Effects of hydrogen adsorption on single-wall carbon nanotubes: Metallic hydrogen decoration
O. Gu¨lseren,1,2T. Yildirim,1 and S. Ciraci3 1
NIST Center for Neutron Research, National Institute of Standards and Technology, Gaithersburg, Maryland 20899 2Department of Materials Science and Engineering, University of Pennsylvania, Philadelphia, Pennsylvania 19104
3Department of Physics, Bilkent University, Ankara 06533, Turkey 共Received 22 July 2002; published 6 September 2002兲
We show that the electronic and atomic structure of carbon nanotubes undergo dramatic changes with hydrogen chemisorption from first principle calculations. Upon uniform exohydrogenation at half coverage, the cross sections of zigzag nanotubes become literally square or rectangular, and they are metallic with very high density of states at the Fermi level, while other isomers can be insulating. For both zigzag and armchair nanotubes, hydrogenation of each carbon atom from inside and outside alternatively yield the most stable isomer with a very weak curvature dependence and a large band gap.
DOI: 10.1103/PhysRevB.66.121401 PACS number共s兲: 73.22.⫺f, 68.43.Bc, 68.43.Fg
Single wall carbon nanotubes 共SWNTs兲1,2 are among the most attractive systems for fabricating nanodevices because they exhibit many unusual mechanical and electronic prop-erties. Variation in the chiral vector or a small radial defor-mation result in marked changes ranging from insulating to ideal one-dimensional 共1D兲 conducting properties.3–7 The physical and chemical properties SWNTs can also be effi-ciently engineered by the adsorption of atoms or molecules on nanotubes.8 –15 Recently, it has been shown that the chemical activity of carbon nanotubes also depends on the chirality and radius,16,17 denoting tunable absorption of at-oms on SWNTs by structural deformation.17,18The interplay between adsorption and electromechanical properties can give rise to interesting physiochemical properties.17,18 The experimentally observed sensitivity of the electronic proper-ties of SWNTs to the presence of oxygen and hydrogen is clear evidence for the importance of this interplay.19
Motivated by these considerations, we have investigated the structural and electronic properties of hydrogenated SWNTs共H-SWNT兲 as a function of hydrogen coverage and decoration 共i.e. isomers兲 by extensive first principles calcu-lations. Our results indicate that hydrogen adsorption on nanotubes gives rise to many properties which can mediate important applications in molecular electronics. One of our most important results is that upon hydrogenation at uniform half coverage, the zigzag (n,0) SWNTs are metallized with high density of states at the Fermi level and the circular cross sections of the tubes are changed to square or rectangle ones. The carbon atoms near the corners form new diamondlike CuC bonds. Therefore, these carbon atoms are electroni-cally and chemielectroni-cally passive, isolating the four conducting faces of the H-SWNT. Hence, loosely speaking a uniform half-coverage (n,0) H-SWNT is composed of four-wire nanocable.
Our study comprises zigzag„(7,0), (8,0), (9,0), (10,0), (12,0)… and armchair „(6,6), (10,10)… SWNTs which are hy-drogenated at two different coverages. For full coverage (⌰⫽1), we consider two isomers; namely exohydrogenation where each carbon atom is bonded to a hydrogen atom from outside of the nanotube 共labeled by C4nH4n) and endo-exohydrogenation where each carbon atom is bonded to a hydrogen from inside and outside of the tube alternatively
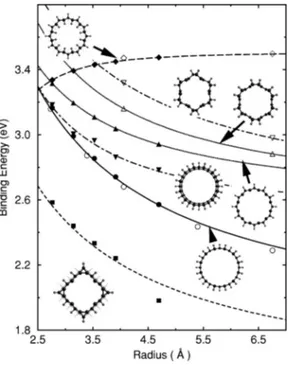
共labeled by C4nH2nH2n). For half coverage (⌰⫽0.5), we consider the three most interesting isomers 共labeled by C4nH2n); namely共i兲 uniform pattern where every other car-bon atom is car-bonded to a hydrogen from outside, 共ii兲 chain pattern where every other carbon zigzag chain is saturated by hydrogen, and 共iii兲 dimer pattern where every other carbon dimer rows perpendicular to the zigzag carbon chains are saturated by hydrogen. Fig. 1共a兲 shows these three isomers at half coverage.
