemical properties of vaginal gels. Terbinafine drug was added to the formulations at the concentration of 1%. After the addition of terbinafine
into Carbopol vaginal gels (G1, G2, G3), a significant alteration was seen in terms of their viscosities.
The pHs of the formulations found between 3.7 4.5 while G1 is the only exception with a pH value of 6.22. These results are approvable for vaginal drug delivery since the vaginal pH value is between 4-5 (Singh et al. 2017). Viscosity and spreadability of gels are very important properties for patient compliance, so the gel given for vaginal application should not be too fluid (Patel & Patel 2015; Velázquez et al. 2019). Various viscosities were revealed. More specifically, the viscosity of G9 could not be measured because of very high viscosity and thus G9 was eliminated from further studies. Also, the viscosities of G2 and G3 were found to be very high. On the other hand, G4 had a very low viscosity. It has been reported that the viscosity of hydrogels increased as polymer concentration increased ( en i it et al. 2014). In general, Carbopol hydrogels (G1, G2, G3) showed the highest viscosity values.
The results of spreadability found between 2.83 9.20 cm. G3 and G7 showed the lowest and the highest spreadability values, respectively. Generally, depending on the increase in polymer proportion, the viscosity of hydrogels can be increased, but their spreadability can be decreased (Okur et al. 2019; st nda Okur, Yo gatl , et al. 2019). From the above preliminary results, three ideal formulations were selected. After terbinafine hydrochloride loading, the drug content uniformities of G5, G7 and G8 were measured. The results were recorded at 81.61±3.45%, 95.61±4.11% and 93.65±1.43%, respectively. In addition, the hydrogels, which were prepared using chitosan, showed higher drug content.
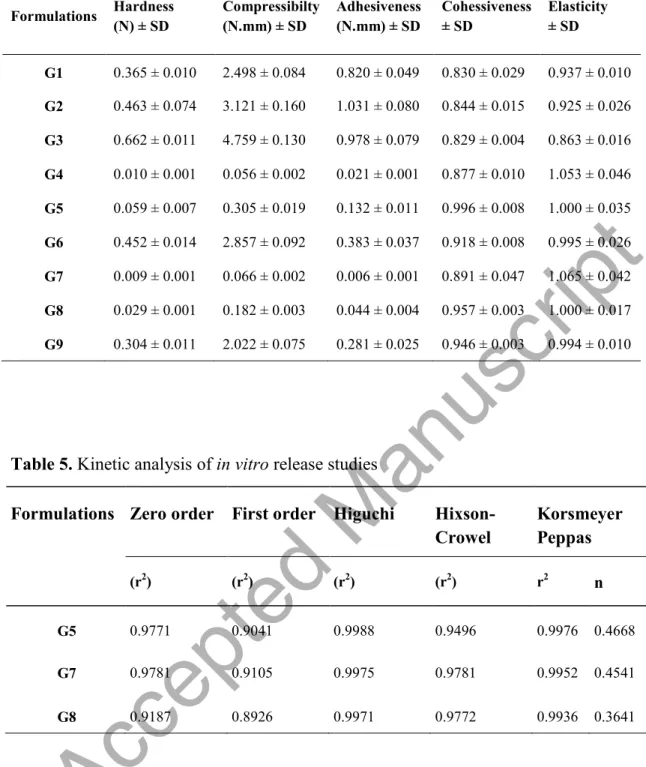
Vaginal hydrogel formulations need to have certain mechanical properties to be easily spread on the application site, to remain in the tissue for the desired time and to be easily removed from the package. Consequently, the mechanical properties of hydrogels such as compressibility, hardness, adhesion, cohesiveness, and elasticity were determined by using TA.XT. PlusC Texture Analysis device. The results of the mechanical properties of hydrogels are shown in Table 4.
The hardness values of the gels found to be between 0.010 and 0.662 N. The hardness value of hydrogels should not be too high for easy application in the vaginal region (Amasya et al. 2012). The hardness results of the gels prepared with Carbopol were found to be quite high (0.365 0.662 N). As in the literature, it is seen that the
hardness value of gels increases with increasing polymer concentration (Cevher et al. 2008; Carvalho et al. 2013).
The lowest and highest compressibility values were observed in G4 (0.056 ± 0.002 N.mm) and G3 (4.759 ± 0.130 N.mm) hydrogels, respectively. Compressibility is defined as the work required for ensuring the product is compressed over a certain distance. It should present low compressibility values for easy removal from its packaging and easy dispersion on the application site (Tu cu-Demiröz et al. 2013). Hydrogel formulations should have good adhesion to remain in the application area for the desired time. The adhesive properties of the polymers used in the preparation of gels are of great importance in this respect. It was determined that as the polymer concentration increased, the adhesive properties of the gels increased (Cevher et al. 2008; Hurler et al. 2012; en i it et al. 2014). For instance, the adhesiveness values of G7 (1% chitosan), G8 (3% chitosan) and G9 (6% chitosan) formulations were calculated as 0.006 ± 0.001 N.mm, 0.182 ± 0.003 N.mm, 0.281 ± 0.025 N.mm, respectively.
Cohesiveness is expressed as the structural reconstitution of the gel after application. If the gel-forming polymers are capable of attracting their molecules, they show high cohesiveness values (Cevher et al. 2008). According to the results of the studies, it was found that the cohesiveness values (0.829 0.996) of the gels were close to each other. The increase in numeric elasticity value means a decrease in the elasticity of the gel (Rençber et al. 2019). In addition, the basic physical mechanism of bioadhesion is associated with the elasticity ability of polymers (Cevher et al. 2008).
Therefore, the decrease in the numerical elasticity values of the gels as the polymer concentration increased was evaluated as a result of increased elasticity.
