KADIR HAS UNIVERSITY
GRADUATE SCHOOL OF SCIENCE AND ENGINEERING
DE NOVO SELECTIVE INHIBITOR DESIGN TO NEURONAL NOS
ENZYME AND EXPLORATION OF THE BINDING SITE
ALİ BORA BÜYÜKTÜRK
ii
DE NOVO SELECTIVE INHIBITOR DESIGN TO
NEURONAL NOS ENZYME
AND EXPLORATION OF THE BINDING SITE
ALİ BORA BÜYÜKTÜRK
B.S. and MS. Teaching Physics, Boğaziçi University, 2004 B.S., Computer Science, Istanbul Bilgi University, 2010
M.S., Computational Biology and Bioinformatics, Kadir Has University, 2013
Submitted to the Graduate School of Science and Engineering in partial fulfillment of the requirements for the degree of
Master of Science in
Computational Biology and Bioinformatics
KADIR HAS UNIVERSITY May, 2013 APPENDIX B Ali B or a B üyüktürk M.S . The sis 20 13 Ali B or a B üyüktürk M.S . The sis 20 13 S tudent’ s F ull Na me P h.D. (or M.S . or M.A .) The sis 20 11
KADIR HAS UNIVERSITY GRADUATE SCHOOL OF SCIENCE AND ENGINEERING
DE NOVO SELECTIVE INHIBITOR DESIGN TO NEURONAL NOS
ENZYME AND EXPLORATION OF THE BINDING SITE
ALİ BORA BÜYÜKTÜRK
APPROVED BY:
Prof. Dr. KEMAL YELEKCI Thesis Supervisor
Kadir Has University
Asst. Prof. Dr. EBRU DEMET AKTEN AKDOĞAN Kadir Has University
Prof. Dr. KUTLU ÜLGEN Boğaziçi University
“I, Ali Bora Büyüktürk, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis.”
_______________________ Ali Bora Büyüktürk
DE NOVO SELECTIVE INHIBITOR DESIGN TO NEURONAL NOS
ENZYME AND EXPLORATION OF THE BINDING SITE
ABSTRACT
Neural Nitric Oxide Synthase (nNOS) is an enzyme that plays a significant role in neural signal transmission among brain cells by carrying on Nitric Oxide(NO) generation. nNOS is one of the member of Nitric Oxide Synthase (NOS) enzyme family and has three isoforms; nNOS, eNOS and iNOS. Since NO is a highly reactive compound, NOS isozymes have many distinct functionalities on neural, endothelial and immune systems respectively. Despite these functionalities, their binding sites show great similarities and hence it became a challenge to design a selective inhibitor to them.
Revealing of crystallographic structures of NOS isoenzymes set a foundation for in silico inhibitor design and computational modeling studies. We applied lead and fragment based de novo design techniques and carried on a series of computational docking operations on the NOS isoforms to discover an nNOS selective leading inhibitor against iNOS and eNOS. Utilizing virtual screening (VS) methods on different software environments we selected suitable lead scaffolds, added fragments, developed candidate ligands and applied leading docking algorithms on these ligands. We compared our ligands with experimental leading compounds defined on the related literature. Finally, we explored the binding sites and stated the properties of cavities and specific amino acids.
NÖRONAL NOS ENZİMİNE DE NOVO SEÇİLİMLİ İNHİBİTÖR
TASARIMI VE BAĞLANMA BÖLGELERİNİN ARAŞTIRILMASI
ÖZET
Nöral Nitrik Oksit Sentaz(nNOS) Nitrik Oksit (NO) üretimini devam ettirerek beyin hücreleri arasında nöral sinyal iletiminde önemli rol oynayan bir enzimdir. nNOS Nitrik Oksit Sentaz (NOS) enzim ailesinin bir üyesidir ve üç izoformu bulunmaktadır; nNOS, eNOS ve iNOS. NO hayli reaktif bir bileşik olduğundan NOS izozimlerinin sırasıyla nöral, endotelyal ve bağışıklık sistemlerinde birçok ayırıcı fonksiyonellikleri vardır. Bu fonksiyonelliklerine ragmen bağlanma bölgeleri büyük benzerlikler gösteirler ve bu yüzden onlar seçici inhibitörler tasarlamak zorlayıcıdır.
NOS izizimlerinin kristalografik yapılarının açığa çıkması in siliko inhibitör tasarımı ve hesapsal modelleme çalışmaları için bir temel oluşturmuştur. iNOS ve eNOS’a karşı nNOS seçimli bir öncü inhibitör keşfetmek için NOS izoformları üzerinde, öncü ve parça temelli de novo tasarım tekniklerini uyguladık ve bir seri hesapsal doklama operasyonu yürüttük. Farklı yazılım ortamlarında Sanal Tarama (VS) methodlarını kullanarak, uygun öncü iskelet yapılarını seçip, parçalar ekleyip, aday ligandlar geliştirdik ve önde gelen doklama algoritmalarını uyguladık. Literatürde tanımlı öncü deneysel bileşiklerin sonuçlarıyla ligandlarımızı kıyasladık. Son olarak, bağlanma bölgelerini araştırıp kavite ve belirli amino asitlerin özelliklerini ortaya koyduk.
Anahtar Kelimeler: nNOS, eNOS, iNO, Nitrik Oksit Sentaz, doking, skorlama,
ACKNOWLEDGEMENTS
Thank you dear Prof. Dr. Kemal Yelekçi. I am glad that I had such an advisor that gave me many handy advices in the most patient and understanding way. Thank you dear Nurdan Kayrak. You never fail to introduce your neat and clear organization abilities during the crucial research steps. Thank you my dear family. You were always positive and supportive for this chronic lifelong student. Lastly, thank you Serkan Altuntaş for your last minute on time help.
