ORIGINAL ARTICLE
The Effects of Scolicidal Agent Propolis
on Liver and Biliary Tree
Kemal Kismet&Sibel Serin Kilicoglu&Bulent Kilicoglu&
Serap Erel&Omur Gencay&Kadriye Sorkun&
Esra Erdemli&Okan Akhan&Mehmet Ali Akkus&
Iskender Sayek
Received: 29 February 2008 / Accepted: 2 May 2008 / Published online: 30 May 2008 # 2008 The Society for Surgery of the Alimentary Tract
Abstract
Background This study was designed to examine the effects of propolis on the liver and biliary system when used as a scolicidal agent.
Materials and Methods Thirty Wistar–Albino rats were divided into two groups. Propolis and 0.9% saline (NaCl) were injected into the biliary tract of the rats. Three rats from control group and four rats from propolis group died within 5 days after the procedure. Blood samples of remaining 23 rats were obtained 1 week after and at the end of the experimental study for liver function tests. Six months after the procedure, retrograde and magnetic resonance cholangiography were performed and liver, common bile duct, and duodenum were excised en bloc for histopathological examination.
Results Liver function tests were slightly elevated 1 week after the procedure and were found to be normal at the end of the sixth month in both groups. No stricture in the biliary tree was found on the retrograde and magnetic resonance cholangiograms. The tissue samples of the propolis group showed no histomorphological difference from the control group. Conclusions Propolis may be used as a scolicidal agent even in the case of cystobiliary communication with no side effects on liver and biliary tree.
Keywords Propolis . Scolicidal agent . Hepatobiliary system . Hydatid disease . Sclerosing cholangitis
Introduction
Echinococcosis is a parasitic tapeworm infection, caused by the larval cestode Echinococcus granulosus. Hydatid disease is endemic in many countries, and the disease remains endemic in Mediterranean countries, the Middle and Far East, South America, Australia, New Zealand, and East Africa. Historically, management of hydatid cysts in the liver typically involved an open surgical approach with meticu-lous operative site packing and employed a variety of conservative and radical operative techniques. Dissemination of protoscolex-rich fluid during surgery is a major cause of recurrence. Various scolicidal solutions have been used for surgical and percutaneous approaches.1,2Caustic sclerosing cholangitis is a dreadful complication after surgical
treat-K. Kismet (*)
:
B. Kilicoglu:
S. Erel:
M. A. AkkusDepartment of General Surgery,
Ankara Training and Research Hospital Ulucanlar, Ankara, Turkey
e-mail: kemalkismet@yahoo.com S. S. Kilicoglu
Department of Histology-Embryology, Ufuk University, Ankara, Turkey
O. Gencay
:
K. SorkunDepartment of Biology, Faculty of Science, Hacettepe University, Ankara, Turkey
E. Erdemli
Department of Histology-Embryology, Ankara University, Ankara, Turkey
O. Akhan
Department of Radiology, Hacettepe University, Ankara, Turkey
I. Sayek
Department of General Surgery, Hacettepe University, Ankara, Turkey
ment. The development of sclerosing cholangitis had been attributed to the passage of the scolicidal solutions into the biliary tree through a cystobiliary fistula.3,4
Propolis is a resinous material collected by bees from various plants. Once collected, this material is enriched with salivary and enzymatic secretions of bees. Propolis is used by bees to cover hive walls, fill cracks or gaps, and embalm killed invader insects.5Propolis contains a variety of flavonoids, phenols, alcohols, terpenes, sterols, vitamins, and amino acids.6 In a previous study, we found that propolis was totally effective on protoscolices in low concentrations and short exposure time.7
This study was conducted to investigate the effects of propolis on the liver and biliary tree by direct injection of propolis into the common bile ducts of the rats.