In summary, it is concluded that G1, G2, G3, G6 and G9 hydrogels showed very high hardness values, G4 and G7 formulations have very low adhesiveness value and G7 has the lowest elasticity because it shows the highest numerical elasticity value. According to all these results, G5 and G8 formulations are considered as the most suitable formulations for vaginal application.
FT-IR spectroscopy is a handful tool in pharmaceutical technology. It has been reported that when novel formulations are designed their stability, in vitro release and drug crystalline nature can be affected by the interaction between the polymers and
the drug. Herein, FT-IR studies are shown in Figure 1. Terbinafine HCL spectrum shows several characteristic bands at 3043, 2967, 2448, 1631, 1508, 1463, 1409, 1362, 1260, and 957 cm 1. At 2967 cm-1 appeared the aliphatic C-H deformation bands from the methyl and methylene groups. The amine group stretching is seen at 2448 cm 1 (Alberti et al. 2001; Kuminek et al. 2013). The spectrum of chitosan demonstrated the characteristic absorption bands at 1659 (amide I). Moreover, the broad band at 3422 cm-1 corresponds to the hydroxyl group. At 1582 cm-1 and 1381 cm-1 are depicted the NH2 bending and CH2 bending, respectively (Bhattarai et al.
2006; Filippousi et al. 2015; Siafaka et al. 2015; Siafaka, Zisi, et al. 2016). The spectrum of Carbopol depicted a broad band at 3500 cm 1 (OH stretching vibration) and 2940 cm 1 (N H stretching vibrations), a sharp peak at 1692.9 cm-1 (due to asymmetric COOH stretching). In further, an absorption band at 1171 cm-1 is related to the C-O-C stretching. The peaks at 1450.9 cm 1 correspond to C O stretching and O H in plane bending whereas at 1242 cm 1 is associated with the C- O -C asymmetric stretching for acrylates (Singh et al. 2013; st nda Okur, H kenek, et al. 2019). In further, the Na-CMC spectrum demonstrated at 3429 cm-1 the broad absorption band of hydroxyl group stretching. The band at 2910 cm-1 corresponds to C H stretching vibration while the strong absorption band at 1599 cm-1 is attributed to the carboxylgroup. The bands at 1422.9 and 1324.5 cm-1 are associated with CH2
scissoring and hydroxyl bending vibration (Biswal & Singh 2004; Girija Aswathy et al. 2012; Haleem et al. 2014).
The formulations free of drugs present similar spectrums to their main components. In the case of the loaded hydrogels due to the absence of active groups of Terbinafine, there are no depicted interactions between the carrier and the drug (Bernabeu et al. 2014). Thus, the prepared hydrogels are stable ( st nda Okur, Yo gatl , et al. 2019).
3.3. HPLC assay
The experimental data showed the level of linearity, which was proved with the value for the correlation coefficient (r2=0.99801). The retention time of terbinafine hydrochloride was about 4.345 min. Low values of standard deviation, which denotes very good repeatability of the measurement were detected. Thus, the equipment used for the study was correct and hence the developed analytical method is highly repetitive ( st nda Okur et al. 2016).
3.4. Evaluation of in vitro release
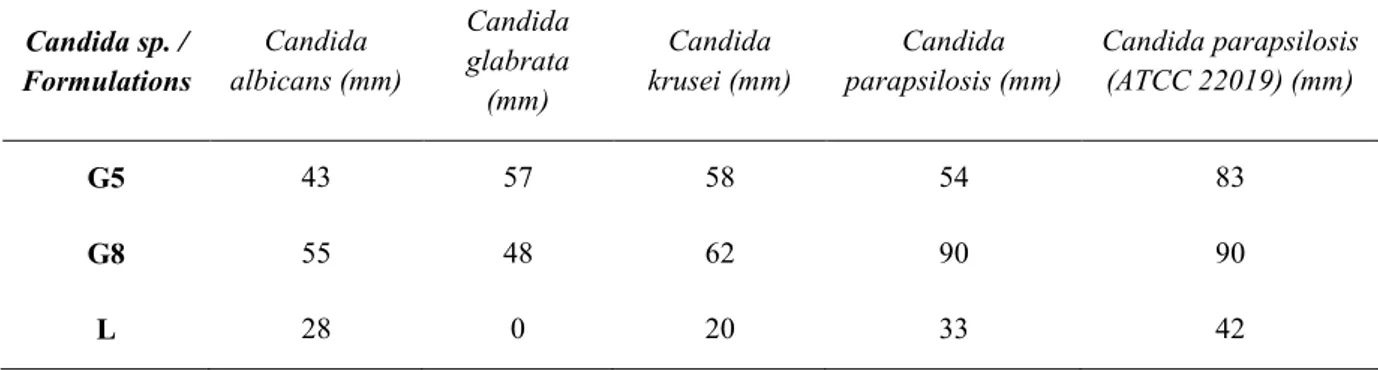
For in vitro release studies three formulations (G5, G7 and G8) were selected for further analysis due to its better viscosity, hardness and good spreadability. In vitro release of terbinafine hydrochloride from the vaginal gels (Figure 2) was studied using the dialysis membrane in phosphate buffer (pH =4) at 37 ± 2 °C. As it can be seen, drug release percentages of hydrogels were between 21 28 % at 8th hour. The percentages of the release of terbinafine hydrochloride from the hydrogels (G5, G7, G8) was recorded to be 34.41±4.49%, 39.83±2.14% and 46.42±6.98% at the end of 24 hours, respectively. It can be said that the hydrogels prepared with chitosan (G7, G8) showed higher drug release. It has been reported that when the viscosities of semi-solid dosage forms are increased, the drug release might be decreased due to slowing down ( en i it et al. 2014). However, in our study, the results showed that the highest viscous formulation (G8) showed higher drug release than G5 and G7. In other words, no significant relationship was found between the results of drug release and their viscosities (p>0.05). There was also no significant difference between the release profiles of hydrogels (p>0.05). Tunca Tanr erdi et al. developed melatonin loaded hyaluronic acid and poly(vinyl alcohol) hydrogels. They evaluated in vitro drug release of melatonin from hydrogels by using the dialysis bag diffusion technique. In vitro release studies showed that after 24 h, the release percentage was found to be 13.240% ± 1.094 for hydrogel (Tunca Tanr erdi et al. 2018).
