Ankara Ünıv Vet Fak Derg
47, 135-ı43. 2000
EOSINOPHIL GRANULOCYTES AND PLASMA CELLS IN
JEJUNAL MUCOSA OF DOGS NATURALLY INFECTED
WITH OR WITHOUT INTESTINAL PARASıTES
ÜlkerEREN! Mustafa SANDıKÇı!
Muharrem BALKA YAı
Şadiye ERCÜLDÜRENLER!
Barsak parazitleri ile doğal enfekte ve eııfekte olmayaıı köpekleri1ıjejulıum mu-kozasmda eoziııofil graııulositler ve plazma hücreleri
Özet: Bu ara,çtırma, barsak parazitleri ile dogal enfekte ve enfekte olmayan köpeklerin jejunum mukozasmda eozinojil granulositler ve plaznıa hücrelerinin da-gılınılannm belirlenmesi amacıyla [{erçekleştirildi. Aynca periler kanda total lö-kosit ve eozino/il granulosit sayılan da belirlendi. Araştırmada, barsak parazitleri ile dogal enfekte olduklan belirlenen 7 ve [{aitasmda parazit belirlenemeyen 4 adet olmak üzere toplam II adet, belirgin bir ırk özelligi [{östermeyen köpek kulla11lldl.
K(jpeklerin jejunumundan doku örnekleri operatil' olarak almdı. Doku ör-neklerinin tespiti için %10 tamponlu nötr formalin kuIla111ldl. Kesitler eozinojil [{ıwıulositlerin demonstrasyon u için Con[{o red, plazma hücrelerinin de-numstrasyonu için metil green pyronin ile boywıdllar. Her iki hücre de jejunal nıu-kozada villus- hipt ünitede sayıldı. Perifer kan lökosit ve eozino/il [{,wıulosit koıı-santrasyonlan standart tekniklerle hemositometrede gerçekle,çtirildi.
Incelenen doku kesitlerinde eozino/il granulositlerin lamina propriya 'da özel-likle villuslarda, kriptlerin bazal klSlmlannda ve lamina subglandularis 'te yogun olarak bulunduklan görüldü. Aynca [{enellikle kriptlerde ve nadiren villuslann ta-banmda intraepiteliyal eozino./il granulositlerin bulundugu g(jzlendi. Bazı eo-zino/il granulositlerin de barsak lumeninde bulunduklan dikkati çekti. Pi-/'Onino/ilik hücrelerin özellikle villuslarda yogun olarak bulunduklan, daha az olarak da hipt bii/[{esinde yer aldıklan gözlendi.
Barsak parazitleri ile enjekte olan ve [{aitasmda parazit belirlenerneyen grup-lar arasmda totallökosit ve kan eozino/il granulosit konsantrasyonları ile doku eo-zino/il [{ranulositleri ve plazma hücreleri için elde edilen veriler kar-,çt!aştlrtl(lLgmda, sadece e'~fekte [{rupta doku eozinojil [{ranulosit sayılarının fazlaııgı anlamlı bulundu (p< 0.05).
Anahtar kelimeler: Eozinofil [{ranulosit, intestinal parazitozis, jejunum, k(jpek, plazma hücresi
i. The University of Adnan Menderes, Facıılty of Veterinary Medicine, Department of Histology- Embryology. Aydın/ TÜRKIYE
136
Ü. EREN, M. BALKAYA, M. SANDıKÇı, Ş.ERGÜLDÜRENLER
Summary: This study was carried out to determine the distribution ol' eo-sinophil granulocytes and plasma cells in the jejunal mucosa (~f the dogs naturally inl'ected or not infected with intestinal parasites. /n addition, the total leukocyte and eosinophil granulocyte concentrations were determined in peripheral blood. For this pUlposes, II mongrel dogs were used where 7 ol'them were determined to be inl'ected naturally with intestin al parasites, and in 4 of them there were no evi-dence ol'an actual intestinal parasitic inl'ection.
