appended rhodamine derivative in aqueous media
Serkan Erdemir
Selcuk University, Science Faculty, Department of Chemistry, Konya, 42031, Turkey
A R T I C L E I N F O Keywords: Triphenylamine Rhodamine PET FRET A B S T R A C T
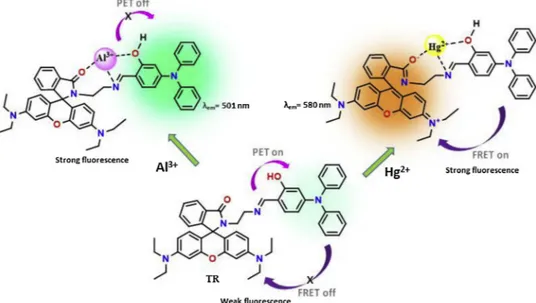
Triphenylamine appended rhodamine (named probe TR) was built as a selectivefluorescent probe for Al3+and
Hg2+ions through different sensing mechanisms. TR demonstrated a distinct fluorescence enhancing at 501 and
580 nm towards Al3+and Hg2+ions due to the“PET-off” (Photoinduced electron transfer-off) and “FRET-on”
(Fluorescence resonance energy transfer-on) processes, respectively. The binding modes betweenTR with Al3+
and Hg2+were found to be 1:1 by job plot analysis. The limits of detection ofTR for sensing Al3+and Hg2+are
down to 71.8 nM and 0.48μM, respectively. At the same time, the complexation details between the probe TR with Al3+and Hg2+ions were investigated by FTIR,1HNMR experiments and DFT calculations. Moreover,
simple test papers coated probeTR were successively developed for the rapid monitoring of Al3+and Hg2+ions.
1. Introduction
Selective monitoring of the metal ions is of great importance, as they play critical tasks in biological systems and the environment. Aluminum is the third abundant element in the lithosphere and used widely in various forms like - food packaging, light alloys, pharma-ceuticals, deodorants etc. [1,2]. However, the toxicity of aluminum may be caused to a number of diseases, including dialysis encephalopathy, Parkinson's disease, Alzheimer's disease and osteoporosis, etc. [3]. With regard to WHO, the average daily human intake of Al3+of around
3–10 mg and the weekly tolerable dietary intake of 7 mg/kg body weight [4–7]. On the other hand, mercury is a heavy-transition metal, and has an extremely toxic impact on living and environment systems. Mercury pollution is a global problem, highly hazardous and wide-spread, causing serious environmental [8] and health issues [9], such as severely damage of the human heart, kidney, stomach, and genes [10–13]. For these reasons, it is important to monitor the concentration levels of aluminum and mercury in the environment and some scientific fields. Recently, research interest has focused on developing new fluorescent chemosensors due to the advantage of high sensitivity, easy application, rapidity, low cost of equipment. In addition, it is a great challenge to develop simple fluorescent probes for the simultaneous detection of Al3+and Hg2+ions
Triphenylamine is one of the most frequently used functional units in opto- and electro-active materials [14,15]. Otherwise, rhodamine based sensors have the advantages of high quantum yield, easily modified structure, long absorption and emission wavelength. The
rhodamine derivatives in spirocylic form are colorless and non-fluor-escent, whereas metal ion caused spirolactam ring-opening leads to strongfluorescence and color changes [16]. Variousfluorescent sensors containing triphenylamine or rhodamine for different cations have been reported [17–22]. Unfortunately, one major limitation of these rhoda-mine and triphenylarhoda-mine chemosensors is that they can detect one or two analyte using a single test method with same signal channels. In recent years, a new design concept of“single sensor for multiple tar-gets” for a sensor received increasing attention due to its the ability to detect more than one target simultaneously with multiple signal channels. In this regard, we think that the integration of rhodamine and triphenylamine rings is very interesting for multiple ion sensing.
Here, it was reported that the synthesis of a novel triphenylamine appended rhodamine derivative (TR) and its cation sensing properties. TR showed high selectively towards Hg2+
and Al3+ions via two dif-ferent sensing mechanisms i.e. Hg2+ion via spirolactam ring-opening
in rhodamine and Al3+ion via PET process in triphenylamine moiety in
MeCN/H2O (v/v, 9/1). Hg2+ and Al3+ions induced the remarkable
enhancement in fluorescence emission of TR centered at 580 and 501 nm with the low detection limits.
