Plant Physiol. (1997) 113: 527-534
AI
Partitioning Patterns and Root Growth as Related to
AI
Sensitivity and
AI
Tolerance in Wheat'
Timmy D. Samuels*, Koksal Küçükakyüz3, and Magaly Rincón-Zachary*
Department of Biology, Midwestern State University, 341 O Taft Boulevard, Wichita Falls, Texas 76308
Studies of AI partitioning and accumulation and of the effect of AI on the growth of intact wheat (Trificum aesfivum 1.) roots of cultivars that show differential AI sensitivity were conducted. The effects of various AI concentrations on root growth and AI accu- mulation in the tissue were followed for 24 h. At low externa1 AI concentrations, AI accumulation in the root tips was low and root growth was either unaffected or stimulated. Calculations based on regression analysis of growth and AI accumulation in the root tips predicted that 5 0 % root growth inhibition in the AI-tolerant cv Atlas 66 would be attained when the AI concentrations were 105 p~ in the nutrient solution and 376.7 pg AI g-' dry weight in the tissue. In contrast, in the AI-sensitive cv Tam 105, 50% root growth inhibition would be attained when the AI concentrations were 11
p~ in the nutrient solution and 546.2 pg AI g-' dry weight in the tissue. The data support the hypotheses that differential AI sensitiv- ity correlates with differential AI accumulation in the growing root tissue, and that mechanisms of AI tolerance may be based on strategies to exclude AI from the root meristems.
~
A1 is a major growth-limiting factor of plants in acid soils (Foy, 1988; Kochian, 1995) because A1 solubility in the soil solution increases as the soil p H decreases. A1 inhibits plant growth by interfering with the regulatory processes of root growth and development (for reviews see Foy, 1988; Taylor, 1988a; Kochian, 1995). Nonetheless, varieties of the same species have developed strategies to avoid or tolerate A1 stress. These strategies are genetically controlled (Foy, 1988) and severa1 genes may be involved (Berzonsky, 1992). The mechanism(s) of differential A1 sensitivity is a subject of much discussion and debate and has been re- viewed recently (Taylor, 1988a, 198813; Kochian, 1995).
To better understand the principles of AI tolerance mech- anisms and A1 sensitivity, it is necessary to elucidate whether the concentration of AI in the tissue is responsible for the onset of root growth inhibition and to understand how A1 is taken up and transported by the roots at both the cellular and tissue levels. Although AI binds mainly to the components of the cell wall (Zhang and Taylor, 1990,1991), there is evidence that A1 is transported across the root
This work was supported by National Science Foundation grant no. IBN-931 8660 and the Samuel Roberts Noble Foundation. Present address: Agronomy Department, University of Mis- souri, Columbia, MO 65211.
Present address: Biology Department, Mugla Üniversitesi, 48000 Mugla, Turkey.
* Corresponding author; e-mail frinconm@nexus.mwsu.edu; fax 1- 817- 689 - 4689.
plasma membrane after a short exposure of the tissue to A1 (Lazof et al., 1994). A1 accumulation inside the cell may be required for growth inhibition (e.g. binding to DNA, mi- crotubules, enzymes, etc.); however, A1 may inhibit growth by disrupting the signal transduction pathways without entering the protoplast.
The primary site of A1 toxicity is the root meristem (Foy, 1988; Bennet and Breen, 1991; Ryan et al., 1993), and re- cently, Rincón and Gonzales (1992) and Delhaize et al. (1993a) have found that the major site of A1 accumulation in wheat is the growing root region. Their observations indicate that a differential AI accumulation between the root tips of sensitive and tolerant wheat cultivars correlates with the differential sensitivity to AI.
The objective of this study was to spatially and tempo- rally characterize the differential AI accumulation between the roots of Al-tolerant and AI-sensitive wheat cultivars and to explain the relationship between the content of A1 in the root tissues and growth.
MATERIALS AND METHODS
Atlas 66 seeds (Cargill Hybrid, Fort Collins, CO) were surface-sterilized with 5% (w / v) commercial bleach and 0.1% ( w / v ) SDS for 5 min and then rinsed well with distilled water and deionized water. Scout 66 seeds (kindly provided by Dr. James Petterson, Department of Agron- omy, University of Nebraska, Lincoln) and Tam 105 seeds (Texas Foundation Seed Stock, College Station, TX) were coated with Heptachlor. Before germination the seeds were put on autoclaved paper towels saturated with 0.1 mM CaC1, and placed in a refrigerator for 24 h. The seeds were then transferred to a growth chamber and kept in the dark at 23OC for 3 d. The paper towels were kept saturated with 0.1 mM CaC1,. The seedlings were grown further hydro- ponically as described previously (Rincón and Gonzales, 1992), except that the hydroponics were set under fluores- cent lights (117 pmol photons m-'s-') with a light/dark cycle of 16/8 h at room temperature. The NS consisted of 0.4 mM CaCl,, 0.65 mM KNO,, 0.25 mM MgCl,, and 0.08 mM NH,NO, (pH 4.2).