The tissue specimens ol' the jejunum were obtained by abdominal surgery fi"om both groups. Specimens were fixed with i 0% neutral bu.ti'eredformalin. The
tissue sections were stained with either Congo red for eosinofil granulocytes or methyl green pyroninfor plasma cells. Tissue sections were examined by light mic-roscopically. Both cells were counted in villus-crypt units olJejunal mucosa. The determination ol' the leukocyte and eosinophil granulocyte concentrations in pe-ripheral blood were carried outfrom blood samples with standart technics.
/t was observed that eosinophil granulocytes were located in lamina propria ofjejunal mucosa, especialfy within vilfus intestinalis. basal parts of crypts and la-mina subglandularis. FUl1hermore. eosinophil granulocytes were also located int-raepithelially in crypts and rarely at the base of the villus. Some eosinophil gra-nulocytes were also seen within intestinal lumen. The pyroninophilic ce lls were identified in jejunum. They were intensively prominent in villus intestinales, but they werefound also in crypts in a lesser extent.
A t-Testfor independent groups showed that the eosinophil granulocyte count
in jejunal mucosa was higher in parasitized dogs than in jejunal mucosa ol' no-ninl'ected dogs (p< 0.05). But there were no sign(ficant differences in
CO/1-centrations (JI' leukocytes and eosinophil granulocytes in peripheral blood, as well
as in the number ofplasma cells in jejunal mucosa when compared the dogs with intestinal parasite with those showing no parasite infeces.
Key words: Dog. eosinophil granulocyte, intestinal parasitosis, jejunum. plasma celfs.
Introduction
Eosinophil granulocytes are ceııs with numerous membranc-bound specific granulcs some of them having usuaııy electron-dense cristaııoid internum, crystaııoid cores, also called "central core". These specific granules contain Iysosomal enzymes as weıı as most of the cationic proteins unique to eosinophil granulocytes. The cristaııoid corc of the granule, when present, composed of major basic protein (MBP), and the noncore matrix contains eosinophil cationic protein (ECP), eosinophil peroJi.idase and eosinophil-derived neurotoxin (l2, 31).
Eosinophil granulocytes defend against
large, nonphagocytable organisms, most
notably the multicellular helminthic parasites (6), fungal agents (20) and foreing proteins (4). One of the components of Iysosomal gramıles of eosinophil granulocytes, the MBP, is a potent cytotoxin for certain parasites (28). Eosinophil granulocytes react to the helmiths when a sensitivity to the protein of the parasite has developed (aııergic state) or the protein or secretory product of the parasite is relcased in the body. Initial binding of eosinophil granulocytes to parasitic targets can bc mediated by antiparasite IgG or IgE antibodies or by C3b deposited on the surfaces of parasites
EOSINOPHIL GRANULOCYTES AND PLASMA CELLS IN JEJUNAL MUCOSA OF DOGS NATURALLY... 137
(38). A1though single-ceııed protozoan
parasites can be killed by eosinophil
granulocytes, eosinophilia is heightened not by infections with protozoa except Isospora belli hut rather by helminthic parasites (6). Eosinophil granulocytes can kill a wide number of helminthic parasites, especiaııy in their larval stages (6,21,36). Although other ceııs can a!so kiıı such parasites, eosinophil granulocytes are particularly toxic to helminths for several reasons. First, the cationic proteins they deposite after binding to the surface of the parasite, especiaııy MBP and ECP are potent helminthotoxins (i). Eosinophil peroxidase gencrates hypohalous acids that also kill parasites. Finaııy, eosinophil oxydatİve products also mediate helminthotoxicity (6, 45). Plasma ceııs are regularly found in the lamina propria of the gastro-intestinal tract according to the demand for local antibody production (33). Investigations of the canine intestinal lract have shown the immunoglobulin (Ig)-posiıive ceııs in .either smaıı or large intestine (LS, 18,43). But, there is no difference in the number of plasma ceııs in different intestinal regions under normal circumstances
(15).
In many parasitic disease of intestine both plasma ceııs and eosinophil granulocytes are
prominent (8, 34, 35, 46). There is an
interacıion between the se two cell types in opsonisation procedure of parasitic agents. Eosinophil granulocytes undergo exocytosis to expeıı their granular constituents when they come in close contact with an opsonised (antibody- and complement-coated) parasite
(38).