2. Experimental 2.1. Materials and methods
FTIR (ATR) spectra were measured by using a Bruker FTIR instru-ment. NMR spectra were collected with a Varian 400 MR spectrometer
https://doi.org/10.1016/j.snb.2019.04.037
Received 23 February 2019; Received in revised form 27 March 2019; Accepted 8 April 2019 E-mail address:serdemir82@selcuk.edu.tr.
Available online 08 April 2019
by using chloroform (CDCl3) and dimethylsulfoxide (DMSO-d6) as the
solvents. The UV–vis and fluorescence spectra were gained on a Shimadzu 1280 and Perkin Elmer LS 55 spectrophotometer, respec-tively. All pH measurements were made with a Crison Basic 20 pH meter. The reagents used in experiments were purchased from com-mercial suppliers.
2.2. Synthesis of TR probe
As depicted in Scheme 1, compound1 was synthesized via Vils-meier-Haack reaction by using POCl3in DMF [23], then the methoxy
group in1 was converted to hydroxyl group with BBr3via hydrolysis
reaction to give compound2 [24]. On the other hand, the ethylene diamine appended rhodamine derivative (3) was obtained according to known procedure [22]. For probeTR, a solution of compound 3 (0.20 g, 0.41 mmol) and compound2 (0.125 g, 0.43 mmol) in methanol (10 mL) was refluxed for 24 h in presence of molecular sieve (4 Å). Then, the mixture wasfiltered, cooled and the solid product as dark-orange was produced and washed with anhydrous methanol for three times, dried to giveTR. Yield: 75%; Mp: 108–109 °C; FTIR (ATR): 1690 cm−1(C]
O), 1638 cm−1(C]N);1H NMR (400 MHz, 25 °C, CDCl
3):δ 13.35 (br s,
1H, OH), 7.92–7.95 (m, 2H, CHN, ArH), 7.46 (m, 2H, ArH), 7.27–7.31 (m, 4H, ArH), 7.08–7.15 (m, 7H, ArH), 6.93 (d, 1H, J = 7.80 Hz, ArH), 6.40–6.47 (m, 6H, ArH), 6.28 (d, 2H, J = 7.80 Hz, ArH), 3.29–3.42 (m, 12H, -NCH2CH3 and –NCH2CH2), 1.19 (t, 12H, J = 6.31 Hz, -NCH2CH3). 13C NMR (100 MHz, 25 °C, CDCl3) δ 168.17, 164.67, 163.82, 153.57, 153.34, 151.68, 148.85, 146.79, 132.41, 131.86, 131.13, 129.35, 128.75, 128.01, 125.95, 124.09, 123.80, 122.85, 112.83, 111.57, 108.65, 108.13, 105.53, 97.85, 64.91, 56.06, 44.37, 41.08, 12.64; Anal. Calcd for C49H49N5O3(755.38): C, 77.85; H, 6.53;
N, 9.26. Found: C, 77.93; H, 6.61; N, 9.53. 2.3. Analytical procedure and calculation methods
The stock solution ofTR was prepared in DMSO at 0.01 M and then diluted in MeCN/H2O (v/v, 9/1) for spectroscopic analysis. The
per-chlorate forms of metal ions (Na+, Cs+, Ca2+, Mg2+, Ba2+, Sr2+, Zn2+, Fe2+, Mn2+, Hg2+, Cd2+, Al3+, Co2+, Ni2+, Pb2+, Cr3+, Ag+,
Cu2+, Fe3+) were used and their solutions were prepared in deionized
water at 10 mM. Spectral data were recorded at 1- and 10-min fol-lowing addition of Al3+and Hg2+ions, respectively. From the
fluor-escence titration data, the association constants (logK) and detection limits (DL) were established by using Benesi-Hildebrand and DL = 3 s/ k, respectively. In addition, the density functional theory (DFT) analysis was applied for the theoretical calculations ofTR and its complexes. The optimization ofTR and its complexes were performed using the
Gaussian 16 program with the 6–31 G (d,p) and LANL2DZ basis sets [25–27].
3. Results and discussion 3.1. Fabricating of probe TR
As depicted in Scheme 1, the formylation of 3-methoxy-triphenylamine via Vilsmeier-Haack reaction wasfirstly performed, and then proceed by its hydrolysis in BBr3to give2. To afford probe TR, the
aldehyde derivative of triphenylamine (2) was interacted with ethyle-nediamine appended rhodamine (3) in methanol. The characterization of TR was performed in detail by using spectroscopic techniques (Supporting information, Figs. S1-S6).