AI Treatment
To determine the A1 accumulation in intact roots, 5-d-old seedlings were floated on 200 mL of aerated NS containing Abbreviations: AI, total aluminum; AI"+, ionic aluminum; NS, nutrient solution. 527 www.plant.org on February 12, 2016 - Published by www.plantphysiol.org Downloaded from
528 Samuels et al. Plant Physiol. Vol. 11 3 , 1997
AlC1,.6 H,O (Sigma) at different concentrations for various periods of time. The roots were rinsed briefly with deion- ized H,O and transferred to 200 mL of aerated ice-cold H,O for 10 min. The ,roots were excised in consecutive segments from the apex, including the cap (in mm): O to 2, 2 to 5, and 5 to 15.
In some experiments a 30-min wash in ice-cold 0.5 mM citric acid (Sigma; anhydrous) at p H 4.5 (adjusted with 5 N NaOH) was used to remove A1 from the free space and the cell wall compartments as described by Zhang and Taylor (1989, 1990). To determine A1 accumulation in excised root tissue, 5-d-old roots were excised prior to the A1 treatment as described above.
AI3+ Chemical Activity
Table I shows the A13+ activities and ionic strength of the NS as calculated by the computer program GEOCHEM-PC version 2 (Parker et al., 1995) at different total A1 concen- trations used in this study.
Effect of AI on C r o w t h
The primary roots of 5-d-old seedlings were measured to the nearest millimeter using a ruler and the seedlings floated on NS with or without Al. After 24 h of AI exposure the primary roots were measured again and O- to 2-mm root tips were excised from the primary and seminal roots for A1 content determination. Regression analyses were performed to explain the relationships between root growth and tissue AI content, and between A1 concentra- tion in the solution and tissue A1 content.
AI Determination
A1 analysis was done by ion chromatography as de- scribed by Rincón and Gonzales (1992). Briefly, the tissue was dried in an oven at 75°C for 24 to 48 h and then digested with HNO, (70%; Baker Instra analyzed; VWR Scientific, Media, PA) and H,O, (50%; Fisher Scientific) (1:1, v / v ) at 75°C for 30 to 60 min. Ion chromatography was performed with an HPLC model DX 500 (Dionex, Houston,
Table 1. Total A/ concentration, A/3' activity, and ionic strength of
the NS
The activity of Ai3+ in the NS (pH 4.2; see "Materiais and Meth- ods") and the ionic strength were estimated using the computer software program CEOCHEM-PC version 2.
Total AI AI3+ Activity lonit Strength
PM O 0.5 1
.o
5.0 10 25 50 75 1 O0 PM 0 0.26 0.53 2.63 5.24 1 3 25.75 31.72 31.65 mM 2.71 2.72 2.72 2.74 2.77 2.86 2.99 3.09 3.1 2TX) equipped with a full control Peaknet software
/
inter- face system (Dionex).A11 samples and A1 standards were contained in polypro- pylene tubes that were soaked in 20% (w / v) HNO, for 48 h and rinsed with distilled H,O and ultrapure water (Milli-Q, Millipore). A11 solutions were prepared with ultrapure wa- ter. A11 treatments were duplicated or triplicated and a11 experiments were repeated at least twice.
RESULTS
AI Partitioning in lntact Roots Exposed to Various AI Concentrations
Figure 1 illustrates the A1 partitioning along the intact roots of the tolerant cv Atlas 66 exposed to increasing A1 concentrations for 6 h. AI accumulation in the more mature 5- to 15-mm root region was 1.8 times that in the O- to 2-mm root tips at an externa1 A1 concentration of 5 p~ (Fig. 1). The same A1 accumulation pattern was observed with in- creasing concentrations of Al. The magnitude of differen- tia1 A1 accumulation between the O- to 2-mm region and the mature regions declined with the increasing A1 concentra- tion in the NS; for instance, the A1 content in the mature region (5 to 15 mm) was 4 times that in the O- to 2-mm root tip when the AI concentration in NS was 25 ~ L M Al, but the
A1 content was the same in a11 root regions when the A1 concentration in the NS was 100 p ~ .
Figure 2 shows A1 partitioning along the intact roots of the Al-sensitive cv Scout 66 when exposed to either 10 p~ or 50 p~ Al. A differential A1 accumulation among the various root regions was evident. When the A1 concentra- tion in the NS was 10 p ~ , AI accumulation in the O- to 2-mm tips was 1.2 times higher than in the 2- to 5-mm segments and 6 times higher than in the 5- to 15-mm
segments. When the AI concentration in the NS was 50 FM,
A1 accumulation in the O- to 2-mm tips was 1.3 times higher than in the 2- to 5-mm segments and 3 times higher than in the 5- to 15-mm segments. Figure 3 shows the results of time-course experiments of A1 accumulation in cv Scout 66 intact roots. AI continued to accumulate throughout the 24-h time course in the O- to 2-mm, 2- to 5-mm, and 5- to 15-mm root regions. A1 concentration in the different re- gions was about the same after 1 h of A1 exposure but differed after 6 h of AI exposure, and the differences in- creased throughout 24 h.