Materials and Methods
Animals
Thirty Wistar–Albino female rats, weighing 200±25 g, were included in this study. Animals were deprived of food 12 h before anesthesia but had free access to water 2 h before operation. No enteral or parenteral antibiotics were administered at any time. Rats were housed under constant temperature (21 ±2°C) individually in wire cages with 12-h light–dark cycle. The rats that died during the study were excluded. The procedures in this experimental study were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals, and ethical approval was obtained from the Animal Ethics Committee of Ankara Research and Training Hospital.
Surgical Procedure
The rats were randomly divided into two equal groups of 15 rats each. Rats were anesthetized by intramuscular injection of 40 mg/kg body weight ketamine HCl (Ketalar, Parke-Davis, Eczacibasi, Istanbul, Turkey) and 5 mg/kg body weight xylazin (Rompun, Bayer, Leverkusen, Ger-many). All animals were allowed to breath spontaneously during the experiments. After the abdomen was shaved and cleaned with povidone iodine, a midline laparotomy was carried out, and the intestines were covered with sterile gauze pads soaked with isotonic saline at 36–38°C. In addition, 5 ml Ringer’s lactate solution was given subcu-taneously to prevent dehydration in animals during the experimental period. A 3-mm duodenotomy was performed and 0.15 ml of test solutions, either sterile isotonic saline solution (control group) or 1% dilutions of propolis (study group) (Anzer propolis, Rize, Turkey), were injected without pressure into the common bile duct with a
27-gauge syringe. Immediately after the injection, the common bile duct was clamped with an atraumatic vascular clamp. The catheter was then withdrawn. The clamp was removed 5 min later, and the duodenotomy was closed with a 6-0 polypropylene suture. There was no operative mortality. The study animals were kept for 6 months, during which time they were fed with rat chow ad libitum and tap water and kept at room temperature (18–20°C) in separate cages. Blood samples were obtained 1 week after the surgical procedure and at the end of the experimental study (6 months after the procedure) for liver function tests including bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and gamma glu-tamyl transferase.
Six months after the procedure, 0.3 ml of radiopaque solution was injected from the tail vein of the rats, and magnetic resonance cholangiograms were performed under ketamine hydrochloride anesthesia. After this procedure, retrograde cholangiography was performed, midline lapa-rotomy was carried out, and a 3-mm duodenotomy was performed. Radiopaque solution 0.15 ml per rat was injected without pressure into the common bile duct with 27-gauge syringe. Immediately after the injection, antero-posterior cholangiograms were obtained. After cholangiog-raphy, blood samples for determination of liver function tests were obtained by cardiac puncture. Liver, common bile duct, and duodenum were excised en bloc for histopathological examination.
Biochemical and Histopathological Examinations
The biochemical analyses were made by an autoanalyzer (Olympus AU 640, Japan) using commercial kits.
The liver specimens of the right and left lobes and common bile duct were taken and immediately fixed in 10% neutral-buffered formaline solution for 1 week. Tissues were washed in flowing water and dehydrated with rising concentrations of ethanol (50%, 75%, 96%, and 100%). After dehydration, specimens were put into xylene to obtain transparency and were then infiltrated with and embedded in paraffin. Histological sections of the specimens in thickness of 6 μm from all the groups were stained with hematoxylin and eosin. The whole tissue blocks were sectioned, and histopathological exami-nations were performed on systematically randomly sampled preparations by a blinded researcher. Liver specimens were evaluated to assess the morphology of the hepatocytes, portal areas, sinusoidal lesions, cellular infiltration in the lobule or portal spaces, and parenchymal lesions. Histopathological examination of the common bile duct was performed to assess the histomorphology of the epithelium, connective tissue, inflammation, fibroblas-tic proliferation, and necrosis.
Statistical Analysis
Differences between the groups were analyzed with Mann– Whitney U test. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 13.0 for Windows (SPSS Inc., Chicago, USA). p values less than 0.05 were considered to be significant.
Results
General
Three rats from control group and four rats from propolis group died within 5 days after the procedure. Three of seven died in the early postoperative period possibly due to anesthesia, and the others died because of trauma to the common bile duct and leakage into the peritoneum. The remaining 23 rats were alive until the end of the study without any problem.