The first principles total energy and electronic structure calculations have been carried out within the generalized gra-dient approximation 共GGA兲20 using the pseudopotential plane wave method21 in a supercell geometry. Details of the parameters used in this work are the same with those given in Ref. 17. Fully relaxed geometries are obtained by optimiz-ing all atomic positions and the lattice constant c along the tube axis until the maximum force and stress are less than 0.01 eV/Å and 0.1 GPa, respectively.
We find that geometric and electronic structures and bind-ing energies of H-SWNTs strongly depend on the pattern of hydrogenation 共i.e. decoration兲. The most remarkable effect is obtained when zigzag nanotubes are uniformly exohydro-genated at half coverage (⌰⫽0.5). Upon hydrogenation the structure undergoes a massive reconstruction, whereby circu-lar cross section of the (7,0) SWNT changes to a rectangucircu-lar one, and those of (8,0), (9,0), (10,0) and (12,0) change to square ones as shown in Fig. 1共b兲. These new structures are stabilized by the formation of new diamondlike CuC bonds with dCC⬃1.51–163 Å near the corners of rectangular or
square H-SWNTs. Hence, triangular and pentagonal C rings are formed instead of hexagonal. Depending on (2n mod 4), either one bond is formed just at the corners or two bonds at either side of the corners. Most interestingly, all these struc-tures are found to be metallic with a very large density of states at the Fermi level. The uniform adsorption at⌰⫽0.5 for zigzag nanotubes are metastable. Such a local minimum does not exist for armchair nanotubes, since uniformly ad-sorbed H atoms are rearranged upon relaxation by concerted exchange of CuH bonds to form zigzag chains along the tube axis. Several snap shots for hydrogen dimerization on an armchair tube are shown in Fig. 1共c兲. The cross sections
RAPID COMMUNICATIONS
PHYSICAL REVIEW B 66, 121401共R兲 共2002兲
of chain isomer at ⌰⫽0.5 of armchair tubes are polygonal where the corners are pinned by the zigzag H chains along the tube axis. For other isomers at half coverage as well as exo- and endo-exohydrogenations at full coverage, the cross sections remain quasicircular共see Fig. 2兲.
The average binding energies of H are obtained from the expression,
Eb⫽共ET,C4n⫹mEH⫺ET,C4nHm兲/m 共1兲
in terms of the total energies of the bare SWNT, ET,C4n, the
hydrogen covered SWNT, ET,C4nHm, and the energy of
atomic hydrogen, EH(m is the number of H atoms per unit
cell兲. According to the above definition, stable structures have positive binding energies. The average binding energies as a function of nanotube radius are shown in Fig. 2. The variation of Eb with the radius of the bare nanotube can be
fitted to a simple formula,16,17
Eb⫽Eo共⌰兲⫹
Cp共⌰兲
Rp . 共2兲
Note that 1/R form共i.e. p⫽1) is quite common to SWNTs and scales various properties.4,5,7,16,17 Here Eo(⌰) is the
binding energy when R→⬁, and hence corresponds to the adsorption of H on graphene at a given coverage, while Cp(⌰) is a constant that depends on coverage ⌰, and
rep-resents the curvature effect. Calculated Eb’s are fitted to Eq.