3.2. Dual-sensing of Al3+and Hg2+by probeTR
We implemented the spectroscopic properties of TR for various metal ions using the fluorescence spectroscopy studies. The fluores-cence responses ofTR to different metal ions were observed in MeCN/ H2O (v/v, 9/1) by exciting at 365 nm. As seenFig. 1a,TR showed a
weak emission band at about 501 nm owing to PET process without metal ion. The significant fluorescence enhancing was observed only with addition of Al3+ within 1 min. at 501 nm, while no significant fluorescence changes in presence of other metal ions, except for Hg2+,
which shows that Hg2+have capacity to cause the spiro-lactam
ring-opening ofTR due to lack of time. Under same conditions,fluorescence spectral data were also recorded at 10 min following addition of metal ions. After this time, the probeTR indicated an obvious‘turn-on’ re-sponse toward only Hg2+at 580 nm, confirming the presence of ring-opening process from the spirolactam (nonfluorescent) to acyclic xan-thene (fluorescent), as well as green-fluorescence response for Al3+at
501 nm upon excitation atλex= 365 nm (Fig. 1b). Thus, the interaction
time ofTR with Al3+
and Hg2+ions were selected as 1 and 10 min in following experiments, respectively.
To study the sensitivity of probeTR with respect to Al3+and Hg2+, fluorescence titration studies of TR were realized with Al3+
and Hg2+ ions (Fig. 2). Titration data were recorded at 1- and 10-min following addition of Al3+and Hg2+ions, respectively. As seen inFig. 2a, upon
the addition of Al3+into a solution ofTR (2.0μM) in MeCN/H2O (v/v,
9/1), the emission intensity at 501 nm steadily increased owing to the inhibition of PET process and it reached a maximum after the addition of 1.0 equiv. of Al3+, which implied a 1:1 binding ratio between Al3+ andTR (inset inFig. 2a). The imine (CH]N) unit in TR act as electron donors, which quenches thefluorescence of triphenylamine in the ex-cited state. When the imine unit coordinated with Al3+, PET process Scheme 1. Chemical structure and synthetic route of probe TR.
occurring from the imine unit to triphenylamine moiety is blocked and therefore the strong fluorescence emission was appeared at 501 nm belong to triphenylamine fluorophore. Also, it clearly indicates the formation of a new species by the interaction of Al3+withTR. On the other hand, while in case of Hg2+ion, the weakfluorescence emission
at 501 nm completely quenched, while the band centering around 580 nm became prominent (Fig. 2b). The emission intensity at 580 nm stabilized after the amount of added Hg2+ion reached 20 equiv. with a
defined emission point. This selectivity can be explained by FRET (fluorescence resonance energy transfer) process, which was enabled the energy to transfer from the triphenylamine donor to the rhodamine acceptor. The characteristic emission of a rhodamine-based dye in the spiro-ring closed form is inhibited, and the FRET dyad shows only the emission of the donor. Consequently, the FRET process of the system is prohibited, whereas, after complexation with metal ion, the spiro-ring of the rhodamine dye opens, which gives a strong emission, conse-quently activating the FRET process“ON” [28]. The spectral overlap of the triphenylamine emission with the absorption of rhodamine clearly shows the possibility of a FRET process (Fig. S7). In addition, the stoichiometric ratios ofTR with Al3+and Hg2+ions were determined by Job's plot analysis and found to be 1:1 (Fig. 3). The association constants (logK) were calculated to be 4.91 for Al3+and 5.26 for Hg2+
according to Benesi–Hildebrand plots (Fig. S8) [29]. The plot of emis-sion intensities at 501 for Al3+ and 580 nm for Hg2+ vs the
con-centration of Al3+and Hg2+ions was linear. From the linear relation
grapies (Fig. S9), the limits of detection (LOD) ofTR were calculated to be 71.8 nM for Al3+and 0.46μM for Hg2+. In addition, the sensing
properties ofTR are comparable to those of some PET and FRET based
fluorescent sensors for Al3+
and Hg2+ [30–36], in terms of sensing method (single and dual wavelength), sensing mechanism and detec-tion limit, which indicate thatTR has many advantages (Table 1).