Table of Contents
1 Introduction ... 12
1.1 Drug Discovery ... 12
1.2 Nitric Oxide (Nitrogen Monoxide, NO) ... 13
1.3 Nitric Oxide Synthase (NOS) Enzyme ... 15
1.3.1 NOS Structures ... 15 1.3.2 NOS Cofactors ... 17 1.3.3 NOS Functionality ... 19 1.4 NOS Literature ... 20 2 Methodology ... 24 2.1 Tools ... 26 2.1.1 Spartan ... 26 2.1.2 AccelrysDS ... 26 2.2 Docking Methodologies ... 27
2.2.1 Protein and ligand Preparation ... 27
2.2.2 AutoDock Methodology ... 27
2.2.3 CDock Methodology ... 28
2.3 De Novo Design ... 30
2.3.1 Library Generation ... 30
2.3.2 De Novo Lead Discovery ... 31
2.3.3 Fragment-based Lead Discovery... 32
2.3.4 Parameters and Scoring ... 33
2.4 ADMET ... 34
2.5 In silico VS In vitro Case ... 34
2.6 Structural Alignment ... 38
3 Results ... 39
3.1 In silico vs. In vitro Case ... 39
3.1.1 Docking Results for nNOS ... 40
3.1.2 Docking Results for eNOS ... 43
3.2 De Novo Results ... 45
3.2.1 Lead Discovery Results ... 45
3.2.2 De Novo Fragment Based Discovery Results ... 46
3.3 ADMET Results ... 49
3.4 2D & 3D Results of De Novo Evolved Ligands ... 50
List of Tables
Table 1NOS Receptors (resolutions units are in Angstrom A0) ... 25 Table 2: ZINC Library properties ... 31 Table 3: 16 prominent selected ligands from recent NOS articles ... 37 Table 4: AutoDock results of 16 prominent ligands on 5different nNOS crystal
structures ... 40 Table 5: Log values of AutoDock Results of Table 4 ... 40 Table 6: C-Dock results of 16 prominent ligands on 5different nNOS crystal structures ... 41 Table 7: LibDock results of 16 prominent ligands on 5different nNOS crystal structure ... 42 Table 8: AutoDock results of 16 prominent ligands on five different eNOS crystal structures ... 43 Table 9: Log values of AutoDock Results of Table 8 ... 43 Table 10: C-Dock results of 16 prominent ligands on 5different eNOS crystal structures ... 44 Table 11: LibDock results of 16 prominent ligands on 5different eNOS crystal structures ... 45 Table 12: De Novo Lead Discovery ( best ZINC molecules that will be used as a Lead in the Fragment based De Novo Stage) ... 46 Table 13Fragment Based Discovery drug candidates and their, Ludi Scores and ... 48 Table 14: AutoDock results of evolved drug candidates in Fragment based De Novo Design. ... 66
List of Figures
Figure 1: Nitric Oxide displayed in "CPK" and "Ball & Stick" modes ... 13
Figure 2 : eNOS dimer and NtoC (blue to red) structures (1FOI) ... 15
Figure 3: iNOS dimer and NtoC (blue to red) structures (1NOD) ... 16
Figure 4 : nNOS dimer and NtoC (blue to red) structures (3N2R) ... 16
Figure 5 : NOS isozyme Active Sites, ligands and cofactors ... 17
Figure 6: NOS Cofactor Tetrahydrobiopterin (H4B) *5,6,7,8-Tetrahydrobiopterin *2-Amino-6-(1,2-ihydroxypropyl)-5,6,7,8-tetrahydoro-4(1H)-pteridinone... 18
Figure 7 NOS Cofactor Heme C34H32FeN4O4 Protoporphyrin IX Containing FE ... 18
Figure 8-Conversion of L-Arginine to L-citrulline and nitric oxide (NO) catalyzed by the enzyme nitric oxide synthase. ... 19
Figure 9: Stereo views of crystal structures of nNOS and eNOS binding to L-Arginine 22 Figure 10 Docking in AccelrysDS: Cavity and chosen binding site sphere is shown ... 29
Figure 11: nNOS AutoDock results chart of Table 5 denoting logarithmic behavior of inhibition constants ... 41
Figure 12 : eNOS AutoDock results chart of Table 9. denoting logarithmic behavior of inhibition constants ... 44
Figure 13: ADMET results of molecules evolved in fragment De Novo ... 49
Figure 14 : 2D&3D images of 1NSI and zinc00151524_evo3 interaction ... 50
Figure 15: 2D&3D images of 1NSI and zinc00151524_evo7 interaction ... 51
Figure 16: 2D&3D images of 1NSI and zinc00151524_evo10 interaction ... 52
Figure 17: 2D&3D images of 1RS7 and 1RS7_zinc04859564_evo5 ... 53
Figure 18: 2D&3D images of 1RS7 and 1RS7_zinc04859564_evo6 interaction... 54
Figure 19 : 2D&3D images of 1RS7 and 1RS7_zinc04859564_evo10 interaction... 55
Figure 20: 2D&3D images of 1RS7 and 1RS7_ zinc42689701_evo5 interaction... 56
Figure 21: 2D&3D images of 1RS7 and 1RS7_ zinc42689701_evo6 interaction... 57
Figure 22: 2D&3D images of 1RS7 and 1RS7_ zinc42689701_evo10 interaction... 58
Figure 23-2D interaction image 3DQS and 3DQS_zinc00081090_Evo8 ... 59
Figure 24 nNOS active site cavity and positioning of the close residues ... 68
Figure 25 Five structurally aligned eNOS enzymes ... 69
Figure 26eNOS enzymes with its cofactors and cavity ... 69
Figure 27 Two structurally aligned iNOS enzymes ... 70
Figure 28 iNOS enzymes with its cofactors and cavity ... 70
Figure 29Ten structurally aligned nNOS enzymes ... 71
Figure 30 nNOS enzymes with its cofactors and cavity ... 71
Figure 31: nNOS Cavity ... 72
Figure 32: eNOS Cavity ... 73
List of Abbreviations
Nitric Oxide ... NO Nitric Oxide Synthases ... NOS Neuronal Nitric Oxide Synthases ... nNOS Endothelial Nitric Oxide Synthases ... eNOS Inducible Nitric Oxide Synthases ... iNOS Tetrahydrobiopterin ... BH4 L-arginine ... L-Arg Accelrys Discovery Studio ... AccelrysDS Root Mean Square Deviation/Distance ... RMSD Nicotinamide adenine dinucleotide phosphate ... NADP Flavin adenine dinucleotide ... FAD Flavin mononucleotide ... FMN Tetrahydrobiopterin ... BH4 Human Intestinal Absorption ... HIA Interferon-gamma ... IFN-γ Tumor necrosis factor ... TNF Transforming growth factor beta ... TGF-β Interleukin-4 ... IL-4 Food and Drug Administration ... FDA New Drug Application ... NDA High Throughput Screening ... HTS
1 Introduction
1.1 Drug Discovery
Drug discovery and travel of a drug to a pharmacy shelves are long processes taking average twelve to fourteen years. Thousands of molecules have to be screened and tested on computers, in laboratories and on the living bodies. The successful drug candidates should be tested on firstly on tissues, animals and then finally on humans. Several thousands of patients are observed in clinics to learn more about toxicity, absorption, insolubility, metabolic reactions to overcome the side effects, deciding safety and tolerability and clarifying the dosage regimens. Elimination of unwanted activities and amplification of desired activities of a drug are the main concerns before NDA and FDA approvals. Luckily, recent computer advancements accelerated the initial step of these long procedures that more probable drug candidates are chosen in a more efficient way for the further steps.