Effect of Different Concentrations of AI on Root G r o w t h and Correlation of G r o w t h and AI Accumulation in the O- to 2-mm Root Region
The experiments described above and elsewhere (Rincón and Gonzales, 1992; Delhaize et al., 1993a) indicate that differential A1 sensitivity in wheat is related to the accu- mulation of A1 in the root meristems. To determine the lowest tissue concentration of A1 that inhibits growth, seed- lings of both AI-tolerant and Al-sensitive cultivars were exposed to different concentrations of AI for 24 h, and the root growth and A1 content in the O- to 2-mm root tips were determined. The effects of A1 on root growth and AI con- centration in the 2-mm root tips of both the Al-tolerant cv
www.plant.org on February 12, 2016 - Published by
www.plantphysiol.org Downloaded from
AI Accumulation as Related to AI Sensitivity in Wheat Roots 529 *
3
600 Z w8n
9
4002
200 O 0-2 2-5 5-15 Root Region (mm)Figure 1. AI partitioning in intact roots of AI-tolerant cv Atlas 66
exposed to various AI concentrations. A, lntact roots were submerged in aerated NS in the absence (controls) or in the presence of different AI concentrations for 6 h at room temperature. The results obtained from the intact roots exposed to 5 ~ L M AI are from experiments conducted in different days from those in which the roots were exposed to 25 to 100 p ~ . Mean ? SD are of two separate experi- ments. No AI was detected in the controls. DW, Dry weight.
Atlas 66 and the Al-sensitive cv Tam 105 are illustrated in Figures 4 and 5, respectively. AI stimulated root growth in both the AI-tolerant and Al-sensitive cultivars when the AI concentrations in the NS were low (Figs. 4A and 5A). The tissue AI content that corresponded with stimulation or no inhibition of root growth is referred to as the stimulatory A1 content. The stimulatory AI content was often low and in some experiments it was difficult to determine because of the detection limits of the ion chromatograph.
In the tolerant cv Atlas 66, the stimulatory AI content varied from O to 6.5 pg A1 g-' dry weight when the AI concentration in the NS was between 1 p~ and 5 p ~ . Doubling the AI concentration in the NS from 5 p~ to 10
p~ caused slight growth inhibition (4%) and a 7-fold in- crease in the AI content in the tissue (i.e. from 6.5 pg AI 8-l dry weight to 46.6 Fg A1 g-' dry weight). Root growth inhibition of 51% was observed when the AI content in the tips was 348.5 pg AI g-' dry weight (inhibitory A1 content) at an external AI concentration of 100 p ~ . The inhibitory A1 content in the tissue represents a 54-fold increase over the stimulatory A1 content. Regression analyses indicated that the relationships between root growth and the tissue A1
750 - 600 -
-
5
9
450 - 0 6 q2
300
--
150 - 0 - I,
I 0-2 2-5 5-1 5 Root Region (mm)Figure 2. AI partitioning in the intact roots of AI-sensitive cv Scout 66 exposed to different AI concentrations. lntact roots were submerged in aerated NS in the absence (control) or in the presence of 1 O and 50
p~ AI for 6 h at room temperature. Mean 2 SD are of two separate experiments. N o AI was detected in the controls. DW, Dry weight.
-o- 0-2 mm +2-5 mm
O 6 12 18 24
Time (h)
Figure 3. Time course of AI accumulation in the intact roots of AI-sensitive cv Scout 66. The intact roots were incubated in NS
containing 50 p~ AI for 1, 6, and 24 h. Mean ? SD are of two separate experiments. No AI was detected in the control. DW, Dry weight.
content and between the tissue A1 content and A1 concen- tration in the NS were linear (Fig. 4, B and C).
Figure 5 displays data from similar experiments per- formed with the Al-sensitive cv Tam 105. Stimulation of growth was observed at AI concentrations in the NS of 0.5
FM and 1 FM (Fig. 5A). Root growth inhibition of 54% was observed when the AI content in the tips was 481 pg A1 g-'
dry weight at an external A1 concentration of 10 p ~Re- . gression analyses of these data indicated that the relation- ships between root growth and the tissue AI content and between the tissue A1 content and the A1 concentration in the NS were best described by a polynomial equation (Fig. 5, B and C).