Moreover, the conversions in the absorption spectra ofTR were measured in presence of Al3+ and Hg2+. As shown inFig.4, the
ab-sorption bands belong toTR (20μM) are observed at 275, 324 and 350 nm, which could be attributed toπ–π* and n–π* transitions in TR. Upon addition of Al3+(5.0 equiv), the absorbance bands at 275 and
324 nm decreased and the absorption band peaked at 350 nm were bathochromically shifted to 380 nm upon cation recognition. Accord-ingly, Hg2+(5.0 equiv) induced the decrase of absorbance intensity at
324 nm and the shift to 388 nm of the absorbance band at 350 nm. Differenlty, a new band at 560 nm was generated in presence of Hg2+
as a result of hydrolysis of probeTR followed by the ring opening. The selectivity ofTR towards Al3+and Hg2+was estabished by the
comparative tests through Fluorescence spectroscopy in the presence of different metal ions. For this, TR (2.0 μM) was treated with 1.0 equiv. of Al3+and 5.0 equiv. of Hg2+in the presence of other competitive metal
ions (10 equiv.), and the emission intensities at 501 and 580 nm were measured. As depicted in Fig. S10, the other metal ions did not cause any obvious interference with the detection of Al3+and Hg2+ions.
These investigated results showed clearly that probe TR has an ex-cellent selectivity towards Al3+ and Hg2+ ions over the other com-peting cations. Also, the effect of pH on the fluorescence of TR and its Al3+/ Hg2+complexes was observed. As seen inFig.5a, the
fluores-cence of metal ion-freeTR was weak which shows that the spiro-lactam form ofTR is stable in the pH range from 5.0–10.0. However, the ad-dition of Al3+and Hg2+ions led to thefluorescence enhancement at
Fig. 1. Fluorescence spectra of TR (2.0μM) in presence of different metal ions (Na+, Cs+, Ca2+, Mg2+, Ba2+, Sr2+, Zn2+, Fe2+, Mn2+, Cd2+, Co2+, Ni2+, Pb2+,
Cr3+, Ag+, Cu2+, Fe3+, Al3+, Hg2+) in MeCN/H
2O (v/v, 9/1) at 1 min (a) and 10 min (b) atλex= 365 nm.
Fig. 2. Fluoresence spectra of TR (2.0μM) with various concentrations of Al3+(a) and Hg2+(b) in MeCN/H
2O (9/1, v/v). Insets: the relationship between the
501 and 580 nm between pH 5.0–8.0, but fluorescence intensity of TR-Al3+and TR-Hg2+complexes decreased rapidly in the pH > 8.0, since
−OH ions interacted with Al3+
and Hg2+ion. These data clearly de-monstrated thatTR can be used for Al3+and Hg2+ions detection in
this pH range (5.0–8.0). We also performed the test paper studies of probeTR in order to achieve more convenient detection. In this process, the test papers were immersed in the solution ofTR in MeCN and then dried in air. Later, these papers were interacted by Al3+ and Hg2+
solutions with different concentration. As shown inFig. 5b, under the 365 nm UV lamb, the color of test papers became orange-, and green-fluorescent in presence of Hg2+
and Al3+, respectively which indicate the potential use of probeTR.
3.3. 1H NMR/FTIR studies and DFT calculations 1
H NMR experiments were conducted in DMSO-d6to examine the
Fig. 3. Job’s plots of the complexation between TR with Al3+(a) and Hg2+(b).
Table 1
Comparison of some PET and FRET basedfluorescent sensors for Al3+and Hg2+.