Human body is a huge system of molecules. A compound may have many interaction with many other molecules in the body. It is not easy to let a compound function only in one way without harming or interacting other molecules and systems in the body. Hence, one of the most challenging aspects of drug design is to find a drug which is selectively acts on the intended specific mechanism of a system without throwing any side effect. For instance, the NO molecule is used in neurotransmission, endothelial vasodilation and immune defense system mechanisms in the body. Very active molecule with many regulatory effects nitric oxide is selected as the molecule of the year in 1992 (Figure 1). So it is very difficult to develop a drug which is selectively effective only on one of
these NO containing system mechanisms. For instance, the drug “Sildenafil” citrate, popularly known as Viagra is discovered while working on heart diseases. It is
discovered as a side effect because it was stimulating penile erections through the nitric oxide pathway.
In this study, we would like to contribute to the search of a noval selective drug inhibitor molecule acting on NO. Contrary to Viagra, we wish to find a drug candidate, which is effective on neural NOS which process on brain neurotransmitters rather than those process on immune or endothelial systems.
1.2 Nitric Oxide (Nitrogen Monoxide, NO)
Nitric oxide is an important intermediate free radical molecule that functions as a signaling and regulatory molecule in various pathological and physiological processes. NO is a subject of neuroscience, physiology, and immunology. NO is produced by NOS enzyme family in the body from L-Arginine amino acid. NO is also made by reduction of inorganic nitrate in bacteria.
causing vasodilation. It decreases blood pressure in endothelial cells (Fleming & Busse, 2003). The production of nitric oxide is elevated in populations living at high altitudes, which helps these people avoid hypoxia by aiding in pulmonary vasculature
vasodilation. Effects include vasodilatation, neurotransmission, modulation of the hair cycle, production of reactive nitrogen intermediates and penile erections. Nitric oxide can contribute to reperfusion injury when an excessive amount produced during reperfusion goes to a reaction with superoxide producing peroxy nitrite, which is a damaging oxidant (KA, 2012 ) . Contrary, inhaled nitric oxide has recovery effects against paraquat poisoning. This poisoning obstructs NOS metabolism by producing superoxide and damages lung tissues (Drummond, Cai, Davis, & Ramasamy, 2000). Low levels of nitric oxide production are important in protecting organs such as the liver from ischemic damage. Nitric oxide is considered an antianginal drug. it causes
vasodilation, which can help with ischemic pain, known as angina, by decreasing the cardiac workload. By expanding the veins, nitric oxide drugs lower arterial pressure and left ventricular filling pressure. (Chirkov, 2001)
One of the generations of Nitric oxide (NO) is by monocytes, macrophages, and neutrophils as part of the human immune response destroys microorganisms and
pathogens. NO is a free radicals and a toxic compound. When secreted against bacteria as an immune response, causes DNA damage and degradation of iron sulfur centers. NO might serve as an inflammation measurement device in asthma case. The level of
exhaled NO reduction can be compared to air pollution exposure. (Batra, Chatterjee, & Ghosh, 2007)
NO regulates the release of neurotransmitters and is involved in synaptic plasticity, memory function and neuroendocrine secretion in neuronal cells. In our brain, under certain pathological conditions after certain ages produced excessive NO, causes tissue damage and oxidative stress. These complications are basis of diseases such as including rheumatoid arthritis, Alzheimer’s disease, and Parkinson’s disease. (Silverman R. B., 2009)
1.3 Nitric Oxide Synthase (NOS) Enzyme
Nitric Oxide Synthases (NOS) enzyme family members are enzymes, which catalyze the L-Arginine amino acid to nitric oxide (NO) and L-citrulline molecules. nNOS, eNOS and iNOS are the most common isozymes in the family (Figure 8).
1.3.1 NOS Structures
On average, NOS enzymes are 420 to 430 amino acid long proteins found mostly in dimer complexes. The structures are given in Figure 2, Figure 3 and Figure 4 as follows:
Figure 3: iNOS dimer and NtoC (blue to red) structures (1NOD)
Figure 5 : NOS isozyme Active Sites, ligands and cofactors
1.3.2 NOS Cofactors
A cofactor is a molecule that is required for the biological activity of a protein. Loosely binding cofactors named coenzymes and tightly binding cofactors termed prosthetic groups. Apoenzyme is an inactive enzyme without the cofactor, whereas the
holoenzyme is the complete enzyme with cofactors. NOS cofactors contribute
conversion of guanidino nitrogen of L-Arg to NO. They are a Zn atom, a Heme and a Tetrahydrobiopterin (BH4), Nicotinamide adenine dinucleotide phosphate (NADP), Flavin adenine dinucleotide (FAD) and Flavin mononucleotide (FMN) molecules.