To further identify, the release mechanism and behavior, several kinetic models were studied. The drug release mechanism was determined by fitting the in vitro release profile in various releases kinetic models and the values of release exponent (n) and regression coefficient (r2) are shown in Table 5. The model that gave a higher r2 value was considered as the best fit model. The release data of the three formulations were fitted to the Higuchi model. This could indicate that the diffusion of Terbinafine retards in the late stage of its pharmacokinetics. It has been reported that most of the topical formulations could follow Higuchi kinetics ( en i it et al. 2014; Siafaka, Barmbale is, et al. 2016; st nda Okur et al. 2018).
3.5. Stability of vaginal gels
The stability tests are performed to determine if gel formulations can be used until their expiration date. The selected three vaginal gels (G5, G7, G8) were packed in an
amber glass bottle and kept at 5 ± 1°C in the refrigerator and at 25±2°C for three months. It was revealed that G7 formulation exhibited phase separation within the first month, so G7 was excluded from the stability study design. The changes in pH, appearance, and drug content of the G5 and G8 were evaluated every month. No significant changes in drug contents, pHs of vaginal gels (G5, G8) were observed (p>0.05). Also, their appearance did not show any change.
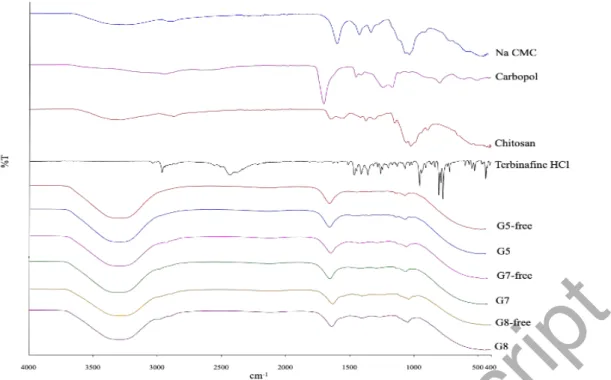
3.6. Antifungal activity of vaginal gels
Antifungal activity of the chosen formulations was evaluated against C. albicans, C.
glabrata, C. parapsilosis and C. krusei, which are reported as species found in the
vagina. Given that G5 and G8 showed the best in vitro release, they were assessed for their antifungal properties. Figure 3 and Table 6 depict the inhibition zones of the vaginal gels. It can be concluded that both of the formulations present greater inhibition zones for C. albicans and C. glabrata which are most common species causing vaginal candidiasis (Gonçalves et al. 2016) whereas C. parapsilosis, an emerging major human pathogen, especially for immunocompromised patients (Trofa et al. 2008) showed the greatest susceptibility to our formulations. Although C. krusei is seen as an unusual case for vaginal candidiasis, it is found to cause 1% of the candidiasis cases (Singh et al. 2002) and it is known as an opportunistic pathogen in hematologic malignancies and for transplant recipients (Pfaller et al. 2008).
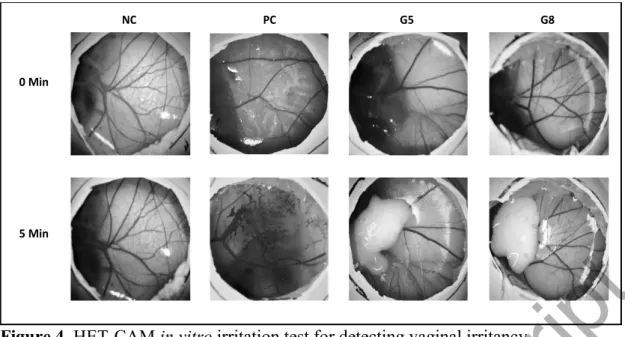
3.7. Vaginal irritation in vitro
Regarding topical toxicity assessment of medical devices, ISO 10993-10 states that any skin or eye irritant material shall be directly labelled as a potential vaginal irritant without animal testing, suggesting that the irritation potentials for the eye and the vaginal epithelia are similar (Palmeira-de-Oliveira et al. 2018). According to HET-CAM test method, 0.3 mL of NC, PC and test substances were applied directly onto the CAM surface after removal of the inner membrane. The reactions on the CAM surface were observed and scored for of 300 seconds with intervals of 0.5, 2 and 5 minutes. The results were given in Table 7.
When using the IS analysis method, a value greater than 9 indicates the severe irritancy potential of a substance. According to the results, the IS value of NC (0.9% NaCl) was scored as 0 and PC (0.1 N NaOH) was scored as 17. These results are considered within the acceptable range according to the ICCVAM protocol. The
analyzed substances G-5 and G-8 did not cause lysis, hemorrhage and coagulation therefore classified as non-irritant according to HET-CAM scoring (Figure 4).
Similarly, Patel et al. developed clindamycin loaded hydroxypropyl methylcellulose and gellan gum based in situ gel systems for vaginal application. They tested the developed in situ gel using HET-CAM method and the result was compared with normal saline. They found that chitosan/gellan gum-based formulation was non-irritant up to 1 h (mean score 0) while the mean score was found to be 0.33 up to 24 h. They concluded that the developed formulation are non-irritant to mild irritant (Patel & Patel 2015).