In an another study in which the same animals were used, we found that the number of mast ceııs were significantly higher in naturaııy infected dogs compared with uninfected dogs (13). AIso, the aim of the present study was to identify the distribution of eosinophil granulocytes and plasma ceııs wilhin jejunal mucosa of the dogs naturaııy infected with
intestİnal parasites. In addition, the toıal
leukocyte and eosinophil granulocyte
concentrations were also determined ın
peripheral blood.
Materials and Methods Animals
In this study, II mongrel dogs (6 females and 5 males) were used weighing 12-24 kg and age of i to 2,5. The dogs had been used in the other study (13). The dogs were divided into two groups; the first group was determined as naturally infected with intestina! parasites (n
=
7), and the second group showed no an aclual in-testİnal parasitic infection (n=4).Surgery
Jejunal biopsi es were obtained from both groups by abdominal surgery. For this purpose the dogs were anesthesized with LO mglkg ketamin hydrochlonır (Kctanes, Alke) given intramuscularly. Jejunal biopsi es were removed and than, additiona! anesthesia was İnduced
with 2 mglkg i.m. xylazin hidrochlonır
(Rompun@, Bayer). Ampisilin trihydrat (5- ıo mglkg, Alfasilin IAbfar) was used for three days as antibiotİc to protect possible infections in post-operative period which was started at the day of operation.
Tissue processing and staining
Tissue samples were fixed in 1O~, neulral buffered formaline (NBF) for 24 hours, and than embedded in parafin. Serial 5 mm thick sections were cut with 30 mm intervals. The tİssue sections were stained with either Congo red for eosinophil granulocytes (17) or with methyl green pyronin for plasma ceııs (10). The counting of ceııs in tissue sections
For counting the ceııs, the mucosa was diveded into "villus-cript" (VC) units. The number of eosinophils and plasma eeııs Iying between two gl and crypts and in the lamina propria of the viııus above were counted. The
138 Ü. EREN. M. BALKAY A. M. SANDıKÇı, Ş. ERGÜLOl;RENLER
whole area comprised a
ve
unİt and wasdelimited basally by the muscularis mucosa (27). The eosinophils and plasma cells at three
ve
units (x400 magnification) werc counted for each section and five seperate slides were counted for each tissue block.B100d sampling and determination of eoncentrations of leukoeytes and eosinophil granuloeytes
The blood samplcs were withdrawn from cach dog into the test tube with EDTA before anaesthesia, and eosinophil granulocytes and leukocyte counts were carried out within following 2 hours. Leukocyte counts were
determined in improved Ncubauer
hemocytometry with the standard technique using a solution ad modum Türk (44), and cosinophil granulocyte concentration was determincd in Fuchs-Rosenthal hemocytometry with a solution ad modum Pilot (32).
Statistical evaluation
The data were statistically evaluated by
t-Test for unpaired groups using SPSS
computer program (37).
Results
In grup I, the dogs were naturally infected with intestinal parasites (Table I).
The total white blood ccll and eosinophil granulocyte consentrations are seen in Table 2.
The tissue sections were examined light microscopically. It was seen that eosinophil granulocytes and prccursors were located intensively in lamina propria of jejunal mucosa. especially within villus intestinales (Figure 1),
in basal parts of crypts and in lamina
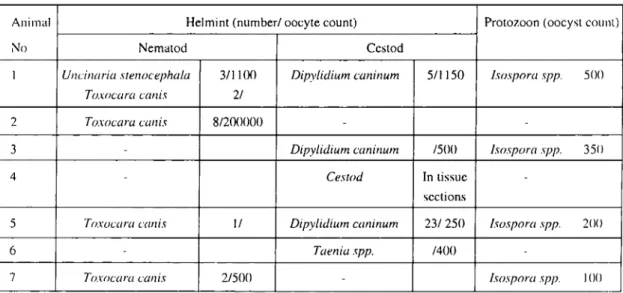
subglandularis (Figure 2). Furthermore, eosinophil granulocytes were located also intraepithelially, especially in crypts and rarely Table i. The intestİnal parasites of naturally infeeted dogs.