Removal of Exchangeable AI by Citric Acid
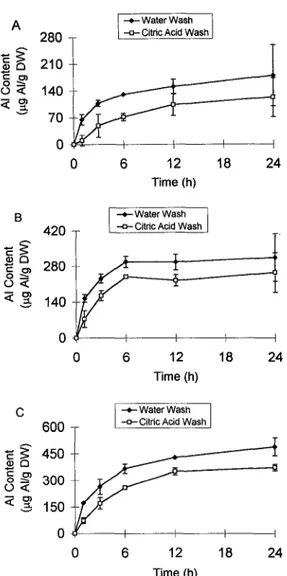
Figure 6 illustrates the results of experiments in which a 30-min citric acid wash was used to remove "exchange- able" AI, presumably from the free space and cell wall (Zhang and Taylor, 1989, 1990). In cv Atlas 66 citric acid removed exchangeable AI from all three root regions, O to 2, 2 to 5, and 5 to 15 mm, and AI partitioning along the roots was the same in both the water- and citric acid- washed roots; in both washes the A1 content in the 2- to 5-mm and 5- to 15-mm root regions was higher than in the O- to 2-mm tips. The proportion of A1 removed by citric acid decreased as the time of AI accumulation increased. Citric acid removed 84% of the A1 accumulated in the O- to 2-mm region in 1 h; however, the fraction of A1 removed by citric acid declined to 55% at 3 h and to 32% at 24 h. As the time of A1 absorption increased AI either became tightly bound to the cell wall or entered a compartment that was inaccessible to citric acid. The rates of A1 accumulation for both water- and citric acid-washed root tissue were calcu- lated by linear regression analysis and are shown in Table 11. During the first 6 h the apparent rate of AI accumulation in the O- to 2-mm root tips washed with citric acid was 63% of that in the root tips washed with water. After 6 h the rates of A1 accumulation dropped and the values were the same in both citric acid- and water-washed tissue. The drop in the rates of AI accumulation with time was also
www.plant.org on February 12, 2016 - Published by
www.plantphysiol.org Downloaded from
530 Samuels et ai. Plant Physiol. Vol. 1 1 3, 1997 T 500
(5%i3ZFl
A 120 T 1 5 10 25 50 100 AI Concentration in Nutrient Solution (pM) R2 = 0.9419 B O 100 200 300 400 AI Content (pg Al/g DW) CIv
= 3.618~-
3.32421O
O 25 50 75 100 AI Concentration in the Nutrient Solution (pM)Figure 4. Root growth and AI accumulation in the meristematic region (O to 2 mm) of the AI-tolerant cv Atlas 66. A, The intact roots were submerged in the aerated NS in the absence and jn the presence of different concentrations of AI for 24 h at room temperature. Growth of the intact primary root was determined and O- to 2-mm tips from the seminal and primary roots were excised and pooled for AI determinations. The control roots grew 16.6 mm in 24 h. Mean ? SD are of two experiments. 6, Linear regression analysis of the data presented in A. C, Linear regression analysis of the tissue AI content and the AI concentration jn the NS. DW, Dry weight.
observed in the more differentiated 2- to 5-mm and 5- to 15-mm root regions.
AI Accumulation in lntact and Excised Tolerant Root Tips A time course of A1 accumulation in excised O- to 2-mm root tips of cv Atlas 66 exposed to 50 p~ A1 is shown in Figure 7. We included data from Figure 6A to compare the rates of A1 accumulation in excised root tips with that in intact tissue. A1 accumulation in the excised root tips in- creased with time, and the rate of A1 accumulation was much higher than in the intact tissue. The rate of A1 accu- mulation between O h and 3 h was 130.8 pg A1 g-' dry weight h-' and linear (r2 = 0.96) in the water-washed
excised root tips. Citric acid removed 30% of the A1 accu- mulated at 1 h, however, after 3 h remova1 of exchangeable A1 by citric acid varied from 7% to 14%. The rate of A1 accumulation between O h and 3 h was 114.2 pg A1 g-' dry weight h-' and also linear (Y' =0.99). After 6 h the rates of A1 accumulation in both water- and citric acid-washed tissues were linear and dropped to 21.8 pg A1 8-l dry weight h-l (Y' = 1.00) and 18.4 pg A1 g-' dry weight h-l
( r 2 =0.96), respectively.
DISCUSSION
In this study we have characterized AI partitioning in roots of both an Al-tolerant and an Al-sensitive wheat
150% 1800
q
z g
EF
100% 1200 B O 05 9
2z
a 3
Y 0% O 6002
-.I- o o 50% 0.5 1 5 10 25 50 AI Concentration in Nutrient Solution (pM) B c c O 02 E
120 1 O0 80 60 40 20 y=5E-O5?-0.1412~+ 110.72 R' = 0.9662 O 500 1000 1500 AI Content (pg Al/g DW) CIY
= -0.5061? + 52.841~ + 13.8731 1500 O Y O 20 40 60 AI Concentration in Nutrient Solution (pM)Figure 5. Root growth and AI accumulation in the meristematic region (O to 2 mm) of AI-sensitive cv Tam 105. A, The intact roots were submerged in the aerated NS in the absence and in the presence of different concentrations of AI for 24 h at room temperature. Growth of the intact primary root was determined and O- to 2-mm tips from the seminal and primary roots were excised and pooled for AI determination. The control roots grew 12.6 mm in 24 h. Mean t
SD are of two experiments. B, Polynomial regression analysis of the data presented in A. C, Regression analysis of the tissue AI content and the AI concentration in the NS. DW, Dry weight.