3.8. Cytotoxicity results
The cytotoxic effect on the L929 cell line, of the G5 and G8 gels was studied using the direct contact assay according to the ISO norms. Figure 5 demonstrates the percent viability of L929 cells treated with G5 and G8 gels for 24 h. Relative cell viabilities on the L929 cell line after 24-h exposure to G5 and G8 were 39.5 ± 5.9 % and 48.3 ± 5.9 %, respectively. The results showed that two gels were cytotoxic to the L929 cells by direct contact assay. First of all, the assay we used is a very sensitive method for testing the cytotoxicity of the medical devices that even with weak cytotoxicity (LI et al. 2015). Besides, in previous studies, terbinafine has been reported to have similar results on cell growth, with a decrease in cell number dose-dependently. These results are interpreted as the antiproliferative and/or apoptotic activities effect of antifungals agents in various cell lines (Lee et al. 2003). Similarly, the results of a study by Kempf et al. show that many antimicrobial agents and moisturizers used for treatment are cytotoxic (Kempf et al. 2011). Duc et al. indicates in a study that even though their tested antiseptic preparations have shown cytotoxicity in vitro, they may be slightly less cytotoxic in vivo (Duc et al. 2007). Due to the above-mentioned studies, cytotoxicity finding of terbinafine in our study does not mean it is not safe for in vivo use.
Accepted Manuscript
CONCLUSION
In this study, vaginal gels comprised of chitosan, Carbopol and sodium carboxymethylcellulose were loaded with Terbinafine, which is a known antifungal drug. The new prepared hydrogels were evaluated for their physicochemical characteristics such as viscosity and found to present desirable properties. Three of the prepared vaginal hydrogels were chosen for further studies. These hydrogels demonstrated good in vitro release. Nonetheless, stability studies showed that only two formulations were stable for 3 months and were further evaluated for their anti-candida activity against several Candida species. It can be concluded that the antimycotic studies against Candida species yielded better results than the marketed product Lamisil. G-5 and G-8 gels did not cause any irritation according to HET-CAM experiment. It can be concluded that the prepared gels present potent characteristics and they can alternatively be used against vaginal candidiasis.
Declaration of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors wish to thank the Amino Chemicals Limited, Malta for providing Terbinafine hydrochloride. The authors wish to thank Primex, Iceland for the kind gift the Chitosan. The authors are grateful to Dr. Panoraia Siafaka for FTIR studies.
REFERENCES
Abruzzo A, Bigucci F, Cerchiara T, Saladini B, Gallucci MC, Cruciani F, Vitali B, Luppi B. 2013. Chitosan/alginate complexes for vaginal delivery of chlorhexidine digluconate. Carbohydr Polym. 91(2):651 658.
Ahmad F, Alam M, Khan Z, Khar R, Ali M. 2008. Development and in vitro
evaluation of an acid buffering bioadhesive vaginal gel for mixed vaginal infections. Acta Pharm. 58(4):407 419.
Ahmed SM, Ibrahim MA, Sarhan HA, Amin MA. 2007. Formulation and
characterization of biodegradable chitosan films for topical application of terbinafine HCl. Bull Pharm Sci Assiut Univ. 30(2):111 129.
Alberti I, Kalia YN, Naik A, Bonny JD, Guy RH. 2001. In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release. 71(3):319 327.
Amas a G, en T, Tarimci N, Kara ana SY, Balo lu E. 2012. Bioadhesi e and mechanical properties of triamcinolone acetonide buccal gels. Turkish J Pharm Sci. 9(1):1 11.
Andrews GP, Laverty TP, Jones DS. 2009. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 71(3):505 518.
Asane GS, Nirmal SA, Rasal KB, Naik AA, Mahadik MS, Rao YM. 2008. Polymers for Mucoadhesive Drug Delivery System: A Current Status. Drug Dev Ind Pharm. 34(11):1246 1266.
Bachhav Y, Patravale V. 2009. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int J Pharm. 365(1 2):175 179. Bassi P, Kaur G. 2015. Bioadhesive vaginal drug delivery of nystatin using a derivatized polymer: Development and characterization. Eur J Pharm Biopharm. 96:173 184.
Bernabeu E, Helguera G, Legaspi MJ, Gonzalez L, Hocht C, Taira C, Chiappetta D a., Dhiman S, Singh TG, Rehni AK. 2014. Paclitaxel-loaded PCL-TPGS nanoparticles: In vitro and in vivo performance compared with Abraxane®. Int J Pharm Pharm Sci. 3(5):43 50.
Bhattarai N, Ramay HR, Chou SH, Zhang M. 2006. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int J Nanomedicine. 1(2):181 187.
Biswal DR, Singh RP. 2004. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr Polym. 57(4):379 387.
Caramella C, Ferrari F, Bonferoni MC, Rossi S, Sandri G. 2010. Chitosan and its derivatives as drug penetration enhancers. J Drug Deliv Sci Technol. 20(1):5 13. Carvalho FC, Calixto G, Hatakeyama IN, Luz GM, Gremião MPD, Chorilli M. 2013. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev Ind Pharm. 39(11):1750 1757.
Cevher E, Sensoy D, Taha MAM, Araman A. 2008. Effect of Thiolated Polymers to Textural and Mucoadhesive Properties of Vaginal Gel Formulations Prepared with Polycarbophil and Chitosan. AAPS PharmSciTech. 9(3):953 965.
Chandra M V., Shamasundar BA. 2015. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int J Food Prop. 18(3):572 584.
Chang JY, Oh Y-K, Choi H, Kim YB, Kim C-K. 2002. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. 241(1):155 163.