Tablo i.Oogal enfekte köpekleri n barsak parazitleri.
Animal Helmint (number/ oocyte eount) Protozoon (ooeyst eoum)
No Nematod Cestod
1 Ullcilıaria sreııocephala 3/1LLX) Dipylidium caııiııum 5/1150 /sospora spp. SOO
TO.wcara cwıis 2/
2 Toxocara cwıis 8/200000
-3 Dipylidium caııiııum /500 /sospora spp. 350
4 Cestod In tissue
seetions
5 Toxocara c({lıis 1/ Dipylidium cwıiııum 23/250 /sospora spp. 200
6 Taeııia spp. /400
7 Toxocara caııis 2/500 - /sospora spp. ı()(ı
Table 2. Total leukocyte and eosinophil granuloeyte coneentrations in peripheral blood of dogs (per ını of bload). Tablo 2. Köpeklerde perifer kanda, ml'de total lökosit ve eozinofil granulosit konsantrasyonları
GroLIps Leukocytes Eosinophil granulocytes
X :t SEM X :t SEM
Groııp i (n=7) 11226 2486 84.00 17.40
EOSINOPI-IIL GRANULOCYTES AND PLASMA CELLS IN JEJUNAL MUCOSA OF DOGS NATURALLY... 139
Fıgııre I. Eosınophıl granııloeyıes observed ıııVIIillS ınıesııııales (arro\\,). Cım;:,) red. x 12.HJ. Şekil ı.ViIIııs intesıinaIis'de gözlenen cozinoril granıılositler (oklar) Con;:o red. x i 24().
Figııre 2. Eosinophil granuloeytes in lamina sııbglandıılaris (arrows). Congo red. xııım Şekİl 2. Lamina sııbglandıılaris'de cozİnofıI granıılositler (oklar) Congo red. x 1180.
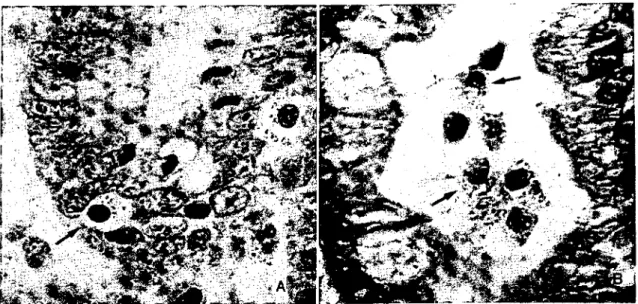
at the base of the villus (Figure 3A). il was also observed that some eosinophil granulocytes were present within intestinal lumen (Figure
3B).
The pyroninophilic cells were identified in JeJunum, especially in viIlus intestinalis
intensivcIy. But they were found also in crypts in a lesser extent (Figure 4).
Eosinophil granulocytes and plasma cell counts per
ve
unit is given in Table 3.The statistical cvaiuatian of the data showed that the dirrerence of the mean values for eosinophil granuloeyte and ieukoeyte eoncentraLİons found in peripheral hlood were not statislicaIIy significant. AIso, the differcnce of plasma ecIl counts in Lİssues wcre not staLİsticaIly significanl. But the differcnce between mean tissue eosinophil granulocyte counts of dogs with parazites in feces and those showing no parazites were signilicant (p< O.05)(Tablc 3).
140 Ü. EREN. M. BALKA YA. M. SANDıKÇı. Ş.ERGÜLDÜRENLER
rıgııre 3. A. Intraepilhelıal eosiııophiI graııu!oeytes (arrow). Congo red. x 1200. B. Eosıııophıl graııulocytcs in intcstinal Iumen (arrows). Congo red. x i 180.
Şekil 3. A. Intraepiteliyal eozinofil granulositlcr (ok). Congo red. x1200. B. Barsak lumeninde eozinofi! granulositler (oklar) Congo red. x 11SO.
Fıgure 4. Plasma eclIs iiivıIIııs intcstınalcs (arrows).
Methyl green pyronin xı220.