Metal(s) Detection Limit (M) Solvent Sensing Mechanism Sensing Method Ref
Al3+ 6 × 10−7 EtOH:H
2O (4:1) PET Single wavelength [30]
Al3+ 3.4 × 10−7 EtOH:Tris (1:1) PET Single wavelength [31]
Al3+ 7.21 × 10−8 HEPES PET Single wavelength [32]
Al3+
Hg2+
1.9 × 10−7 1.26 × 10−7
MeCN:H2O (3:1) FRET Single wavelength [33]
Hg2+ 1.16 × 10−6 MeOH:H
2O (7:3) FRET Single wavelength [34]
Fe3+ Hg2+ 5.7 × 10−7 2.72 × 10−6 EtOH:H2O (1:1) PET FRET Dual wavelength [35] Al3+ Hg2+ 1.6 × 10−7 1.9 × 10−8
MeCN:H2O (1:1) Complexation Single
wavelength [36] Al3+ Hg2+ 7.18 × 10−8 0.48 × 10−6 MeCN:H2O (9:1) PET FRET
interaction between TR and Al3+ or Hg2+. As seen in Fig.6a, the
phenolic OH (Ha) and imine proton (Hb) signals of TR in DMSO-d6
appeared atδ 13.23 and δ 7.85 ppm, respectively. Upon the addition Al3+, the imine proton signal was shifted downfield (δ 8.00 ppm), while
the phenolic OH signal was shifted upfield (δ 11.78 ppm). Also, the Hc
proton atδ 7.74 was slightly downfield shifted to δ 7.77 ppm. Other-wise, after the addition Hg2+, the phenolic OH and imine protons ap-parent upfield and downfield shifts of 1.41 and 0.20 ppm, respectively which showed the coordination of the phenolic OH and imine units. Moreover, the different splits were observed for the Hcand other
pro-tons (ArH) in rhodamine ring, due to the change in electron density resulting from the spiro-lactam ring open induced Hg2+ion [37]. The
spectral changes in1H NMR suggest the complexation betweenTR and Hg2+and Al3+through the imine, phenolic OH and carbonyl units. The
binding ofTR and Al3+ and Hg2+ was further supported by FT-IR
analysis. The FTIR spectra of TR, TR-Al3+ and TR-Hg2+ were also
collected, and depicted inFig. 6b. The characteristic carbonyl (C]O) band ofTR at 1690 cm−1disappeared in the presence of Hg2+,
sup-porting the spirolactam ring-open mechanism, and a new band belong to C]N imine observed at 1658 cm−1[38,39]. In presence of Al3+, the
vibration of the carbonyl (C]O) band at 1690 cm−1
belong toTR was shifted to lower frequency (1673 cm−1), while the imine (C]N) at 1638 cm−1was shifted to higher frequency (1646 cm−1), indicating that the carbonyl and imine units inTR are coordinated to Al3+ion. Based on these results, the proposed interaction model betweenTR and Al3+and Hg2+ion was shown inScheme 2.
In addition, the DFT and TDDFT calculations of probeTR and its Al3+and Hg2+complexes have been performed to acquire their HOMO
Fig. 4. UV–vis changes of TR (20 μM) with 5 equiv. of Al3+and Hg2+in in MeCN/H
2O (9/1, v/v).
Fig. 5. (a) The influence of pH on the fluorescence of TR (2.0 μM) in absence and presence of Al3+and Hg2+ions; (b) Photographs of the test papers coatedTR for
detecting Al3+and Hg2+ions with different concentrations.
and LUMO energy levels. The orbital energies were detected using the Gaussian 16 program at the B3LYP with 6-31 G and LANL2DZ basic sets [25–27]. As seen inFig. 7, the HOMO and LUMO of probe TR were spread over the triphenylamine and imine moieties. However, the electronic dispersal in TR-Al3+was relatively different. The HOMO was
centered on the triphenylamine moiety, while the LUMO localized around the Al3+and imine center. Otherwise, the HOMO and LUMO of
the TR-Hg2+complex was both distributed over the xanthene part. The
calculated energy gap between HOMO and LUMO orbitals ofTR, TR-Al3+and TR-Hg2+complexes are 3.83, 3.05 and 2.81 eV respectively, confirming a red shift in the absorption. These results also revealed that the binding of Al3+and Hg2+toTR stabilizes the system as evident
from the lower HOMO–LUMO energy gap of the complexes compared to TR (Fig. 7).
4. Conclusion
In summarize, it was designed and synthesized a novel triphenyla-mine appended rhodatriphenyla-mine derivative (TR) as fluorescent sensor for Al3+and Hg2+ over other metal ions in MeCN/H2O (9/1, v/v).TR
indicated the dual sensing behaviour forfluorescent detection of Al3+
via“PET-off” at 501 nm and Hg2+via“FRET-on” at 580 nm. The limits
of detection (LOD) for Al3+and Hg2+were measured to be 71.8 nM
and 0.48μM, respectively which is sufficiently low to enable the de-tection of these ions in practical applications. In addition, the design strategy of twofluorophores with different recognition signals or the identicalfluorescent responses would help to extend the development of more analogues FRET and PET-basedfluorescent sensors.