Cook MT, Brown MB. 2018. Polymeric gels for intravaginal drug delivery. J Control Release. 270:145 157.
Duc Q Le, Breetveld M, Middelkoop E, Scheper RJ, Ulrich MMW, Gibbs S. 2007. A cytotoxic analysis of antiseptic medication on skin substitutes and autograft. Br J Dermatol. 157(1):33 40.
Filippousi M, Siafaka PI, Amanatiadou EP, Nanaki SG, Nerantzaki M, Bikiaris DN, Vizirianakis IS, Van Tendeloo G. 2015. Modified chitosan coated mesoporous strontium hydroxyapatite nanorods as drug carriers. J Mater Chem B. 3(29):5991 6000.
de Freitas ALD, Kaplum V, Rossi DCP, da Silva LBR, Melhem M de SC, Taborda CP, de Mello JCP, Nakamura CV, Ishida K. 2018. Proanthocyanidin polymeric tannins from Stryphnodendron adstringens are effective against Candida spp. isolates and for vaginal candidiasis treatment. J Ethnopharmacol. 216:184 190.
Gajdo o M, Vetch D, Dole el P, Gajd iok J, Lando H, Musel k J, Zeman J, Knotek Z, Hauptman K, Jekl V. 2016. Evaluation of mucoadhesive oral films containing nystatin. J Appl Biomed. 14(4):247 256.
Gianni C. 2010. Update on antifungal therapy with terbinafine. G Ital Dermatol Venereol. 145(3):415 24.
Girija Aswathy R, Sivakumar B, Brahatheeswaran D, Raveendran S, Ukai T, Fukuda T, Yoshida Y, Maekawa T, Nair Sakthikumar D. 2012. Multifunctional
Biocompatible Fluorescent Carboxymethyl cellulose Nanoparticles. J Biomater Nanobiotechnol. 03(02):254 261.
Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. 2016. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol. 42(6):905 927.
Haleem N, Arshad M, Shahid M, Tahir MA. 2014. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr Polym. 113:249 255. Hani U, Shivakumar H. 2013. Development of Miconazole nitrate Thermosensitive Bioadhesive Vaignal Gel for Vaginal Candidiasis. Am J Adv Drug Deliv. 1(3):358 368.
HET-CAM. 2010. ICCVAM-Recommended Test Method Protocol: Hen s Egg Test-Chorioallantoic Membrane (HET-CAM) Test Method.
Hurler J, Engesland A, Poorahmar Kerman B, kalko-Basnet N. 2012. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J Appl Polym Sci. 125(1):180 188.
Hussain A, Ahsan F. 2005. The vagina as a route for systemic drug delivery. J Control Release. 103(2):301 313.
Ibrahim MM, Hafez SA, Mahdy MM. 2013. Organogels, hydrogels and bigels as
transdermal delivery systems for diltiazem HCL. Asian J Pharm Sci. 8(1):46 54. ISO 10993-10. International Standard ISO 10993-10. Biological evaluation of medical devices. Part 10: Tests for irritation and skin sensitization.
(september):10993.
ISO 10993-5. International Standard Biological evaluation of medical devices-Part 5: Tests for in vitro cytotoxicity. 61010-1 © Iec2001. 2006:13.
Kadajji VG, Betageri G V. 2011. Water soluble polymers for pharmaceutical applications. Polymers (Basel). 3(4):1972 2009.
Kempf M, Kimble RM, Cuttle L. 2011. Cytotoxicity testing of burn wound dressings, ointments and creams: A method using polycarbonate cell culture inserts on a cell culture system. Burns. 37(6):994 1000.
Korkmaz E, Gokce EH, Ozer O. 2013. Development and evaluation of coenzyme Q10 loaded solid lipid nanoparticle hydrogel for enhanced dermal delivery. Acta Pharm. 63(4):517 29.
Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. 2008. Mucoadhesive
nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target. 16(10):806 814.
Kumar MK, Nagaraju K, Satyabrata B, Sudhakar M. 2014. Formulation and Evaluation of sublingual tablets of terazosin hydrochloride. Int J Pharm Sci Res. 5(10):4117 4128.
Kuminek G, Rauber GS, Riekes MK, Campos CEM De, Monti GA, Bortoluzzi AJ, Cuffini SL, Cardoso SG. 2013. Single crystal structure, solid state characterization and dissolution rate of terbinafine hydrochloride. J Pharm Biomed Anal. 78 79:105 111.
Kyle AA, Dahl M V. 2004. Topical therapy for fungal infections. Am J Clin Dermatol. 5(6):443 51.
Langasco R, Spada G, Tanriverdi ST, Rassu G, Giunchedi P, Özer Ö, Gavini E. 2016. Bio-based topical system for enhanced salicylic acid delivery: preparation and
performance of gels. J Pharm Pharmacol. 68(8):999 1009.
Lee W Sen, Chen RJ, Wang YJ, Tseng H, Jeng JH, Lin SY, Liang YC, Chen CH, Lin CH, Lin JK, et al. 2003. In vitro and in vivo studies of the anticancer action of
terbinafine in human cancer cell lines: G0/G1 p53-associated cell cycle arrest. Int J Cancer. 106(1):125 137.
LI W, ZHOU J, XU Y. 2015. Study of the in vitro cytotoxicity testing of medical devices. Biomed Reports. 3(5):617 620.
Mahmoudabadi AZ, Najafyan M, Moghimipour E, Alwanian M, Seifi Z. 2013. Lamisil versus clotrimazole in the treatment of vulvovaginal candidiasis. Iran J Microbiol. 5(1):86 90.
Mansuri S, Kesharwani P, Jain K, Tekade RK, Jain NK. 2016. Mucoadhesion: A promising approach in drug delivery system. React Funct Polym. 100:151 172. Mendes Giannini MJS, Bernardi T, Scorzoni L, Fusco-Almeida AM, Sardi JCO. 2013. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 62(1):10 24.