www.plant.org on February 12, 2016 - Published by
www.plantphysiol.org Downloaded from
AI Accumulation as Related to AI Sensitivity in Wheat Roots 531 A 280 T
ti
O F1 I I I I I,
I O 6 12 18 24 Time (h) B 4 2 0 T T O 6 12 18 2 4 Time (h)-1
C 600 T I II
d
O
6 12 18 24 Time (h)Figure 6. Time course of AI accumulation in intact roots of Al- tolerant cv Atlas 66. lntact roots were submerged in the aerated NS
in the presence of 50 p~ AI for different periods of time. Following AI exposure, the roots were transferred to ice-cold 0.5 mM citric acid (pH 4.5) for 30 min or to ice-cold water for 10 min. The roots were then excised in different lengths. A, AI content in the O- to 2-mm tip.
B, AI content in the 2- to 5-mm region. C, AI content in the 5- to 15-mm region. Mean t SD are of two experiments. DW, Dry weight.
cultivar. We present quantitative data that support the hypothesis that A1 accumulation in the root-growing re- gions is related to A1 sensitivity.
In the Al-tolerant cv Atlas 66, A1 accumulation in the apical O- to 2-mm root region was always lower than in the more mature regions at concentrations between 1 PM and
75 PM (Fig. 1). However, at a high A1 concentration (100
PM) the accumulation of A1 in a11 regions was approxi- mately the same. On the other hand, AI accumulation in the apical O- to 2-mm root region of the Al-sensitive cv Scout 66 was always higher than in the mature regions (Figs. 2 and 3). The same pattern was observed in the Al-sensitive cv Tam 105 (Rincón and Gonzales, 1992).
Rincón and Gonzales (1992) and Delhaize et al. (1993a) reported results that support the hypothesis that differen-
Table II. Rates of A / accumulation in intact roots of the A/-tolerant
cv Atlas 66 at different time periods
The rates of AI accumulation were calculated by regression anal- ysis of the data in Figure 6. The numbers in parentheses are the coefficients of determination ( F ) .
AI Accumulation Water wash Citric acid wash Root Region Time Period
mm h pg A / g-'dry wt h-' o -2 O- 6 19.6 (0.83) 12.4 (0.96) 6-24 2.7 (0.90) 2.7 (0.98) 2-5 O- 6 44.1 (0.83) 38.6 (0.94) 6-24 0.96 (0.85) 1 .O7 (0.41) 5-1 5 O- 6 55.2 (0.88) 41.9 (0.96) 6-24 6.5 (0.96) 5.7 (0.74)
tia1 A1 sensitivity between the AI-tolerant and Al-sensitive cultivars of wheat was related to a differential A1 accumu- lation in the root tips. The results presented here clearly demonstrate that A1 sensitivity, measured as an effect of A1 on root growth, correlated with the concentration of A1 in the root tips (Figs. 4 and 5 ) . In the tolerant cv Atlas 66, the
accumulation of A1 in the O- to 2-mm root tips strongly correlates with the concentration of A1 in the NS (r2 = 0.97; Fig. 4C), and more importantly, there was a strong linear relationship between root growth and tissue A1 concentra- tion (r2 = 0.94; Fig. 4B). Low A1 concentration in the O- to 2-mm root tissue caused either a small stimulation or no inhibition of root growth. The growth-stimulatory tissue A1 content varied from O to 6.5 Fg A1 g-' dry weight. Stimu- lation of growth in other wheat cultivars and other plant species by A1 has been observed (discussed by Foy, 1988; Rincón and Gonzales, 1991; Kinraide, 1993), which might be due to an alleviating H + toxicity (Kinraide, 1993), in- creasing the P0,3- uptake (Macklon and Sim, 1992; Nicho1 et al., 1993), and redistributing the P 0 2 - pool inside the plant (Miranda and Rowell, 1989). Based on the regression analyses shown in Figure 4, B and C, it was calculated that in Atlas 66 a 10% inhibition of root growth would corre- spond to 86.05 Pg A1 g-' dry weight A1 in the root tips and
~ ~ .- -
-e- Excised Waler Wash
[-A- Exwsed Cilnc Acld Wash . . . . = * . . IntaCt lnlact Water Wash CltnC Acld Wash I
- F
cS n
SP
o a
a q
v iO
10 20 30 Time (h)Figure 7. Time course of AI accumulation in excised cv Atlas 66 root tips (O to 2 mm). Excised root tips were submerged in the aerated NS containing 50 p~ AI for different periods of time. Following AI exposure, the tips were transferred to ice-cold 0.5 mM citric acid (pH 4.5) for 30 min or to ice-cold water for 10 min. The results from Figure 6A are plotted for a comparison (dotted lines). Mean 2 SD are of two experiments. DW, Dry weight.
www.plant.org on February 12, 2016 - Published by
www.plantphysiol.org Downloaded from
532 Samuels et al. Plant Physiol. Vol. 1 13, 1997
to 24.7 PM AI in the NS, and 50% root growth inhibition would correspond to 376.7 pg AI g-' dry weight in the root tips and to 105 p~ in the NS.