Acknowledgements
I am grateful for the financial supports from the Research Foundation of Selcuk University (BAP).
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.snb.2019.04.037. References
[1] S. Goswami, S. Paul, A. Manna, Selective naked eye detection of Al(III) and PPi in aqueous media on a rhodamine–isatin hybrid moiety, RSC Adv. 3 (2013) 10639–10643.
[2] S.V. Verstraeten, L. Aimo, P.I. Oteiza, Aluminium and lead: molecular mechanisms of brain toxicity, Arch. Toxicol. 82 (2008) 789–802.
[3] (a) G. Berthon, Aluminium speciation in relation to aluminium bioavailability,
Scheme 2. Proposed mechanism for Al3+and Hg2+detection by probeTR.
fluorescent molecular sensors for cation recognition, Coord. Chem. Rev. 205 (2000) 3–40.
[6] Z. Krejpcio, R.W. Wojciak, The influence of Al3+ions on pepsin and trypsin activity in vitro, Pol. J. Environ. Stud. 11 (2002) 251–254.
[7] J.C. Qin, T.R. Li, B.D. Wang, Z.Y. Yang, L. Fan, A sensor for selective detection of Al3+based on quinoline Schiff-base in aqueous media, Synth. Met. 195 (2014) 141–146.
[8] (a) P.B. Tchounwou, W.K. Ayensu, N. Ninashvili, D. Sutton, Review: environ-mental exposure to mercury and its toxico pathologic implications for public health, Environ. Toxicol. 18 (2003) 149–175;
(b) Q. He, E.W. Miller, A.P. Wong, C.J. Chang, A selectivefluorescent sensor for detecting lead in living cells, J. Am. Chem. Soc. 128 (2006) 9316–9317; (c) X. Liu, X. Shu, X. Zhou, X. Zhang, J. Zhu, Ultra-sensitivefluorescent sensor for Hg2+based on a donor–acceptor–donor framework, J. Phys. Chem. A 114 (2010) 13370–13375.
[9] (a) S. Ando, K. Koide, Development and applications offluorogenic probes for mercury (II) based on vinyl ether oxymercuration, J. Am. Chem. Soc. 133 (2011) 2556–2566;
(b) Z. Gu, M. Zhao, Y. Sheng, L.A. Bentolila, Y. Tang, Detection of mercury ion by infraredfluorescent protein and its hydrogel-based paper assay, Anal. Chem. 83 (2011) 2324–2329;
(c) S. Voutsadaki, G.K. Tsikalas, E. Klontzas, G.E. Froudakis, H.E. Katerinopoulos, A“turn-on” coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media, Chem. Commun. (Camb.) 46 (2010) 3292–3294. [10] O. Brummer, J.J. La Clair, K.D. Janda, Practical screening of mercury
contamina-tion infish tissue, Bioorg. Med. Chem. 9 (2001) 1067–1071.
[11] A. Mitra, A.K. Mittal, C.P. Rao, Carbohydrate assistedfluorescence turn-on gluco-imino-anthracenyl conjugate as a Hg (II) sensor in milk and blood serummilie, Chem. Commun. (Camb.) 47 (2011) 2565–2567.
[12] P. Grandjean, P. Weihe, R.F. White, F. Debes, Cognitive performance of children prenatally exposed to“safe” levels of methylmercury, Environ. Res. 77 (1998) 165–172.
[13] E.M. Nolan, S.J. Lippard, A“Turn-On” fluorescent sensor for the selective detection of mercuric ion in aqueous media, J. Am. Chem. Soc. 125 (2003) 14270–14271. [14] R. Tarsang, V. Promarak, T. Sudyoadsuk, S. Namuangruk, N. Kungwan,
S. Jungsuttiwong, Modification of D-A-π-A configuration toward a high-perfor-mance triphenylamine-based sensitizer for dye-sensitized solar cells: a theoretical investigation, Z. Fã¼r Phys. Chemie/international J. Res. Phys. Chem. Chem. Phys. 15 (2014) 3809–3818.
[15] M.P. Balanay, C.M. Enopia, S.H. Lee, D.H. Kim, Theoretical design of triphenyla-mine based derivatives with asymmetric D-D-pi-A configuration for dye-sensitized solar cells, Spectrochim. Acta A. Mol. Biomol. Spectrosc. 104 (2015) 382–391. [16] X.Q. Chen, T. Pradhan, F. Wang, J.S. Kim, J. Yoon, Fluorescent chemosensors based
on spiroring-opening of xanthenes and related derivatives, Chem. Rev. 112 (2012) 1910–1956.