Şekil 4. ViIIus intestinalis'de plazma hücreleri (oklar). Methyl green pyronin xi220.
Table 3. The eosinophil granulocytes and pyroninophilic eell counLs in vC ıınit in the jejıınal mucosa of dogs (means with SEM).
Tablo 3. Köpeklerde jejıınum Intıkozasında. villus-kript ünitede plazma hiicresi ve eozinofil gramılosit sayıları.
Groups Eosinophil granulocytes Plasma Cells
X :t SEM X :t SEM
Group i (n=7) 133.26* 11.90 90.91 13.50
Group ıı(n=4) 72.07* 18.90 72.15 24.40
EOSINOPHIL GRANULOCYTES AND PLASMA CELLS IN JEJUNAL MUCOSA OF DOGS NATURALL Y... ı4ı
Dİscussİon
The present study provides information about distribution of eosinophil granuloeyte and plasma ecııs within the jejunal mueosa of do gs with or without intestinal parasites.
There are relative little studies about the distribution of eosinophil granuloeytes and plasma eeııs in the body under physiologieal or physiopathologieal eonditions, and only a smaıı part of these studies were eoneemed with the distribution of thcse eeıı types within intestinal waıı (15, 22, 23, 40, 43).
Experiments in the mı ce (7, 11, 14, 41, 46), rats (24, 25, 27, 29, 39), guinea pigs (16), turkeys (8), sheep (19) and human (9) have shown that infeetions with helmints and protozoan parasites are assoeiated with
pronouneed intestinal mastoeytosis,
eosinophilia, plasmaeytosis and increased antibody produetion.
In this study a signifieantly higher intestinal eosinophil granulocyte population in dogs naturaııy infccted with intestinal parasites were determined. The eosinophil granuloeytes were observed intensively in lamina propria of jejunal mueosa, espeeiaııy within viııus
intestinales, in basal parts of erypts and in lamina subglandularis. Furthermore, eosinophil granuloeytes were loeated intraepitheliaııy in erypts and rarely at the base of the yillus. It was
alsa observed that same eosinophil
granulocytes had been passed in intestinal lumcn. These findings are in aeeordanee with earlier studies stating that eosinophil granuloeytes in intestinal traet are loeated mainly in lamina propria (3, 5, 21). Also, the
inerease of the numbers of eosinophil
granuloeytes in jejunum of parasitized do gs eonfirms the previous findings about response to parasitic agents of intestinal mueosa (14, 16, 34,41,42).
In sheep infeeted with Trichostrongylus eolubriformis, increases in the populations of IgA- and IgG I-eontaining plasma ceııs in the
lamina propria was identified (2). Morever, the distribution of plasma eeııs in intestinal mueosa with Toxaeara eanis and Aneylostoma eaninum infeeted dogs were also deseribed (26, 35). Soh and Kim (35) suggested that more plasma eeııs oeeurred only in the viııi intestinales. In this study, the plasma eeııs were seen espeeiaııy in villus intestinales intensivcly, but they were found also in erypts in a lesser extent. However, the inerease of plasma eeııs in intestinal traet in respons to the intestinal parasites described by different authors (2, 35) was not supported by this study, than the plasma eeııs in jejunal mueosa of dogs with intestinal parasites did not differe signifieantly from those without parasites. But it is in good agreements with the results of Lloyd et al (26)
who observed no changes in plasma ceıı
numbers during infeetion with Toxaeara canis in puppies.
On the other site, the statisticaı cvaIuatian of the data showcd that the differenee of the me an values for eosinophil granuloeytes and
leukoeytes of peripheral blood are not
statistieaııy signifieant. These results alsa eonfirm the previous study results about leueoeyte formula and gastrointestinal parasites in goats (30).
In summary, we have identified
distribution of eosinophil granuloeyte and plasma eeııs within jejunal mueosa of dogs with or without intestinal parasites.
References
i. Ackerman S.•, Gleich G", Locgering DA, Ric-hardson BA, BuUerworth AE (I 985) A mınparalive toxicity of purifı.ed human eosinophil granulc ealionie protcins for schistosomula of Schi<;tosoma mansoni. J Trop Med Hyg, 34, 735-745.