The relationship between growth and tissue A1 concen- trations was not linear in the AI-sensitive cv Tam 105 (Fig. 5); the estimated AI concentration, which would cause 50% root growth inhibition, corresponded to 546.2 pg A1 g-'
dry weight (Fig. 58) and to a NS AI concentration of 11.4 p~ (Fig. 5C). Saturation of growth inhibition occurred when the tissue A1 concentration was higher than 1 mg AI g-' dry weight. The inhibitory AI content in cv Tam 105 was 145% of that in cv Atlas 66. These results however, differ from those reported by Tice et al. (1992); they calcu- lated that the inhibitory AI content that caused 50% root growth was approximately 160 pg A1 g-' dry weight in both the AI-sensitive cv Tyler and the AI-tolerant cv Yecora Rojo. The apparent discrepancy between their results and ours may be because of the differences in the experimental conditions (duration of the AI treatment, NS composition, seedling age, etc.) and because of the degree of sensitivity to A1 exhibited by the AI-sensitive cvs Tyler and Tam 105 and the AI-tolerant cvs Yecora Rojo and Atlas 66. Our results clearly demonstrate that the AI-sensitive cv Tam 105 exhibits higher rates of AI accumulation than the Al- tolerant cv Atlas 66 (Figs. 4 and 5). However, further re- search is needed to establish the cellular compartment(s) where A1 is accumulated and the signal transduction mech- anism that leads to root growth inhibition.
A1 accumulation in the O- to 2-mm root tips of the toler- ant cv Atlas 66 was faster during the first 6 h of AI exposure than during the time interval of 6 h to 24 h (Fig. 6; Table 11). The rates of AI accumulation in the 2- to 5-mm and 5- to 15-mm root regions were higher than in the O- to 2-mm root tips. The low AI content in the growing root region of the Al-tolerant wheat cv Atlas 66 may be due to an AI efflux mechanism coupled to an Al-induced organic acid and a
P0,-3 efflux. Reves and Rincón-Zachary (1995) have re- ported that in cv Atlas 66, the loss of AI from excised O- to 2-mm is evident and depends on the metabolism. Delhaize et al. (1993b) and Ryan et al. (1995a, 199513) identified and characterized an AI-induced malate efflux from the root tips of AI-tolerant cultivars of wheat. AI did not induce malate efflux in AI-sensitive cultivars (Delhaize et al., 199313; Ryan et al., 1995a). Also, in Al-tolerant cultivars of
snapbeans and corn, exudation of citric acid was evident (Miyasaka et al., 1991; Pellet et al., 1995). Furthermore, AI-induced P 0 4 - 3 exudation from wheat root tips has been observed (Pellet et al., 1996). These results support the working hypothesis that malate, citrate, and P 0 4 - 3 chelate external AI; consequently, the external chemical activity of AI in the rhizosphere and its accumulation in the root tips are reduced (Kochian, 1995). Active AI-Pi and Al-malate (or citric acid) efflux may be involved in excluding A1 from tolerant roots (Taylor, 198813, 1991; Lindberg, 1990), how- ever, direct experimental evidence to support these Al- exclusion mechanisms is lacking. In summary, there may be severa1 strategies operating in the root cells of Al- tolerant cultivars to lower the tissue A1 content in the growing region.
We also compared the effectiveness of a citric acid wash and a water wash to remove the desorbable AI from the root surface. The fraction of AI removed by citric acid decreased as the time of AI uptake increased (Fig. 6). Thus, under the conditions of these experiments, A1 entered a compartment that was inaccessible to exchange (tightly bound in the cell wall or in the symplast). Zhang and Taylor (1989, 1990) and Tice et al. (1992) identified the cell wall as the main site of A1 accumulation. Zhang and Taylor (1989, 1990) used citric acid washes to remove AI from the cell wall and showed that over a 3-h period there were two phases of AI uptake; a rapid, linear phase followed by a slower phase of accumulation. The authors suggested that the second linear phase of AI uptake represented transport of AI across the plasma membrane.
In cv Atlas 66 the rates of AI accumulation in the O- to 2-mm region dropped with time in both water- and citric acid-washed root tips (Fig. 6A; Table 11). The rates of AI accumulation in the presence of 50 p~ AI were 19.6 and 12.4 pg AI g-' dry weight h-' during the first 6 h for water- and citric acid-washed root tips, respectively, and they dropped to 2.7 pg AI 8-l dry weight h-' between 6 h and 24 h. In cv Tam 105 the rate of AI accumulation in the presence of 10 ~ L M AI in the NS was almost twice as high as in the cv Atlas 66 root tips during the early phase of AI uptake (i.e. 31.6 and 34.3 A1 g-' dry weight h-l for water- and citric acid-washed tips, respectively), and as in cv Atlas, the fraction of AI removed by citric acid decreased with time (data not shown).