Heme (Figure 7) is a an iron ion containing prosthetic group contained in the center of a large heterocyclic organic porphyrin ring, composed of four pyrrolic groups and
methine bridges. Hemes are recognized as part of hemoglobin in blood, hemo-proteins such as myoglobin, cytochrome and catalase, etc.
phenylalanine and in the biosynthesis of the neurotransmitters serotonin, melatonin, dopamine, noradrenaline, adrenaline. The role of BH4 in this enzymatic process is very critical that being a core cause of the neurovascular dysfunction that is the hallmark of circulation-related diseases such as diabetes.
Figure 6: NOS Cofactor Tetrahydrobiopterin (H4B) *5,6,7,8-Tetrahydrobiopterin *2-Amino-6-(1,2-ihydroxypropyl)-5,6,7,8-tetrahydoro-4(1H)-pteridinone
Figure 7 NOS Cofactor Heme C34H32FeN4O4 Protoporphyrin IX Containing FE
Crystal structures of these double-headed amino pyridine inhibitors in complexes with nNOS show unexpected and significant protein and Heme conformational changes induced by inhibitor binding that result in removal of the Tetrahydrobiopterin (H4B) cofactor and creation of a new Zn2+ pterin binding site. In
the dimer interface, Zinc tetrathiolate center helps dimer stabilization (Igarashi, et al., 2009). These changes are due to binding of a second inhibitor molecule that results in the displacement of H4B and the placement of the inhibitor pyridine group in position to serve as a Zn2+ ligand together with Asp, His, and a chloride ion. Binding of the second inhibitor molecule and generation of the Zn2+ site do not occur common in some of the eNOS and iNOS. (Silverman, Poulos, Jamal, Li, Xue, & Delker, 2010)
1.3.3 NOS Functionality
Nitric oxide producing NOS enzyme has three isoenzymic forms which of two is structural and the last one is inducible. While eNOS in endothelial cells produces NO to regulate blood pressure, nNOS in neuronal cells produces NO for neurotransmission and iNOS in macrophage cells being stimulated by pathogens produces NO to fight against infections and microorganisms. Under the presence of cofactors NADPH, FAD, FMN and BH4 (Figure 6) NOS enzyme produces NO by oxidation of L-Arginine terminal guanidino group. (Silverman & Zhu, 2008)
Figure 8 Conversion of L-Arginine to L-citrulline and nitric oxide (NO) catalyzed by the enzyme nitric oxide synthase.
reductase domain and transfers two electrons to FAD. FMN transfers one electron to Heme in oxygenase domain. BH4 in oxygenase domain facilitates catalysis from L-Arginine to L-Citrulline. Zn, Glycerol (GOL) and two-water molecules’ interactions are the other facilitators. (Silverman & Zhu, 2008)
Phagocytes are armed with iNOS. It is activated by IFN-γ or TNF as first and second signals respectively. Alternatively, TGF-β provides a strong, whereas IL-4 and IL-10 provide weak inhibitory signals. This way, the immune system regulates the armament of phagocytes playing role in inflammation and immune responses. (Teng, Zhang, Snead, & Catravas, 2002)
1.4 NOS Literature
From the beginning of 90’s, many research-based pharmaceutical companies commence programs targeting nNOS selective compounds detection, because of the potential benefit of neurodegenerative diseases treatment (Erdal, Litzhger, Seo, Zuhu, Ji, & Silverman, 2005) . Before crystal structure of NOS enzyme discovered, basic approach was using L-Arginine substrate as lead compound and applying structural changes on it with the hope of selective binding analogues to nNOS, eNOS and iNOS (Igarashi, et al., 2009). Researches denote that the lack of selectivity is because of high similarity of substrates, active sites and reactions of these 3 enzymes. Any modifications on
L-Arginine binding the active sites will have similar effects. A few compounds designed to bind as an anchor to active site looking for a difference from distant iron cofactor site tries to reach out second cavity of amino acids (Igarashi, et al., 2009).
responsible with selectivity carried on. Biggest surprise in these studies was to show dipeptide analogue has weak potential and low selectivity. A sharp decrease noted by the need to amino group adding peptoid composed by adding carboxamide group. In
addition, carboxamide excision lowers the selectivity. Consequently, the changes on carboxamide or nitroguanidine groups of lead compound will bring on selectivity increase (Silverman, Martasek, Roman, Huang, & Hui, 2000).
First crystal structures of iNOS and eNOS discovered at the end of 1990’s by X-Ray Crystallography technique. Both isozymes’ active sites are extremely similar. nNOS structure which will enlighten inhibitor selectivity was not definite until 2002. The important difference between nNOS and eNOS enzymes, with respect to eNOS in nNOS ligand places vertically whereas in eNOS there is an inclination (Figure 9). Lacking effects of absence of Nitric Oxide enzyme are observed on the transgenic mice for all NOS isoenzymes on (knock-out) as it is expected. (Silverman R. B., 2009) In the lights of these experiments, we can claim that without hypertensive effect of eNOS inhibition or without attenuating immune system strength iNOS inhibition, obtaining a selective nNOS inhibition will be protective effect upon neurodegenerative diseases.
Figure 9: Stereo views of crystal structures of nNOS and eNOS binding to L-Arginine
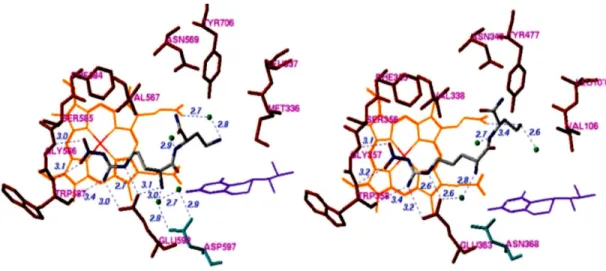
The alpha-amino primer L-Nitroarginine group cannot be modified because electrical interaction between Asp597 and Glu592 amino acids of nNOS is active. All nNOS inhibitors nitroguanidino groups are bound to the same guanidino groups of nNOS arginine analogs (Poulos, Li, Raman, Martasek, & Masters, 2001). The main differences among nNOS to eNOS and iNOS and among active site amino acids can help designing selective inhibitors.