Biochemical and Radiological Results
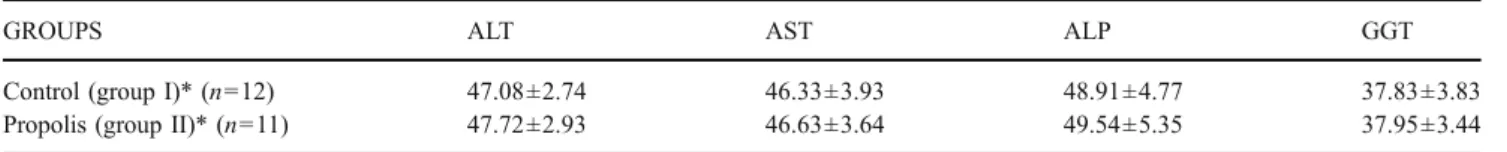
Liver function tests were slightly elevated 1 week after the procedure in both groups, and this might be due to the cannulation and injection of common bile duct. There was no difference between the groups (Table1). At the end of the sixth month, liver function tests were found to be normal in both groups (Table2).
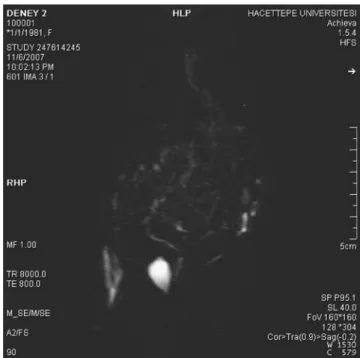
No stricture in the biliary tree was found on the retrograde and magnetic resonance (MR) cholangiograms (Fig.1).
Histopathological results
Both control and the propolis groups did not show any difference from normal lobul structure of liver tissue. There was no enlargement of hepatocytes or/and dilatation of canalicular spaces in both groups. In addition, there was no bile pigment accumulation in the tissue sections of both groups (Fig.2A,C). Tissues from the both groups presented no morphological alterations in the portal tract. A typical portal tract containing terminal branch of the hepatic portal vein, terminal branches of the hepatic artery with the structure of arterioles, and bile ductules were in regular architecture in propolis and control groups (Fig.2B,D). The infiltration with mononuclear cells was not seen in the liver parenchyma of the propolis group, while it was visualized around the terminal hepatic venules of the control group (Fig. 2A,C). The mononuclear cell infiltration in the portal areas was recognized in the liver specimens of control group, while it could be neglected in the propolis group (Fig. 2B,D). When we examined the ductus choledochus specimens of the propolis group, we clearly observed the lining epithelium consisted of a single layer of tall columnar cells above with the connective tissue. The lumen was wide-opened, as it was in the control group. The sacculi of Beale, the appearance of the infoldings of the surface epithelium, were observed in common aspect. We did not notice any recognizable mononuclear cell infiltra-tion in both groups. As a result, the tissue samples of liver and ductus choledochus of the propolis group showed no histomorphologic difference from the control group, and also, we did not see any adverse effect of the treatment (Fig. 3).