2. Adams DB, MerriU GC, Cripps A W (1980)
IIl-ıestinal lymph and ıhe local uııtihody aııd im-munoglohulin respolise lo iııfectioıı h" Tric-hastraııgylus coluhriformis iıı sheep. Aust J Exp Bio
Med Sci, 58,167-177.
3. Archer RK (ı968) The easiııaphil leukacyıes. Ser Ha-ematol,
ı.
3-32.4. Aschkenasy A (1971) Nutritioıı el Hhnaıopoii'sis. C N Ray Sac, Paris.
142 Ü. ERE:-.ı. M. BALKA YA, M. SANDıKÇı, Ş. ERGÜLDÜRENLER
5. BuUerworth AE (I 977) The eosiııophi! aııd its role in
immuıziı)' ro helmiııth iJ~fectiolı. Current Topics Im-ımıııo\. 77,127-169.
6. BuUerworth AE ( 1984) Cel/-mediated damaRe to
hel-miıııhs Adv Parasito\. 23, 143-235.
7. Carroll SM, Grove DI, Heenan 1'.1 (1986) Kineıics of
cells in ıhe inıeslinal mucosa of mice fol/owiııg oral in-.lecrirJll wiıh Ancylostoma ceylanicum. Inler Areh
AI-lergy App Immunol, 79, 26-32,
8. Cooper GL, Shivaprasad HI., Bickford AA, Nord-hausen R, Munn RJ, Jen'rey .lS (I 995) Enıeritis in
ıurkeys assııciated wiıh an unusual .f/aRellaıed pm-/OZOWl(Cochlosııma wırıtis). Avian Dis, 39, i83- i90.
9. Croese.l Loukas, Opdebeeck A,.l, Provic P (1994)
Occulı eıııeric iııfecıion by Aııcylostoma cwıinum. A previously unrecoRııized zoOlıosis. Gastroenterology.
106,3-12.
iO. Culling CFA, AlIison RT, Bar WT (ı985) Cel/ular
PaıholoR)' Techııique. Buııerwords, London.
i i.Dent LA, Daly CM, Mayrhofer G, Zimmerman T, HallcU A, Bignold Ll', Creaney .I, Parsons .lC (I 999) Inıerleukin-5 IrwısRenic mice show enhaneed resisıwıce Iıı primar)' lııjeclirJllS wiıh NippostrOlıgylus Im/silimsis bul nol primary ilıfectiOlIS wiıh Toxııcara cwıis. Infeeılmmun. 67, 989-993.
12. Egesten A, Alumets O, von Mecklenburg C, Pal-megren
:vI,
Olsson i. (I 986) LocalizatiOlI of catiO/ıicproıeiıı. major basic proıein, and eıısinophi! pe-roxidase iıı human eosinophils by immunoelectron micrascııpic ıechnique. J Hisıochem Cyıochem, 34,
1399- 1403.
13. Eren Ü, (;üzel :'il,Türkütanıt S, Durukan A, Er-güldürenler ş,Kara E (2000) Köpeklerde barsak
mu-kıızas/nda ması hücreleri. Ankara Üniv Yel Fak Derg,
47, 2ı-30
14. Faulkner H, Humphreys N, Renauld .lC, Snick .I van, Grencis R, van-Snick .l (I 997) lııterleukin-9 is involved in hosı proıeclive immUlıity to intestiııal ne-marode in{ecıirııı. Elir J Immuno\. 27, 2536-2540.
i 5. German A.l, Hall E.l, Day MJ (I 999) Anal)'sis of
le-ucııc)'ıe suhseıs iıı ıhe caııine inlesıine. J Comp Path,
120, 129-145
16. Gleich G.l, Olson GM, HerIich H (ı979) The e.fJecl
ııl' anıisemm iii eosinophils on susceptihilit)' and ac-quired immUlıily of the guinea-piR to TrichosırOllRYlus colubrifr}l'/nis. Immunology. 37, 873-880.