Characterization of active sites is done by observing molecular interaction fields derived from 10 different grid probe (GRID, 2002 ) and calculations of these fields by CPCA (consensus principal compound analysis) method. A conclusion is arrived as electrostatic and hydrophobic interactions are most dominant interactions (Westerhuis, Kourti, & Macgregor, 1998).
First computer modeling studies creates effects in the direction of ”De novo” inhibitor design on the selective compounds classes on NOS isozyme caused more broader and new modeling approach. This approach called fragment hopping (H. et al. 2008). The
base of fragment hopping is to produce a minimal pharmacophoric element for each pharmacophore that isozyme selective and ligand binding sites take role. Five libraries are prepared named as base fragment, bioisostere, metabolic stability rules, toxicophore and side chain. These general libraries are used to match each pharmacophore to each minimal pharmacophoric element.
2 Methodology
In this study, we started with a literature scanning for NOS related articles published for the last 2 decades. We selected the best 16 ligands declared on the papers according to experimental laboratory results. We drew them from the scratch on AccelrysDS and minimized them into the energetically best conformations by AccelrysDS’ clean geometry tool. In addition, for some failure docking result we repeated the same operation using Spartan programs and its minimization tool, which also introduces quantum calculations for a better output ligand file. In silico docking operations are carried out on these ligands to mimic the real world. We set CDocker and LibDock docking volumes fixed to 14 to 16nm radius spheres, and 56NM edged square in
AutoDock by setting the center to Fe atom on the Heme. The lab results are compared to identify systems accuracy, calibration and validation for future library screenings. We downloaded 24 different NOS isozyme pdb files from RCSB protein databank. PDB files keeps the 3D coordinates of all the atoms of the molecule. 3PNE, 3PNF, 3PNG, 3PNH, 3SVP, 3SVQ were from latest articles that we only introduced them to the
alignment operations since we were in the middle of study by the time. We used
enzyme pdb files as their resolutions are given on the Table 1. We decided to use the
enzyme pdb files referred in the articles that we have chosen our 16 promising ligands.
Aiming to go for an HTS for de novo we wanted to keep only one enzyme from each isoform enzyme sets. So we docked al promising ligands to all enzymes and distributed
to isoform graphs and pick the each one closest to experimental value which is more
suitable to our in silico environment. We download ZINC fragment database that holds
series of de novo lead scaffold searches on AccelrysDS. Highest scored scaffolds are set
aside and accepted as base scaffolds for the next fragment based de novo design. Succeeding fragments based de novo design resulted with evolved new drug candidates for the cavities. This time, we went for a series of docking operations.
For this purpose, we used two different popular software tools to find the most probable potential inhibitor conformer based on binding affinity. We detected the most selective ligands for nNOS enzyme afterwards. At this point, we concerned metabolic
Functionality of new ligands so that we applied an ADMET test. Brain Blood Barrier passage capabilities are also important, since nNOS is a neural enzyme. Our test resulted with a table displaying five different measurement validation territory including BBB. Successful ligands falling into approved ADMET domains are observed under 2D and 3D visualization analysis for the conformer placement in the active site. Interactions are identified among ligands, cofactors and amino acids are stated. At the end, to observe the cavities in the binding sites of similar NOS isozymes, we applied a few structural alignments.
nNOS Resolutions A0 nNOS Resolutions A0 eNOS Resolutions A0
1OM4 1.75 3B3M 1.95 3DQT 2.54 1P6I 1.9 3B3N 1.98 3DQS 2.03 1P6J 2 3PNE 1.97 1FOI 1.93 1QWC 2.3 3PNF 1.94 2NSE 2.34 1RS7 1.95 3PNG 1.88 3PNH 1.93 3B30 2.05 3SVF 1.98 3DQR 2.4 3SVQ 2.18 iNOS Resolutions A0 3N2R 1.9 3SVP 1.98 1NOD 2.6 3SVP 2.05 1NSI 2.55
2.1 Tools
A RHEL5 server having Intel Quadro processor and nVidia G84 Quadro FX 1700 Graphics card
An IBM workstation having Quadro core right processors.
Three cores of 96core server is allocated for AutoDock processes. The visual screening jobs are submitted with qsub scripts
Discovery Studio 3.1, Spartan 10.1,AccelrysDS CDocker and LibDock protocols, Autodock4.2. Raccoon interface and Spartan molecular modeling software.
2.1.1 Spartan
Spartan is a molecular modeling software program of Wavefunction Inc., which is capable of introducing quantum mechanics to the molecular computations. We utilized Spartan’s minimization function by setting energy profile equilibrium geometries to semi-empiric geometry with PM3 model, which is suitable to identify conformational minima, and for determining the geometries of these minima. Spartan is introduced when a ligand conformation caused a system crash during docking.
2.1.2 AccelrysDS
Accelrys Discovery Studio is a client-server molecular simulation visual programming software suite built on a Pipeline Pilot developed by Accelrys Software Inc. It is very useful in developing novel therapeutic medicines. By licensing, Discovery Studio Accelrys Suite may contain docking protocols such as CDocker and LibDock that are widely used by computational chemists and biologists. (Sousa & Fernandes, 2006)
2.2 Docking Methodologies
2.2.1 Protein and ligand Preparation
Initially NOS crystal structures mentioned in the literature retrieved from protein databank. One of the monomer chains of the protein dimers are selected and the other is removed. Both water molecules and irrelevant substances like ammonium placed in the crystal complex obtained from protein databank are removed from the pdb files. We removed ligands as well without touching cofactors. The charge of central Heme Fe atom is set as +3 instead of +2 after breaking the bond in between cysteine amino acid located behind the Heme cofactor and Fe atom. Then, the hydrogen atoms are inserted. AccelrysDS tools correct distortions of the protein. Under the influence of a clean geometry short minimization, the bond angles and distances are optimized. Hence, these optimized pro-structures became suitable to insert candidate ligands to the active sides in the docking procedures. Pdb files are saved in “sd” format for CDocker and LibDock protocols. For the enzymes causing run time problems in other docking programs, we introduced Spartan’s ligand conformation optimization tools.