[17] B.X. Shen, Y. Qian, Triphenylamine-BODIPYfluorescent dendron: click synthesis andfluorometric chemodosimeter for Hg2+, Fe3+based on the C=N bond, Chemistry Select 2 (2017) 2406–2413.
O. Meth-Cohn, S.P. Stanforth, The Vilsmeier–Haack reaction, Comprehens. Organic Synth. 2 (1991) 777–794.
[24] A. Matoliukstyte, J.V. Grazulevicius, V. Jankauskas, Glass-forming hole-trans-porting TriphenylamineBased hydrazones with reactive functional groups, Mol. Cryst. Liq. Cryst. 466 (2007) 85–100.
[25] M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, et al., Gaussian 16, Revision A. 03. Wallingford CT: Gaussian Inc, (2016).
[26] A.D. Becke, Density‐functional thermochemistry. III. The role of exact exchange, J. Chem. Phys. 98 (1993) 5648–5652.
[27] C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B 37 (1988) 785. [28] M. Maniyazagan, R. Mariadasse, J. Jeyakanthan, N.K. Lokanath, S. Naveen,
K. Premkumar, P. Muthuraja, P. Manisankar, T. Stalin, Rhodamine based turn–on molecular switch FRET–sensor for cadmium and sulfide ions and live cell imaging study, Sens. Actuators B Chem. 238 (2017) 565–577.
[29] H.A. Benesi, J.H. Hildebrand, A spectrophootometric investigation of the interac-tion of iodine with aromatic hydrocarbons, J. Am. Chem. Soc. 71 (1949) 2703–2707.
[30] J.C. Qin, T.R. Li, B.D. Wang, Z.Y. Yang, L. Fan, A sensor for selective detection of Al3+based on quinoline Schiff-base in aqueous media, Synth. Metals 195 (2014) 141–146.
[31] K. Shen, S. Mao, X. Shi, F. Wang, Y. Xu, S.O. Aderinto, H. Wu, Characterization of a highly Al3+‐selective fluorescence probe based on naphthalimide‐Schiff base and its application to practical water samples, Luminescence 33 (2018) 54–63. [32] L. Tian, J. Xue, Z.Y. Yang, A simple quinoline derivative asfluorescent probe with
high sensitivity and selectivity for Al3+in aqueous solution, Tetrahedron Lett. 59 (2018) 4110–4115.
[33] S. Mondal, C. Bandyopadhyay, K. Ghosh, Chromenone-rhodamine conjugate for naked eye detection of Al3+and Hg2+ions in semi aqueous medium, Supramol. Chem. 31 (2019) 1–8.
[34] S. Wang, H. Ding, Y. Wang, C. Fan, G. Liu, S. Pu, Novel multi-responsive fluores-cence switch for Hg2+and UV/vis lights based on diarylethene-rhodamine
deri-vative, Tetrahedron (2019),https://doi.org/10.1016/j.tet.2019.01.071. [35] J. Liu, Y. Qian, A novel naphthalimide-rhodamine dye: intramolecularfluorescence
resonance energy transfer and ratiometric chemodosimeter for Hg2+and Fe3+, Dyes Pigm. 136 (2017) 782–790.
[36] S. Chemate, N. Sekar, A new rhodamine based OFF–ON fluorescent chemosensors for selective detection of Hg2+and Al3+in aqueous media, Sens. Actuators B Chem. 220 (2015) 1196–1204.
[37] J. Hu, Z. Hu, Y. Cui, X. Zhang, H.W. Gao, K. Uvdal, A rhodamine-basedfluorescent probe for Hg2+and its application for biological visualization, Sens. Actuators B Chem. 203 (2014) 452–458.
[38] A. Sikdar, S. Roy, K. Haldar, S. Sarkar, S.S. Panja, Rhodamine-based Cu2+-Selective fluorosensor: synthesis, mechanism, and application in living cells, J. Fluoresc. 23 (2013) 495–501.
[39] P. Venkatesan, N. Thirumalivasan, S.P. Wu, A rhodamine-based chemosensor with diphenylselenium for highly selectivefluorescence turn-on detection of Hg2+in vitro and in vivo, RSC Adv. 7 (2017) 21733–21739.