Mundhey DA, Morris PS, Lohiya GK, Avari JG. 2015. Formulation and evaluation of mucoadhesive vaginal gel containing novel combination of metronidazole and
miconazole nitrate for the treatment of vaginitis [Internet].
das Neves J, Bahia MF. 2006. Gels as vaginal drug delivery systems. Int J Pharm. 318(1 2):1 14.
Okur ME, A la , Batur , Yolta A, Gen E, Pertek S, st nda Okur N. 2019. Evaluation of In Situ Gel Containing Pycnogenol for Cutaneous Wound Healing. Medeni Med J. 34(1):67 75.
Okur N , Er S, a lar E , Ekmen TZ, Sala F. 2017. Formulation of microemulsions for dermal delivery of cephalexin. ACTA Pharm Sci. 55(4):27.
can , Abac , U tan AH, Aksu B, Bo ac o lu H, G neri T, er . 2009. Enhanced Topical Delivery of Terbinafine Hydrochloride with Chitosan Hydrogels. AAPS PharmSciTech. 10(3):1024.
Özer Ö, Özyazici M, Tedajo M, Taner MS, Köseoglu K. 2007. W/O/W Multiple Emulsions Containing Nitroimidazole Derivates for Vaginal Delivery. Drug Deliv. 14(3):139 145.
Oliveira R, Monteiro Machado R, Martinez-de-Oliveira J, Palmeira-de-Oli eira A. 2018. Testing aginal irritation ith the Hen s Egg Test-Chorioallantoic Membrane assay. ALTEX. 35(4):495 503.
Park S-C, Kim Y-M, Lee J-K, Kim N-H, Kim E-J, Heo H, Lee Y, Lee JR, Jang M-K. 2017. Targeting and synergistic action of an antifungal peptide in an antibiotic drug-delivery system. J Control Release. 256:46 55.
Patel Priya, Patel Paresh. 2015. Formulation and evaluation of clindamycin HCL in situ gel for vaginal application. Int J Pharm Investig. 5(1):50.
Pa eli , kalko-Basnet N, Schubert R. 2001. Liposomal gels for vaginal drug delivery. Int J Pharm. 219(1 2):139 149.
Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, Dobiasova S, Rinaldi M, Barton R, Veselov A. 2008. Candida krusei, a Multidrug-Resistant Opportunistic Fungal Pathogen: Geographic and Temporal Trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 46(2):515 521. Rakesh G, Mukesh G, Hemant S. 2014. A Review on Organogels and Fluid Filled Method. Int J Sci Res Rev IJSRR. 3(32):274 288.
Ramchandani U, Sangameswaran B. 2013. Formulation and Evaluation of Topical gel of Ketoprofen using Different Polymers. Int J Pharm Biol Arch. 4(2):323 326.
Rençber S, Cheaburu-Yilmaz CN, Kose FA, Yaprak Karavana S, Yilmaz O. 2019. Preparation and Characterization of Alginate and Chitosan IPC based gel formulation for mucosal application. Cellul Chem Technol. 53(7 8):655 665.
Rowe R, Sheshey P, Quinn M. 2004. Handbook of Pharmaceutical Excipient. 6th ed. [place unknown]: London Pharmaceutical Press.
Russo E, Selmin F, Baldassari S, Gennari CGM, Caviglioli G, Cilurzo F, Minghetti P, Parodi B. 2016. A focus on mucoadhesive polymers and their application in buccal dosage forms. J Drug Deliv Sci Technol. 32:113 125.
Salehei Z, Seifi Z, Mahmoudabadi AZ. 2012. Sensitivity of Vaginal Isolates of
Candida to Eight Antifungal Drugs Isolated From Ahvaz, Iran. Jundishapur J Microbiol. 5(4):574 577.
Sangeetha SS, Pramod Kumar TM, Roopa K. 2012. Formulation and evaluation of bioadhesive miconazole nitrate gel for vaginal candidiasis. Int J Pharm Technol. 4(1):3825 3838.
Sawant B, Khan T. 2017. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed Pharmacother. 96:1478 1490. van Schalkwyk J, Yudin MH, Yudin MH, Allen V, Bouchard C, Boucher M,
Boucoiran I, Caddy S, Castillo E, Kennedy VL, et al. 2015. Vulvovaginitis: Screening for and Management of Trichomoniasis, Vulvovaginal Candidiasis, and Bacterial Vaginosis. J Obstet Gynaecol Canada. 37(3):266 274.
en i it ZA, Kara ana SY, Era B, G rsel , Limoncu MH, Balo lu E. 2014. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 64(2):139 156.
Siafaka PI, Barmbalexis P, Bikiaris DN. 2016. Novel electrospun nanofibrous matrices prepared from poly(lactic acid)/poly(butylene adipate) blends for controlled release formulations of an anti-rheumatoid agent. Eur J Pharm Sci. 88:12 25.
Siafaka PI, Mone M, Koliakou IG, Kyzas GZ, Bikiaris DN. 2016. Synthesis and physicochemical properties of a new biocompatible chitosan grafted with 5-hydroxymethylfurfural. J Mol Liq. 222:268 271.
Siafaka PI, Titopoulou A, Koukaras EN, Kostoglou M, Koutris E, Karavas E, Bikiaris DN. 2015. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int J Pharm. 495(1):249 264.
Siafaka PI, st nda Okur N, Mone M, Giannakopoulou S, Er S, Pa lidou E, Karavas E, Bikiaris D. 2016. Two Different Approaches for Oral Administration of Voricona ole Loaded Formulations: Electrospun Fibers ersus -Cyclodextrin Complexes. Int J Mol Sci. 17(3):282.