17. Grouls V, Helpap B (I 981) Selecrive staining of
eıı-siııopiıds and ıheir imıııalure precul'Sors in ıissue sec-i/!iiis aııd auııımdiogmphs wiıh COIIRO red. Sıain Techno\. 56, 323-325.
I~. Hart IR (I 979) The disırihuıion ııl' immUlıol(lııbulin-conlaininl( cel/s in canine sml/l/ iilıesıine. Res Vet Sei.
27.269-274.
19. HuntIey .lF, Wallace GR, MilIer HRp (1982)
Qu-anıilaıive reccııvel'\, oj'isıılaıed mucoml ması cel/s and K/o/nde lew;ocytes .lrom parasi/ised sheep. Res Vet Sci, 33, 58-63.
20. Ishikawa T, Dalton AC, Arbesman CE (ı972)
Pha-I(ocyıosis ıif Candida albicam by eosinophilic le-ukocyıes. J Allergy clin Immuno\. 49, 3ı1-315. 21. Jain NC (1986) 5chalm's Veterinary Hemmıılııg.". Lea
&Febiger, Philadelphia.
22. Jergens AE, Moore FM, Kaiser MS, Haynes .lS, Kinyon .IM (I 996) Morphomeıric evaluaıiıın of im-munoglobulin A-cmıraiııing and immUlıol(lııhulilı C-cmıtainiııl( eel/s and T cel/s iıı duodenal mucosa .Ii-Ilm healt)' dogs and from dogs wiıh iııflammaııır\, bıııvel disease or ııonspecijic gasıroenıerilis. Am J Yel Res 57,697-704.
23. Kazura .lW, Mahmoud AAF (1983) Proıecril'l' Rııle
of Eosinophils: Thl' Schistosome El(g Crwıuloma.
383-395, In: T. Yoshİda and M, Torisu (Eds.): Inı-munobiology of the Eosinophi1. Elsevier Seieııee Puh-lishing Co Ine, New York,
24, Kelly .ID, Ogilvie BM (I 972) "ııeslinal mmı cell und
eosinophil ııumhen during ıvorm expLllsion iıı IlLlI-liparous and lactaıing mts infected Yo'iıh Nip-postrOlıgylus brasiliensis, Inler Arch Allergy App Im-munol, 43, 497-509,
25, Lindsay MC, BIaies DB, Williams JFSO (1983)
Ta-enia ıal'ıziaefrmnis: ImmUlıoglohulilı E-conl!linin~ cells in inıeslinal and Iymplwıic ıinues of infeetl'd mIs. Inter J ParasİLOl, 13, 9 i-99.
26. Lloyd MK, Wi.lensundera S, Soulshy EJL (i991)
111-ıestinal chaııges in puppies infecıed wiıh ToxoUlm canis. J Comp Paıho\. 105,93- i04,
27, Miller HRp, .larreU WFH (I 97i) Immııııe reactions in mucous ml'mhranes. ı.Inıesıinal mast eell resonse during helminl expulsion in the rat. Immunology. 20, 277- 288.
28, Mills .lN, Valli VEO (1988) The Hemopııieııc System, In: W.F, Robinson and C.R.R, Huxtahle (Eds.): Cli-nicopathologie Principles for veterinary Medicine. Camhridge University Press. Cambridge, [\ew York. 29. Moqbcl R, King S.l, Macdonald AJ, Miller HRp,
Cromwell O, Shaw RJ, Kay B (1986) Eıııemi and
sysıemic release of leukoırienes duriııg il/wphyloxls of Nippostrongylus bmsiliellSis-primed rats. J Immunol.
137,296-301.
30. Palacio M.lF, Montes CAM, Vega FDA, Del-Palacio M.lF, De-Vega FDA (1985) Relaıiıııı beıweeıı 11'-ukocyle formula and gasırointesıinal parasiıes iıı goats. Rev Clin Esp, ı.137-139,
3ı.Peters MS, Rodriguez M, Gleich GJ (1986) Lo-calizalion ol human eosiııophi! grwıule mu;or basic protein, I'osiııophil caıionic prolein, aııd eosiııophil-derived neurolOxin by immunoelecırrııı mieroscopic ıechnique, Lab Invest, 54. 656-662.