Ownby and Popham (1989) showed that inhibition of root growth by A1 in AI-tolerant cv Atlas 66 could be completely reversed by removing AI from the solution or treating the roots with 2 mM citric acid, whereas in Al- sensitive cultivars the recovery of root growth by citric acid was partial. Recently, Lazof et al. (1994) used second- ary ion MS to estimate symplastic AI concentrations at 71 nmol g-' fresh weight (1.92 pg AI g-' fresh weight) in intact roots of an Al-sensitive cultivar of soybean within 30 min of AI exposure. Thus, it is possible that the initial phase of root growth inhibition may be due to A1 inter- ference with the growth processes that occur in the cell wall (e.g. cell wall loosening) and with the signal trans- duction pathways that are involved in cell growth. How- ever, it remains to be demonstrated whether apoplastic or symplastic AI is responsible for the initiation of root growth inhibition.
Most ion transport studies are done with excised root tissue because of the convenience of conducting transport experiments under laboratory conditions (Huang et al., 1992). Usually, the excised root segments are allowed to recover from the initial "injury" of excision during a wash- ing or aging period. The assumption is that aged root tissue behaves like intact root tissue in terms of ion transport (Gronewald and Hanson, 1980). The results in Figure 7 clearly show the differences in A1 accumulation between excised and intact O- to 2-mm root tips of the AI-tolerant cv Atlas 66 at the external AI concentration of 50 p ~ . The data show a 2.8-fold increase of the AI content in the excised tissue at 1 h AI uptake as compared with the intact root
www.plant.org on February 12, 2016 - Published by
www.plantphysiol.org Downloaded from
AI Accumulation as Related to AI Sensitivity in Wheat Roots 533
tips. The tissue A1 concentration in the excised tips reached the inhibitory concentration observed in intact Al-sensitive cv Tam 105 root tips (Fig. 5 ) . However, this apparent
increase of the A1 content in the tips may be due to an increased A1 binding to the cut surface. If the apparent increase in AI accumulation in the excised tips were due to A1 binding to the cut surface, then citric acid should re- move the bound Al. However, a 30-min citric acid wash had little effect on the shape of the time-course curve or on the remova1 of A1 from the tissue (Fig. 7). The citric acid wash removed 36% of the A1 accumulated in 1 h, but after 3 h the citric acid wash removed only a small fraction of the accumulated A1 (7-16%). These results indicate that in excised tissue A1 either binds to the cut surface very tightly or enters the symplasm where it is not available for chela- tion by citric acid.
A preliminary experiment showed that A1 uptake was greater in aged excised O- to 2-mm root tips of cv Atlas 66 than in freshly excised root tips (data not shown). Ion transport researchers have interpreted that increased K+ uptake in washed excised roots is due to a "recovery" of cellular activities (e.g. respiration, protein synthesis, etc.) that regulate ion transport mechanisms damaged by the initial injury of excision. The H+-ATPase activity increases during the washing period and, consequently, the cells regain the electrical and pH gradients across the plasma membrane, and influx of Kt and other ions is restored. Miyasaka et al. (1989) reported that A1 induced hyperpo- larization of wheat root cells. A higher electrical gradient across the membrane (negative inside and positive outside) could increase A1 uptake. Does A1 uptake depend on the activity of the H+-ATPase? More research is needed to explain the nature of A1 uptake into root cells.
Based on the results shown in Figure 7, we conclude that caution must be exercised in interpreting results when excised root tissue is used in studies of A1 sensitivity, A1 transport, and the effects of A1 on root growth. Therefore, whenever possible, intact roots should be used to assess A1 sensitivity. In summary, the data reported here support the hypotheses (a) that differential A1 accumulation in the growing root tissue is related to differential A1 sensitivity; (b) that inhibition of root growth is related to the A1 content in the root tissue; and (c) that mechanisms of A1 tolerance may be based on strategies to reduce or to restrict A1 absorption in the root meristems and are the subject of ongoing studies.
Received July 24, 1996; accepted November 5, 1996. Copyright Clearance Center: 0032-0889/97/113/0527/08.
LITERATURE CITED
Bennet RJ, Breen CM (1991) The aluminium signal: new dimen- sions to mechanisms of aluminium tolerance. Plant and Soill34:
Berzonsky WA (1992) The genomic inheritance of aluminum tol- erance in 'Atlas 66' wheat. Genome 35: 689-693
Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993a) Aluminum tolerance in wheat (Triticum aestivum L.).
153-166
I. Uptake and distribution of aluminum in root apices. Plant Physiol 103: 685-693
Delhaize E, Ryan PR, Randall PJ (1993b) Aluminum tolerance in wheat (Tuiticum aestivum L.). 11. Aluminum-stimulated excretion
of malic acid from root apices. Plant Physiol 103: 695-702 Foy CD (1988) Plant adaptation to acid, aluminum-toxic soils.