共2兲 with the values listed in Table I. While Eb increases with
decreasing R in the case of exohydrogenation, this trend is reversed for endo-exohydrogenation due to increased HuH repulsion inside the tube at small R. Nevertheless, the endo-exohydrogenation of SWNTs, which transforms the s p2 to s p3-like bonding, gives rise to the highest binding energy saturating at 3.51 eV as R→⬁. At this limit, the exo-endohydrogenated graphene 共from above and below兲 is FIG. 1.共a兲 A view of three different isomers of H-SWNT at half
coverage. The left and up arrows indicate the tube axis for armchair and zigzag nanotubes, respectively. Carbon atoms which are bonded to hydrogens are indicated by dark color.共b兲 A side and top view of a (12,0) H-SWNT, indicating the square cross section of a uni-formly exohydrogenated nanotube at half coverage. 共c兲 Several snap shots during the relaxation steps of an armchair (6,6) H-SWNT, indicating that an uniform exohydrogenation at half cov-erage is not stable against forming a chain isomer.
FIG. 2. Average binding energies, Eb, of hydrogen atoms
ad-sorbed on various zigzag and armchair SWNTs versus bare tube radius R. Filled and open symbols are for zigzag and armchair nanotubes, respectively. Circles and diamonds are for exo- and endo-exohydrogenation at full coverage, respectively. The filled squares show the zigzag nanotubes uniformly exohydrogenated at half coverage. The chain and dimer patterns of adsorbed hydrogen atoms at half coverage are shown by down- and up-triangles, re-spectively. Curves are analytical fits explained in the text. Insets show top view of several H-SWNT isomers.
TABLE I. Values of the parameters E0(eV) and Cp(⌰)
(eV Åp) given in Eq.共2兲 to fit the binding energies.
Hydrogen Zigzag Tubes Armchair Tubes
decoration E0 Cp(⌰) Cp(⌰)
Exo⌰⫽1.0 1.75 C1⫽3.87 C1⫽3.87
Exo-endo⌰⫽1.0 3.51 C3⫽⫺3.49 C3⫽⫺3.49 Uniform⌰⫽0.5 1.41 C1⫽3.19 not stable
Chain⌰⫽0.5 2.50 C1⫽0.53 C1⫽3.16
⫹C2⫽3.70 ⫹C2⫽0.73
Dimer⌰⫽0.5 2.55 C1⫽1.41 C1⫽1.87
⫹C2⫽1.94 ⫹C2⫽2.42
RAPID COMMUNICATIONS
O. GU¨ LSEREN, T. YILDIRIM, AND S. CIRACI PHYSICAL REVIEW B 66, 121401共R兲 共2002兲
buckled by 0.46 Å as if two diamond 共111兲 planes with in-terplanar distance of 0.50 Å.
As with the atomic structure, the electronic structure of SWNTs undergo important changes as a result of hydrogena-tion. For bare zigzag SWNTs the variation of the band gap, Eg, with n is rather complex due to interplay between zone folding and curvature induced*–* mixing7,22while bare armchair SWNTs are metallic. Here we find that sizable band gaps are opened as a result of hydrogenation共Fig. 3兲. At ⌰ ⫽1, the band gap displays a similar behavior for both types of nanotubes and hydrogenation, and decreases with inreas-ing R. Relatively larger band gaps共in the range of 3.5–4 eV兲 of endo-exohydrogenated SWNTs (⌰⫽1) can be explained by the fact that the adsorption of H alternatively inside and outside leads to the atomic configuration closer to the dia-mond structure having rather large band gap (Eg⫽5.4 eV).
The effect of hydrogenation on the electronic structure is even more remarkable at⌰⫽ 0.5 and is the most interesting aspect of our study. Depending on the pattern of hydrogen adsorption, an isomer can be either a metal or insulator. For example, all uniform (n,0) H-SWNTs are metallic. On the other hand, the chain pattern realized on the (n,0) SWNTs results in two doubly degenerate, almost dispersionless states at the valence and conduction band edges. The band gap Eg between these states decreases with increasing R. When n is odd, Eg is large关e.g Eg⫽2.1 eV for (7,0)]. When n is even,
the doubly degenerate band at the conduction band edge moves towards the valence band edge and splits as bonding and antibonding states. As a result Egis reduced significantly
becoming only a pseudogap for large and even n. Finally, the dimer row isomers are insulators and the Eg increases with
increasing radius. Surprisingly, there are two dispersive bands with⬃1 eV bandwidth at both band edges and extre-mum moves from the center of Brillioun zone (⌫ point兲 to the zone edge (Z point兲.