2.2.2 AutoDock Methodology
Genetic algorithms are search heuristics that mimics the process of natural evolution that routinely are used to generate useful solutions to optimization and search problems. AutoDock uses a genetic algorithm for the conformational search. AutoDock docking environment uses a semi-empirical force field based on the AMBER force fields.
some examples. Each of these components are multiplied by empirical weights obtained from the calibration against a set of know experimental binding constants. For the conformational search, AutoDock uses a Lamarckian genetic algorithm. 70 independent runs are performed for each molecule. 300 distinct ligand conformers are initially generated and this population positioned randomly in the binding cavity. They are randomly assigned torsion angles to rotatable bonds and an overall rotation. Maximum 5 million energy evaluations are allowed for each docking. AutoGrid program
pre-calculates 3D energy grid of equally spaced discrete points prior to docking for a rapid energy evaluation. We chose a grid box, with dimensions of 56*56*56 angstroms. It is centered at the iron atom contained in the center of a large heterocyclic organic ring of Heme group and covers the entire binding site and its neighboring residues. The distance between 2 grid points is set to 0.375 A. Enzyme pdb files are converted to “pdbqt” files by Autodocktools.1.5.4 by program adding Gasteiger Charges aiming to use in
AutoDock 4.2 program.
2.2.3 CDock Methodology
CDOCKER uses a molecular dynamics-based algorithm for the conformational search. Dynamic-based algorithms obtain their efficiency by solving and storing the answers to small problems that they usually trade off space for increased speed. Discovery Studio 3.0 CDOCKER protocol uses the CHARMM force fields and a molecular dynamics grid based docking algorithm, and elicits full ligand flexibility and reasonable computation times. It involves several random ligand conformations generation inside the target protein active site, following an MD-based simulated annealing composed of many heating and cooling procedures. Final refinement is an energy minimization. CDOCKER
makes use of soft-core potentials. They are effective in exploring the conformational spaces of small organics and macromolecules. The unbounded interactions that include electrostatics and van der Waals are softened at different levels, except during the final minimization step.
In CDOCKER, initially generates 10 random conformations replicas for each inhibitor in the active site of the target protein. Target is created as a spherical region with a diameter of 12A and centered on the HEME molecule. Simulated annealing is performed using a flexible ligand and a rigid protein. The ligand-protein interactions are computed from grid extension 8.0. Random conformations are generated using 1000 molecular dynamic steps, while the system is heated up to 1000 K in 2000 steps. In the simulated annealing, the number of heating steps is set to 2000, the heating target temperature to 700K, whereas cooling steps to 5000, and the cooling target temperature to 300 K. The final refinement step of minimization is performed with full potential. Final minimized docking poses are then clustered, based on a heavy atom RMSD approach using a tolerance of 0.5A. The final ranking is based on the total docking total energy, which is composed of the ligand’s intramolecular energy and the ligand-protein interaction. The Discovery Studio-3.0 visualization tool is used to analyze the 10 top hit conformations (Brooks et al. 1983).
Figure 10: Docking in AccelrysDS: Cavity and chosen binding site sphere is shown
2.3 De Novo Design
In Latin, De Novo means "from the beginning”. In our context, with De Novo design we will make predictions about new drug inhibitors using computational models without comparison to existing drugs. We will use compound libraries to generate scaffolds suitable to NOS cavities and then we will add additional groups to these leads from the fragment libraries. At the end, we will try to come up with new molecules, which will have correlated docking results to original NOS substrate.
2.3.1 Library Generation
In AccelrysDS, we generated standard Ludi libraries for De Novo Receptor and link libraries for De Novo Link and De Novo Evolution protocols by using ZINCv12 compound library. AccelrysDS generated Ludi str files for fragment topologies and trg files for specifying interaction types of functional groups. ZINC is a free database of commercially available compounds for virtual screening. They contain more than a million compounds. The Shoichet Laboratory in the Department of Pharmaceutical Chemistry at the University of California, San Francisco (UCSF), provides ZINC. We also contributed to ZINC library by 400 thousand fragments AccelrysDS libraries.
In ZINC database, there are three library subdomains of compound subsets. These are called “Standard”, “Clean” and “inStock” subdomains. We have mainly used the inStock subdomain. Standard library subsets are popular subsets that appear commonly in the literature approximations. Clean subsets are what is left after problematic compounds in some assays are removed. InStock subsets are composed of the molecules, which
months’ time. InStock library subsets are filtered into three groups. They are called “Lead-Like”, “Fragment-Like” and “Drug-Like” groups. Filtering criteria are as follows:
ZINC Libraries Lead-like
(Lead Now)
Fragment-like (Frags Now)
AccelrysDS De Novo
Lead Discovery Fragment Based Lead Improvement Compounds 1943551 400420 Date 2012-04-20 2012-04-16 octanol/water partition coefficients (xlogp) < 3.5 <=3.5 Molecular weight (mwt) >=250 & <= 350 <=250 Rotational bonds (rb) <= 7 <= 5
Table 2: ZINC Library properties
2.3.2 De Novo Lead Discovery
We tried to find leader scaffolds to our NOS cavities to improve later by adding new small fragments onto them to find new drug candidates. The De Novo Receptor protocol is utilized to identify potential molecules that fit well within a user-specified binding site of a receptor. The De Novo method is advantageous as it is fast and the variety of
molecules that can be generated in extremely large. The protocol suggests how suıtable fragments can be posıtıoned ınto clefts of proteın structures (for example active site of an enzyme) in such a way that hydrogen bonds can be formed wıth the enzyme and hydrophobıc pockets are filled with hydrophobic groups. An advantage to this approach is that it is fast and a large number of fragments can be screened quickly. The protocol
2.3.3 Fragment-based Lead Discovery
Fragment-based Lead Discovery is a new lead discovery approach that molecular weights are in the range of from 120 to 250Da comparing High Throughput Screening., screened molecular compounds are much lower in weight. Fragment-based hits are typically weak inhibitors in the range of 10 µM to mM, hence need to be screened at higher concentration with sensitive detection techniques such as protein crystallography and NMR, rather than bioassays. Fragment-based fragments are simpler, less
functionalized compounds with lower affinity. On the other hand, fragment hits typically possess high binding affinity per heavy atom called ligand efficiency. (RA, Congreve, Murray, & Rees, 2005)
In AccelrysDS we used De Nova Evolution protocol which uses a fragment based approach to suggest novel ligands from scaffold. Protocol adds small fragments from libraries to a scaffold in a protein active site. Fragments are placed in complementary positions to the receptor using a calculated interaction map to produce a collection of high scoring molecules. The nature of fragment selection and construction of new ligands depends on Evolution Mode. (Böhm, 1992) We used Full Evolution as the Evolution Mode of the protocol. In this mode, molecules are built in an evolutionary fashion starting from a scaffold and fragments are fused and then molecules are selected by score for the next iteration. LUDI scores are calculated as the scoring functions. We used FAST as the hit criteria that as soon as a hit has been found with a matching set of interaction sites, no further attempts are made to fit set of fragment target sites to any of the matched interaction.