Commun Soil Sci Plant Ana1 19: 959-987
Gronewald JW, Hanson JB (1980) Sensitivity of the proton and ion transport mechanisms of corn roots to injury. Plant Sci Lett 18: Huang 22, Yan X, Jalil A, Norlyn JD, Epstein E (1992) Short-term
experiments on ion transport by seedlings and excised roots. Plant Physiol 100: 1914-1920
Kinraide TB (1993) Aluminum enhancement of plant growth in acid rooting media. A case of reciproca1 alleviation of toxicity by two toxic cations. Physiol Plant 88: 619-625
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mo1 Biol 46:
Lazof DB, Goldsmith JG, Rufty TW, Linton RW (1994) Rapid uptake of aluminum into cells of intact soybean root tips. A microanalytical study using secondary ion mass spectromztry. Plant Physiol 106: 1107-1114
Lindberg S (1990) Aluminium interactions with K* (86Rb+) and
4 5 c a Z + fluxes in three cultivars of sugar beet (Beta vulgaris).
Physiol Plant 79: 275-282
Macklon AES, Sim A (1992) Modifying effects of non-toxic levels of aluminium on the uptake and transport of phosphate in ryegrass. J Exp Bot 43: 915-923
Miranda LN de, Rowell DL (1989) Aluminium-phosphate inter- actions in wheat. New Phytol 113: 7-12
Miyasaka SC, Buta JG, Howell RK, Foy CD (1991) Mechanism of aluminum tolerance in snapbeans. Root exudation of citric acid. Plant Physiol 96: 737-743
Miyasaka SC, Kochian LV, Shaff JE, Foy CD (1989) Mechanisms of aluminum tolerance in wheat. An investigation of genotypic differences in rhizosphere pH, K+, and H+ and root-cell mem- brane potentials. Plant Physiol 91: 1188-1196
Nicho1 BE, Oliveira LA, Glass ADM, Siddiqi MY (1993) The effects of aluminum on the influx of calcium, potassium, ammo- nium, nitrate, and phosphate in an aluminum-sensitive cultivar of barley (Hordeum vulgare L.). Plant Physiol 101: 1263-1266 Ownby JD, Popham HR (1989) Citrate reverses the inhibition of
wheat root growth caused by aluminum. J Plant Physiol 135:
Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In RH Loeppert, ed, Soil Chemical Equilibrium and
Reactions Models. ASA and SSSA, Madison, WI, pp 253-269 Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exuda-
tion as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 196: 788-795
Pellet DM, Papernick LA, Kochian LV (1996) Multiple aluminum- resistance mechanisms in wheat. Roles of root apical phosphate and malate exudation. Plant Physiol 112: 591-597
Reves L, Rincón-Zachary M (1995) Aluminum efflux in tolerant and sensitive wheat (Triticum aestivum L.) cultivars (abstract no. 407). Plant Physiol 108: S-86
RincÓn M, Gonzales RA (1991) Induction of protein synthesis by aluminum in wheat (Triticum aestivum L.) root tips. In RJ Wright,
VC Baligar, RI' Murrmann, eds, Plant-Soil Interactions at Low pH, Kluwer Academic Publishers, Dordrecht, The Netherlands,
RincÓn M, Gonzales RA (1992) Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat
(Triticum aestivum L.) cultivars. Plant Physiol 99: 1021-1028
Ryan PR, Delhaize E, Randall PJ (1995a) Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta 196: 103-110
Ryan PR, Delhaize E, Randall PJ (1995b) Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Aust J Plant Physiol 22: 531-536
143-150 237-260 588-591 pp 851-858 www.plant.org on February 12, 2016 - Published by www.plantphysiol.org Downloaded from
534 Samuels et al. Plant Physiol. Vol. 11 3 , 1997
Ryan PR, Ditomaso JM, Kochian LV (1993) Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44: 437-446
Taylor GJ (1988a) The physiology of aluminum phytotoxicity. In
AH Sigel, A Sigel, eds, Metal Ions in Biological Systems, Vol24. Marcel Dekker Inc., New York and Basel, pp 123-163
Taylor GJ (198810) The physiology of aluminum tolerance. In AH
Sigel, A Sigel, eds, Metal Ions in Biological Systems, Vol 24. Marcel Dekker Inc., New York and Basel, pp 165-197
Taylor GJ (1991) Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol 10: 57-93
Tice KR, Parker DR, DeMason DA (1992) Operationally defined
apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol 100: 309-318 Zhang G, Taylor GJ (1989) Kinetics of aluminum uptake by ex-
cised roots of aluminum-tolerant and aluminum-sensitive culti- vars of Triticum aestivum L. Plant Physiol 91: 1094-1099 Zhang G, Taylor GJ (1990) Kinetics of aluminum uptake in Triti-
cum aestivum L. Identity of the linear phase of aluminum uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars. Plant Physiol 94: 577-584
Zhang G, Taylor GJ (1991) Effects of biological inhibitors on kinetics of aluminum uptake by excised roots and purified cell wall material of aluminum-tolerant and aluminum-sensitive cul- tivars of Triticum aestivum L. J Plant Physiol 138: 533-539
www.plant.org on February 12, 2016 - Published by
www.plantphysiol.org Downloaded from