Siafaka PI, Zisi AP, Exindari MK, Karantas ID, Bikiaris DN. 2016. Porous dressings of modified chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr Polym. 143:90 99.
Singh B, Sharma S, Dhiman A. 2013. Design of antibiotic containing hydrogel wound dressings: Biomedical properties and histological study of wound healing. Int J
Pharm. 457(1):82 91.
Singh S, Sobel JD, Bhargava P, Boikov D, Vazquez JA. 2002. Vaginitis Due to Candida krusei: Epidemiology, Clinical Aspects, and Therapy. Clin Infect Dis. 35(9):1066 1070.
Singh S, Verma D, Mirza MA, Das AK, Dudeja M, Anwer MK, Sultana Y,
Talegaonkar S, Iqbal Z. 2017. Development and optimization of ketoconazole loaded nano-transfersomal gel for vaginal delivery using Box-Behnken design: In vitro , ex vivo characterization and antimicrobial evaluation. J Drug Deliv Sci Technol. 39:95 103.
Sipahi H, Reis R, Dinc O, Ka a T, Dimoglo A, A d n A. 2019. In itro
biocompatibility study approaches to evaluate the safety profile of electrolyzed water for skin and eye. Hum Exp Toxicol. 38(11):1314 1326.
Tasdighi E, Jafari Azar Z, Mortazavi SA. 2012. Development and In-vitro Evaluation of a Contraceptive Vagino-Adhesive Propranolol Hydrochloride Gel. Iran J Pharm Res IJPR. 11(1):13 26.
Theill L, Dudiuk C, Morano S, Gamarra S, Nardin ME, Méndez E, Garcia-Effron G. 2016. Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina. Rev Argent Microbiol. 48(1):43 49.
Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an Emerging Fungal Pathogen. Clin Microbiol Rev. 21(4):606 625.
Tu cu-Demiröz F. 2017. Development of in situ poloxamer-chitosan hydrogels for vaginal drug delivery of benzydamine hydrochloride: Textural, mucoadhesive and in vitro release properties. Marmara Pharm J. 21(4):762 770.
Tu cu-Demir F, Acart rk F, Erdo an D. 2013. De elopment of long-acting
bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int J Pharm. 457(1):25 39.
Tunca Tanr erdi S, Cheaburu-Yilmaz CN, Carbone S, Özer Ö. 2018. Preparation and in vitro evaluation of melatonin-loaded HA/PVA gel formulations. Pharm Dev Technol. 23(8):815 825.
st nda -Okur N, Ege MA, Karasulu HY. 2014. Preparation and Characterization of Naproxen Loaded Microemulsion Formulations for Dermal Application. Int J Pharm. 4(4):33 42.
st nda Okur N, a lar E , Yo gatli V. 2016. De elopment and Validation of an Hplc Method for Voriconazole Active Substance in Bulk and its Pharmaceutical Formulation. MARMARA Pharm J. 20(2):79.
st nda Okur N, Filippousi M, Okur ME, A la , a lar E , Yolta A, Siafaka PI. 2018. A novel approach for skin infections: Controlled release topical mats of poly(lactic acid)/poly(ethylene succinate) blends containing Voriconazole. J Drug Deliv Sci Technol. 46:74 86.
st nda Okur N, H kenek N, Okur ME, A la , Yolta A, Siafaka PI, Ce her E. 2019. An alternative approach to wound healing field; new composite films from natural polymers for mupirocin dermal delivery. Saudi Pharm J. 27:738 752.
st nda Okur N, Ya c lar AP, Siafaka PI. 2020. Promising pol meric drug carriers for local delivery; the case of in situ gels. Curr Drug Deliv. 17.
st nda Okur N, Yo gatl V, Okur ME, Yolta A, Siafaka PI. 2019. Impro ing therapeutic efficacy of voriconazole against fungal keratitis: Thermo-sensitive in situ gels as ophthalmic drug carriers. J Drug Deliv Sci Technol. 49:323 333.
Vejnovic I, Huonder C, Betz G. 2010. Permeation studies of novel terbinafine formulations containing hydrophobins through human nails in vitro. Int J Pharm. 397(1 2):67 76.
Velázquez NS, Turino LN, Luna JA, Mengatto LN. 2019. Progesterone loaded thermosensitive hydrogel for vaginal application: Formulation and in vitro comparison with commercial product. Saudi Pharm J.:1096 1106.
Vermani K, Garg S. 2000. The scope and potential of vaginal drug delivery. Pharm Sci Technolo Today. 3(10):359 364.
Voltan A, Quindós G, Alarcón K, Fusco-Almeida AM, Mendes-Giannini MJ, Chorilli M. 2016. Fungal diseases: could nanostructured drug delivery systems be a novel paradigm for therapy? Int J Nanomedicine. 11:3715 3730.