Table 1 Mean values of liver function tests at the end of first week
GROUPS ALT AST ALP GGT
Control (group I)* (n=12) 56.66±6.86 58.91±7.02 62.08±9.30 41.25±4.31
Propolis (group II)* (n=11) 60.81±2.22 61.81±2.71 64.63±5.86 41.04±4.47
ALT Alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gamma glutamyl transferase *p>0.05
Table 2 Mean values of liver function tests at the end of sixth month
GROUPS ALT AST ALP GGT
Control (group I)* (n=12) 47.08±2.74 46.33±3.93 48.91±4.77 37.83±3.83
Propolis (group II)* (n=11) 47.72±2.93 46.63±3.64 49.54±5.35 37.95±3.44
ALT Alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gamma glutamyl transferase *p>0.05
Discussion
Propolis, or bee glue, is a brownish resinous material collected by honeybees from various plant sources. The chemical composition of propolis has been clarified to some extent in recent years. Hundreds of chemical com-pounds have been identified in propolis samples. The main chemical classes present in propolis are flavonoids, phe-nolics, and various aromatic compounds. Other compounds in propolis are volatile oils and aromatic acids (5–10%), waxes (30–40%), resins, balms, and pollen grains, which are rich sources of essential elements such as magnesium, nickel, calcium, iron, and zinc. However, its chemical composition varies depending on the site of its collection.8 Antimicrobial properties of propolis seem attributable mainly to the flavonoids, pinocembrin, galangin, and pinobanksin. Pinocembrin also exhibits antifungal proper-ties. Other active compounds are ester of coumaric and caffeic acids. Prenylated p-coumaric and diterpenic acids possess antibacterial and cytotoxic activities. Caffeoyl-quinic acid derivates show immunomodulatory and hep-atoprotective actions and furofuran lignans inhibit the growth of some bacteria.6
The ideal treatment for hepatic hydatid disease should completely eliminate the parasite and prevent recurrence of
Figure 1 The magnetic resonance cholangiogram of a rat from propolis group.
Figure 2 Liver sections stained with hematoxylin and eosin. A, B Control group showing the regular architecture. C, D Propolis group. vc Central vein, pv portal vein, ha hepatic arter, bd bile ductules; arrow, hepatic sinusoids, asterisk cell infiltration.
the disease with minimum morbidity and mortality. There are three available therapeutic modalities for hepatic hydatid cysts: systemic chemotherapy, surgery, and percu-taneous treatment.9 Meticulous packing of the operative field is necessary irrespective of the surgical technique employed. In conventional or minimally invasive hydatid disease surgery, disinfection of the cyst cavity is very important, justifying the routine use of scolocidal solutions. In the presence of cystobiliary communications, the passage of these solutions may cause spotty necrosis in the liver parenchyma, widening of the sinusoids, regenerative changes in hepatocytes, Kupffer cell hyperplasia, pigment accumulation, periductal fibrosis, inflammation, fibroplastic proliferation, and necrosis in the extrahepatic biliary ducts.1,4 Various scolicidal agents such as 95% alcohol, 10% povidone iodine, hypertonic saline, hydrogen perox-ide, 5% formalin, silver nitrate, cetrimperox-ide, and albendazole have been evaluated for scolicidal effects and hepatobiliary complications in the presence of cystobiliary communica-tions.10–13Sahin et al.12evaluated the effects of hypertonic saline (20%), povidone-iodine (1%), and silver nitrate (0.5%) on liver and biliary tree and found that the use of these agents resulted in chronic low-grade biliary
inflam-mation. The intensity of the lesions was more remarkable in the silver nitrate group. In an experimental study, Houry et al.3showed that injection of 20% hypertonic saline or 2% formaldehyde solution into the biliary tracts of the rats was followed by lesions of the biliary epithelium. As compared with 20% hypertonic saline solution, the 2% formaldehyde solution caused more severe lesions of the biliary epithe-lium and, in addition, induced the development of sclerosis. They concluded that that intracystic injection of 2% formaldehyde solution should be abandoned. Belghiti et al.14 reported five cases of caustic sclerosing cholangitis with the use of 2% formaldehyde and 20% sodium chloride solutions, and because of the risk of this complication, they recommended that intracystic injection of a scolicidal solution should be abandoned in the surgical treatment of hydatid disease of the liver. In conclusion, sclerosing cholangitis is a serious complication that develops with the use of some scolicidal agents in the presence of cystobiliary communications, and an ideal scolicidal agent should not cause such a serious side effect.