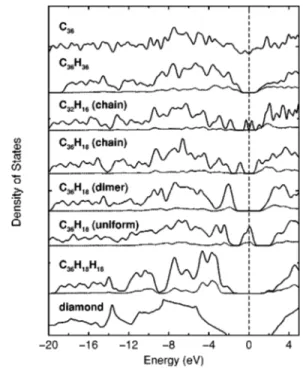
Hydrogen adsorption induced dramatic changes of elec-tronic structure are demonstrated by total density of states 共DOS兲, D(E), of (9,0) nanotubes in Fig. 4. First of all, the small band gap of the bare (9,0) SWNT is opened by⬃2 eV upon exohydrogenation at ⌰⫽1. The band gap is still sig-nificant for⌰⫽0.5 with the chain pattern, and increases to 4 eV for dimer pattern. However, a similar chain pattern in the (8,0) H-SWNT 共see C32H16 in Fig. 4兲 has a much smaller band gap. Suprisingly, all zigzag nanotubes uniformly exo-hydrogenated at⌰⫽0.5 are metals. As displayed in the sixth panel of Fig. 4 for C36H18 共uniform兲, their total density of states are characterized by a peak yielding high state density at EF. While carbon states are pushed apart, yielding a
⬃4 –5 eV gap, a new dispersive metallic band with ⬃1 –2 eV bandwidth crosses the Fermi level. Apart from being an ideal 1D conductor, this very high density of states at EF might lead to superconductivity. Note that, these
uni-form H-SWNTs undergo a massive reconstruction and their circular cross sections change into square ones with the for-mation of new CuC bonds (C4) at the corners. All C atoms without H attached共except those at corners兲 as well as the H atoms at the center of four planar sides contribute to the high D(EF). This way four individual conduction paths are
formed on each side of square tube. It is emphasized that the transformation from the s p2 to the s p3 bonding underlies various effects discussed in this study. The H-SWNTs, espe-cially C4nH2nH2n structures can be conceived as if they are more diamondlike than graphitic. Our arguments are justified by the comparison of D(E) of the endo-exohydrogenated FIG. 3. The band gaps, Eg, versus the bare nanotube radius R.
Filled and empty symbols indicate zigzag and armchair SWNTs, respectively. Squares show nonmonotonic variation of the band gap of the bare zigzag nanotubes. Exo and endo-exohydrogenated nano-tubes (⌰⫽1) and chain and row patterns of adsorbed hydrogen atoms at half coverage are shown by circles, diamonds, down- and up-triangles, respectively. Lines are guide to eye.
FIG. 4. Comparison of the electronic density of states共DOS兲 of a bare (9,0) nanotube (C36) and its various hydrogenated isomers. DOS of a chain pattern 共8,0兲 H-SWNT at half coverage (C32H16) and bulk diamond共bottom panel兲 are also shown for comparison. The zero of energy is taken at the Fermi energy showed by vertical dashed line. The dotted lines are the partial density of states re-solved on hydrogen atoms.
RAPID COMMUNICATIONS
EFFECTS OF HYDROGEN ADSORPTION ON SINGLE- . . . PHYSICAL REVIEW B 66, 121401共R兲 共2002兲
(9,0) with that of bulk diamond in Fig. 4. Apart from open-ing a large band gap, the quasimetallic D(E) of the bare (9,0) is modified to become similar to that bulk diamond. The latter has relatively larger valence band width due to coupling of distant neighbors.