2.3.4 Parameters and Scoring
We selected the radius of the input site sphere at the center of cavity, which defines a sphere in the receptor where receptor-ligands interactions are permitted. Fitting Scoring Function Ludi scoring function to use to prioritize the fragment hits for receptor-based runs. Ludi score is a sum of five contributions.
1. Contributions from ideal hydrogen bonds
2. Contributions from perturbed ionic interactions (donor/acceptor) 3. Contributions from lipophilic interactions
4. Contributions due to the freezing of internal degrees of freedom of the ligand 5. Contributions due to the loss of translational and rotational entropy of the ligand Energy Estimate scoring functions are used for De Novo steps. They estimate the change in free energy upon binding the fragment to the receptor. Each fragment is evaluated as a function of the potential number of hydrogen bonding, hydrophobic and ionic contracts it can make and an estimate is made of the penalty due to freezing the internal degrees of freedom of the ligand. Fitting max Fit Attempts set to a 250. Fitting Maximum RMSD is set to 0.2A0. At each receptor, interaction site there is some range of interaction
geometry between the ligand and receptor that maximizes the interaction. Deviation from this maximum, therefore, constitutes a measure of the quality of fit. Poor fits have high deviations and good fits have low deviations. As Ludi fits each fragment to the interaction sites, the RMSD is computed. From most fragments, if the RMSD exceeds the value of Maximum RMSD, the fragment is discarded. If Ludi is trying to fit a large fragment, it may allow the RMSD to exceed the value of the Maximum RMSD
2.4 ADMET
ADMET is a set of test for human intestinal absorption (HIA). HIA is defined as a percentage absorbed rather than as a ratio of concentrations. A well-absorbed compound is one that is absorbed at least 90% into the bloodstream in humans. The intestinal absorption model in AccelrysDS includes 95% and 999% confidence ellipses in ADMET_PSA_2D, AlogP98 plane. The ellipses define regions where well-absorbed compounds should fall within the 99% ellipse. Note that the location of any particular compound does not necessarily imply whether it will be well, moderately, or poorly absorbed. Absorption drops off quite rapidly outside the 95% ellipse.
ADMET uses a few different models in the analysis. Aqueous solubility model uses linear regression to predict the solubility of each compound in water at 250C. Blood Brain barrier model predicts blood-brain penetration using quantitative linear regression. Cytochrome P450 2D6 model predicts CYP2D6 enzyme inhibition using 2D chemical structure as input. Hepatotoxicity model predicts potential organ toxicity for a wide range of structurally diverse compounds. The plasma protein-binding model predicts whether a compound is likely to be highly bound to carrier proteins in the blood or not.
2.5 In silico VS In vitro Case
In the literature scanning, we accessed laboratory results for NOS enzymes studied in vitro. Here we tried to give a comparison to In silico. We have selected 24 articles containing selective inhibitor candidates from literature. We take the ligand-protein docking Ki values as the sample binding energy values and selected 16 compound to
We draw the ligand models from the scratch and minimized in AccelrysDS. To dock these ligands the enzyme files mentioned on the articles 3B3M, 3B3N, 3B3O, 3DQR, 3N2R and 3SVP nNOS receptors; 1FOI, 2NSE eNOS receptors; and 1NOD iNOS receptor are downloaded from the NCBI protein databank. We aimed to observe how the results changes in silico environments. 16 ligands group mostly having aromatic rings, fluorinated and chlorinated groups which are shown having effective in binding and guanidinium groups which have high cell membrane permeability. Energy minimizations were applied to these ligands by clean geometry and ligand optimization tools of
AccelrysDS.
Enzymes decrease activation energies mainly in transition states. The transition state in a chemical reaction is a specific configuration on the reaction coordinate. The time step is typically at the femtosecond level and this step cannot be simulated. Instead,
computational calculations based on highly improved force fields. Used AutoDock Free Energy Estimations are based on Van der Waals, Hydrogen bond, desolvation,
Electrostatic, Torsional Free Energies and Unbound System Energy. Whereas in AccelrysDS scoring functions’ contributions to evaluation is protein and amino acid related algorithms apart from force fields. LibDock is more concentrated on Hotspots like VDW and electrostatic attractions. For better binding, we are looking for lower AutoDock Ki values and higher CDocker and LibDock scores. In the field scoring
functions used as fast approximation mathematical methods to predict the strength of the non-covalent interaction between two molecules after they have been docked.