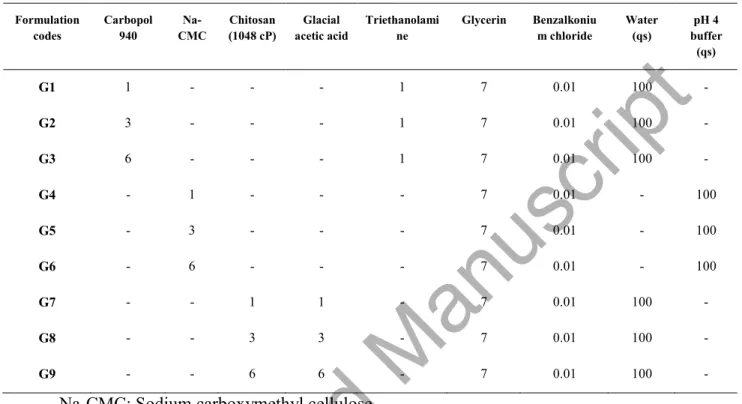
Table 1. The ingredients and ratios of vaginal gel formulations % Component Formulation codes Carbopol 940 Na-CMC Chitosan (1048 cP) Glacial acetic acid Triethanolami ne Glycerin Benzalkoniu m chloride Water (qs) pH 4 buffer (qs) G1 1 - - - 1 7 0.01 100 - G2 3 - - - 1 7 0.01 100 - G3 6 - - - 1 7 0.01 100 - G4 - 1 - - - 7 0.01 - 100 G5 - 3 - - - 7 0.01 - 100 G6 - 6 - - - 7 0.01 - 100 G7 - - 1 1 - 7 0.01 100 - G8 - - 3 3 - 7 0.01 100 - G9 - - 6 6 - 7 0.01 100 -
Na-CMC: Sodium carboxymethyl cellulose
Table 2. Scoring scheme for irritation testing with the HET-CAM test method
Effect Score
0.5 Min 2 Min 5 Min
Lysis 5 3 1 Hemorrhage 7 5 3 Coagulation 9 7 5
Table 3. The characterizations of the vaginal gels
Formulations pH Viscosity (mPa.s) Spreadability (cm)
G1 6.22 ± 0.12 27,230 ± 210 2.83 ± 0.12 G2 4.37 ± 0.17 165,000 ± 1.100 2.87 ± 0.12 G3 3.72 ± 0.09 198,000 ± 2.340 2.48 ± 0.03 G4 4.18 ± 0.11 32.5 ± 2.5 8.60 ± 0.36 G5 4.40 ± 0.12 376 ± 24 3.95 ± 0.23 G6 4.56 ± 0.07 5,800 ± 120 2.73 ± 0.15 G7 4.00 ± 0.13 323 ± 18 9.20 ± 0.10 G8 3.81 ± 0.08 34,500 ± 750 4.57 ± 0.03 G9 3.72 ± 0.15 3.07 ± 0.12 mPa.s: millipascal-second
Accepted Manuscript
Table 4. The results of mechanical properties of hydrogels
Formulations Hardness (N) ± SD Compressibilty (N.mm) ± SD Adhesiveness (N.mm) ± SD Cohessiveness ± SD Elasticity ± SD
G1 0.365 ± 0.010 2.498 ± 0.084 0.820 ± 0.049 0.830 ± 0.029 0.937 ± 0.010 G2 0.463 ± 0.074 3.121 ± 0.160 1.031 ± 0.080 0.844 ± 0.015 0.925 ± 0.026 G3 0.662 ± 0.011 4.759 ± 0.130 0.978 ± 0.079 0.829 ± 0.004 0.863 ± 0.016 G4 0.010 ± 0.001 0.056 ± 0.002 0.021 ± 0.001 0.877 ± 0.010 1.053 ± 0.046 G5 0.059 ± 0.007 0.305 ± 0.019 0.132 ± 0.011 0.996 ± 0.008 1.000 ± 0.035 G6 0.452 ± 0.014 2.857 ± 0.092 0.383 ± 0.037 0.918 ± 0.008 0.995 ± 0.026 G7 0.009 ± 0.001 0.066 ± 0.002 0.006 ± 0.001 0.891 ± 0.047 1.065 ± 0.042 G8 0.029 ± 0.001 0.182 ± 0.003 0.044 ± 0.004 0.957 ± 0.003 1.000 ± 0.017 G9 0.304 ± 0.011 2.022 ± 0.075 0.281 ± 0.025 0.946 ± 0.003 0.994 ± 0.010
Table 5. Kinetic analysis of in vitro release studies
Formulations Zero order First order Higuchi Hixson-Crowel Korsmeyer Peppas (r2) (r2) (r2) (r2) r2 n G5 0.9771 0.9041 0.9988 0.9496 0.9976 0.4668 G7 0.9781 0.9105 0.9975 0.9781 0.9952 0.4541 G8 0.9187 0.8926 0.9971 0.9772 0.9936 0.3641
Accepted Manuscript
Table 6. Inhibition zone diameters in mm of Lamisil and terbinafine hydrochloride loaded hydrogels Candida sp. / Formulations Candida albicans (mm) Candida glabrata (mm) Candida krusei (mm) Candida parapsilosis (mm) Candida parapsilosis (ATCC 22019) (mm) G5 43 57 58 54 83 G8 55 48 62 90 90 L 28 0 20 33 42 L: commercial product
Table 7. Irritation score of formulations according to HET-CAM protocol.
Tested Substance IS Score Result
NaCl 0 No irritation potential
NaOH 17 Strong irritation potential
G5 0 No irritation potential
G8 0 No irritation potential
NC: negative control (0.9% NaCl); PC: positive control (0.1 N NaOH).
Figure 1. FT-IR spectroscopy studies of the sodium carboxymethylcellulose,
Carbopol, chitosan, Terbinafine HCL, and loaded and unloaded formulations (G5, G7 and G8).
Figure 2. In vitro release studies of Terbinafine hydrochloride from the hydrogels
Figure 3. Anti-fungal activity of commercial product (Lamisil) and developed
hydrogels
Figure 4. HET-CAM in vitro irritation test for detecting vaginal irritancy.
NC: negative control (0.9% NaCl); PC: positive control (0.1 N NaOH); HET-CAM: hen s egg test-chorioallantoic membrane.
Figure 5. Effects of G5 and G8 gels on L929 cell viability.
Cells were treated with G5 and G8 gels for 24 h. M: Medium control, NC: Negative control (PBS), PC: Positive control (4%SDS).
NC PC G5 G8
0 Min
5 Min