In conclusion, our study reveals many important and novel effects of hydrogen adsorption on SWNTs, and brings a number of new problems and issues to be explored. For example, one can argue that the band gap of a SWNT can be engineered by the controlled hydrogenation of a single nano-tube as in the alloy of SixGe1⫺x. A number of isomers which can be tailored with different hydrogen decoration provide options in developing new materials. Furthermore, multiple
quantum well structures, or one-dimensional chain of quan-tum dots, can be tailored by periodic and modulated hydro-genation of a single nanotube. Finally, the very high density of states at the Fermi level of uniform pattern isomer at half coverage may result in to superconductivity in SWNT based nanowires. Needless to say, realization of the systems pro-posed here will be an experimental challenge. However, the fact that other carbon clusters such as cubane, dodecahe-drane, and C60H32 have been successfully synthesized sug-gests that this is not impossible.
This work was partially supported by the NSF under Grant No. INT01-15021 and TU¨ BI´TAK under Grant No. TBAG-U/13共101T010兲.
1S. Iijima, Nature共London兲 354, 56 共1991兲.
2R. Saito, G. Dresselhaus, and M.S. Dresselhaus, Physical Prop-erties of Carbon Nanotubes 共Imperial College Press, London, 1998兲.
3N. Hamada, S. Sawada, and A. Oshiyama, Phys. Rev. Lett. 68, 1579共1992兲.
4C.T. White, D.H. Robertson, and J.W. Mintmire, Phys. Rev. B 47, 5485共1993兲.
5J.W.G. Wildo¨er et al., Nature 共London兲 391, 59 共1998兲; T.W. Odom et al., Nature共London兲 391, 62 共1998兲.
6Z. Yao, C.L. Kane, and C. Dekker, Phys. Rev. Lett. 84, 4613 共2000兲.
7O. Gu¨lseren, T. Yildirim, and S. Ciraci, Phys. Rev. B 65, 153405 共2002兲.
8A.C. Dillon et al., Nature 共London兲 386, 377 共1997兲; C. Liu et al., Science 286, 1127共1999兲.
9K.N. Kudin, G.E. Scuseria, and B.I. Yakobson, Phys. Rev. B 64, 235406共2001兲.
10K. Tada, S. Furuya, and K. Watanabe, Phys. Rev. B 63, 155405 共2001兲.
11Y. Ma, Y. Xia, M. Zhao, R. Wang, and L. Mei, Phys. Rev. B 63, 115422共2001兲.
12S.M. Lee and Y.H. Lee, Appl. Phys. Lett. 76, 2877共2000兲. 13S. Chan et al., Phys. Rev. Lett. 87, 205502共2001兲.
14K.N. Kudin, H.F. Bettinger, and G.E. Scuseria, Phys. Rev. B 63, 045413共2001兲.
15A.N. Andriotis, M. Menon, D. Srivastava, and G. Froudakis, Phys. Rev. B 64, 193401共2001兲.
16T. Yildirim, O. Gu¨lseren, and S. Ciraci, Phys. Rev. B 64, 075404 共2001兲.
17O. Gu¨lseren, T. Yildirim, and S. Ciraci, Phys. Rev. Lett. 87, 116802共2001兲.
18D. Srivastava et al., J. Phys. Chem. B 103, 4330共1999兲. 19P.G. Collins et al., Science 287, 1801共2000兲; J. Kong et al., ibid.
287, 622共2000兲.
20J.P. Perdew and Y. Wang, Phys. Rev. B 46, 6671共1992兲. 21M.C. Payne et al., Rev. Mod. Phys. 64, 1045共1992兲.
22X. Blase, L.X. Benedict, E.L. Shirley, and S.G. Louie, Phys. Rev. Lett. 72, 1878共1994兲.
RAPID COMMUNICATIONS
O. GU¨ LSEREN, T. YILDIRIM, AND S. CIRACI PHYSICAL REVIEW B 66, 121401共R兲 共2002兲