32: Pilot ML (ı950) Use r~lbase influidsjfıı- COU1ıtin~eo-siııophils. A meıhod for stainiııg eosiııophils, Am J Ci in Path o\. 20. 871-872,
33, Ross MH, Reith EJ, Romrell L.J(ı989) Histology. 1\
Text and Atlas. Williams aııd Wilkiııs, Ba\timore, 34, Ruitenberg E.l, EIgersma A, Kruizinga W,
Le-enstra F (ı977) Trichiııella sJ?iralis infeCliml in cOIl-genitally aılı)'mic (nude) mice, Pamsi{o/ııgica/,
.re-EOSINOPHIL GRANULOCYTES AND PLASMA CELLS IN JEJUNAL MUCOSA OF DOGS NATURALLY.. 143
rol0t:ical aııd haemalOlogical studies with oh-servatioııs (}Lı iııtestiııal patholog}'. Immunology. 33.
581-587.
35. Soh CT, Kim SJ (1973) Chant:es ofilltestinal mueous memhrane or dog with reference to the immunological respOlISe to parasite inrestation. Yonsei Med J, 4,
27-36.
36. Spry CJF (1988) Eosinophils. A Comprehmsive Re-"iew and Guide to the Scientific and Medical Li-lerature. Oxford Medical Publications. Oxford Uni-versity Press. Oxford. New York.
37. SPSS Advanced StatisticslM• 7.5. (1977) SPSS Ine.
Chicago.
38. SuIIivan TJ (1979) The role of eosinophils in inl lammator)' reaetiollS. Prog Hematol. 11,65-82.
39 Taraschcwski H, Sagani C, Mehlhorn H (1989)
Ult-raslrtlctural sıudy or the host-parasite imerjiıce of Mo-nilirorllıis monilirormis (ArchiaC(/nthocephala) in la-horatorY-in/'ected rats. J Parasitol. 75. 288-296.
40. Torisu M, Iwasaki K, Tanaka .1, Lino H, Yoshida T ( 1983) Anisaki and Eosinophi: PathoKene.üs and Bi-ologic SiKııi/ieance oj'Eosinophilic PleKmon in Human Anisakiasis. 343-367. In: T. Yoshida and M. Torisu (Eds.). Immunohiology of the Eosinophil. Elsevier Sci-ence Publishing Co Ine. New York.
41. Tronchin G, Outoit E, Vernes A, Diguet .1 (1979)
Oral immLllıiwtiOlı or mice and metaholic anıiKem of
Triehinella spiralis larvae: E/feets on the kinetics oj' intesıinal eell response includinI{ ması cells and poly-morphonuclear eosinophils. J Parasitol. 65,685-691.
42. WiIkes SO, Goven AJ (1984) Tissue eosinophil
num-hers and phospholipase B aelivily in miee in/i'cted \IIiıh Triehinella spiralis. Int J Parasitol. 14,479-482.
43. WiIIard MD, Leid RW ( 1981) Noııuni/iJr/n horimnıal
and ı'ertical dstrihutions oj' immunoglohulin A cells ilı canine intestines. Am J Yel Res. 42, 1573-ı580. 44. Wirth D (I 950) GrundlaKen Einer Kliııischeıı
HilmatoloKie der hausıiere. Urban & Schwarzenberg. Wien und Insbnıck.
45. Yazdanbakhsh MA, Tai PC, Spry CJF, GIcich GJ, Roos D (1987) S)'nergism beıween eosinophil catiOlıic
protein and ox)'gen metaholites in killint: or shis-tommula (~j'Schistosoma man so ni. J Imlnlınol. 138. 3443-3447.
46. Yin .lG, Li OC, Zhang XC, Zhou CF, Yang .1, Li JH
(1998) Immune respolISe oj' intestinal mucosa to in-feetion with Cryptosporidium parvum in mice. Chinese
J Vet Sci. 18, 254-256.
Yazışma Adresi:
Doç. Dr. Ülker EREN Adnan Menderes Üniversitesi Veteriner Fakültesi