Sclerosing cholangitis may be due to immunological, infectious, vascular, or chemical factors. In patients with hydatid disease of the liver, various factors, including
Figure 3 Common bile duct (ductus choledochus) sections stained with hematoxylin and eosin. A, B Control group. C, D Propolis group. L Lumen, sb sacculi of Beale, c capillary, s stroma; arrowhead, simple columnar epithelium.
injection of scolicidal agent into the cyst cavity, a communication between the cyst and biliary tree, and a particular sensitivity to the scolicidal agent, seem to be necessary to promote caustic sclerosing cholangitis.12
In a previous study, we evaluated the scolicidal effect of propolis and found that it was totally effective in low concentrations (1%) and short exposure time (3 min). Propolis did not cause any systemic side effects when it was applied intraperitoneally. We concluded that propolis might be used as a potent scolicidal agent if it would not cause caustic sclerosing cholangitis when injected into the biliary tree.7 We also designed another study that would compare propolis with other scolicidal agents for the side effects on liver and biliary tree.
Conclusion
We designed the present study to evaluate the effects of propolis on liver and biliary tree. Propolis did not cause any side effects that have been documented with radiological evaluation, histopathological examinations, and biochemis-try analysis in liver functions tests in 6 months of follow-up period. According to the results of this experimental study, we concluded that propolis might be used as a scolicidal agent even in the case of cystobiliary communication with no side effects on liver and biliary tree.
References
1. Smego RA, Sebanego P. Treatment options for hepatic cystic echinococcosis. Intern J Infect Dis 2005;9:69–76.
2. Ustunsoz B, Akhan O, Kamiloglu MA, Somuncu I, Ugurel MS, Cetiner S. Percutaneous treatment of hydatid cysts of the liver.
AJR 1999;172:91–96.
3. Houry S, Languille O, Huguier M, Benhamou JP, Belghiti J, Msika S. Sclerosing cholangitis induced by formaldehyde solution injected into the biliary tree of rats. Arch Surg 1990;125:1059– 1061.
4. Tozar E, Topcu O, Karayalcin K, Akbay SI, Hengirmen S. The effects of cetrimide- chlorhexidine combination on the
hepato-pancreatico-biliary system. World J Surg 2005;29:754–758.
5. Dimov V, Ivanovska N, Manolova N, Bankova V, Nikolov N, Popov S. Immunomodulatory action of propolis, influence on anti-infectious protection and macrophage function. Apidologie
1991;22:155–162.
6. Orsolic N, Tadic Z, Benkovic V, Horvat A, Lojkic M. Basic I: Radioprotective effect of a water-soluble derivate of propolis in
mice. Mellifera 2004;4:45–52.
7. Kismet K, Kilicoglu B, Koru O, Tanyuksel M, Oruc MT, Sorkun K, Salih B, Akkus MA. Evaluation on scolicidal efficacy of
propolis. Eur Sur Res 2006;38:476–481.
8. Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002;73 Suppl 1:1–6.
9. Sayek I, Tirnaksiz MB, Dogan R. Cyst hydatid disease: Current trends in diagnosis and management. Surg Today 2004;34:987– 996.
10. Garcia JIL, Alonso E, Gonzalez-Uriarte J, Romano DR. Evaluation of scolicidal agents in an experimental hydatid disease model. Eur
Surg Res 1997;29:202–208.
11. Topcu O, Kuzu I, Karayalcin K. Effects of peritoneal lavage with scolicidal agents on survival and adhesion formation in rats.
World J Surg 2006;30:127–133.
12. Sahin M, Eryilmaz R, Bulbuloglu E. The effects of scolicidal
agents on liver and biliary tree. J Invest Surg 2004;17:323–326.
13. Yetim I, Erzurumlu K, Hokelek M, Baris S, Dervisoglu A, Polat C, Belet U, Buyukkarabacak Y, Guvenli A. Results of alcohol and albendazole injections in hepatic hydatosis: Experimental study. J
Gastroenterol Hepatol 2005;20:1442–1447.
14. Belghiti J, Benhamou JP, Houry S, Grenier P, Huguier M, Fékété F. Caustic sclerosing cholangitis. A complication of the surgical treatment of hydatid disease of the liver. Arch Surg 1986;121 (10):1162–1165.