Journal article reference and Ligand codes : ligand names and 2D
conformation
Journal article reference and Ligand codes : ligand names and 2D conformation 1 (Silverman, et al., 2010) Lig1: 2k 2 (Silverman,, et al., 2008) Lig2: ligand2 3 (Silverman,, et al., 2008) Lig2_a : ligand3 4 (Silverman, 2009) Lig3: L-ArgNO2-L-Dbu-NH2
5 (Silverman, Poulos, Jamal, Li, Xue, &
Delker, 2010) Lig4: 3h
6 (Silverman, et al., 2007) Lig6: compound3
7 (Silverman, Roman, Martasek,
Gomez-Vidal, & Ji, 2006) Lig7: ligand 2d
8 (Ji, Li, Flinspach, Poulos, & Silverman, 2003)
Lig8: lig_III_2_CH2CH2NH2
9 (Kowaluk, et al., 1998)
Lig10: L-NNA
10 (Zhang, Fast, Marletta, Martasek, &
Silverman, 1997) Lig12: propyl
11 (Fengtian, et al., 2011)
Lig14: 8c
12 (Lee, Oplinger, Frick, Garve, Furfine, &
Shearer, 1997) Lig15: 39
13 (Shearer, et al., 1998)
Lig16: compound2
14 (Hah, Martasek, Roman, & Silverman, 2003)
Lig18 : RedAm-Ethyl (50)
15 (Silverman, et al., 2007)
Lig19: ligand1
16 (Silverman, Martasek, Roman, Huang, &
Hui, 2000)
Lig20: L-ArgNO2-L-Dbu-NH2d
Table 3: 16 prominent selected ligands from recent NOS articles
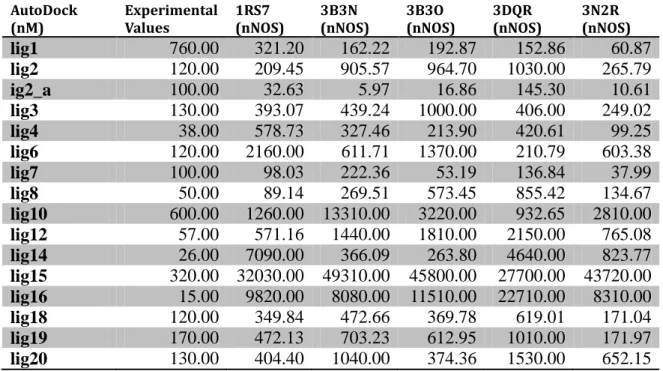
We applied AutoDock docking to these 16 ligands using nNOS, eNOS and iNOS pdb files denoting the NOS enzyme in three groups. Then we compared the experimental values with the in silico values. According to log values we draw a line chart. In the line chart we have chosen closest line to the experimental value line. Among 5 closest nNOS protein we found 1RS7 is closest to experimental data for nNOS. 3DQS is selected as the same way to be used in the Lead De novo design as the enzyme frame. We had only one iNOS candidate so we used 1NSI directly.
2.6 Structural Alignment
Sequence analysis is frequently a first step in characterizing a potential protein target, because proteins with the same or similar functionality mostly share high sequence similarity. We used “Modeler Salign” function of AccelrysDS to structurally align macromolecular enzyme data records. It uses a general dynamic programming based alignment algorithm. The weight matrix used for dynamic programming is a weighted sum of five protein structure and sequence features: residue type, intermolecular distance of residue pairs, fractional side chains accessibility, secondary structure type and local conformation. At the end of alignments, different snapshots are taken from the 3D images. Additionally we used a Perl script on AccelrysDS named
“BindingPocketSASAV2.pl” to display the cavity as a solvent accessible surface area and measure binding site volumes and visualize the positioning of the binding sites with the residues around.
3 Results
3.1 In silico vs. In vitro Case
In the literature there are laboratory results for NOS enzymes studied in vitro. We chose 16 ligands from 24 articles to define experimental ligand-protein docking Ki binding
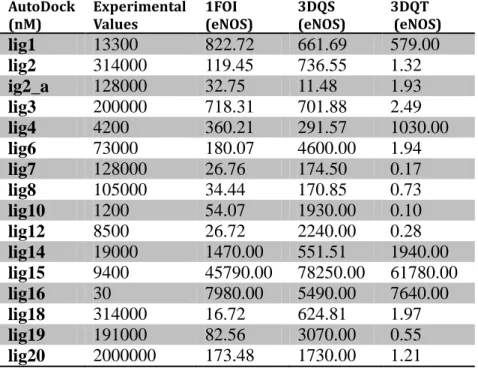
energy values. To dock these ligands we used 3B3M, 3B3N, 3B3O, 3DQR and 3N2R nNOS receptors; 1FOI, 3DQT and 3DQT eNOS receptors; and 1NSI iNOS receptor downloaded from the NCBI protein databank. We aimed to observe how the results changes in silico environments.
In the light of docking results of CDock, LibDock and AutoDock we decided to eliminate enzyme structures, which are not very compatible to our system. 1RS7 for nNOS and 3DQS for eNOS are set the foundation for the Lead discovery step. For iNOS, we did not have enough experimental data to test and compare in silico environment. Hence, we preferred to use 1NSI human enzyme which has a better resolution then 1NOD.
3.1.1 Docking Results for nNOS
AutoDock
(nM) Experimental Values 1RS7 (nNOS) 3B3N (nNOS) 3B3O (nNOS) 3DQR (nNOS) 3N2R (nNOS)
lig1 760.00 321.20 162.22 192.87 152.86 60.87 lig2 120.00 209.45 905.57 964.70 1030.00 265.79 ig2_a 100.00 32.63 5.97 16.86 145.30 10.61 lig3 130.00 393.07 439.24 1000.00 406.00 249.02 lig4 38.00 578.73 327.46 213.90 420.61 99.25 lig6 120.00 2160.00 611.71 1370.00 210.79 603.38 lig7 100.00 98.03 222.36 53.19 136.84 37.99 lig8 50.00 89.14 269.51 573.45 855.42 134.67 lig10 600.00 1260.00 13310.00 3220.00 932.65 2810.00 lig12 57.00 571.16 1440.00 1810.00 2150.00 765.08 lig14 26.00 7090.00 366.09 263.80 4640.00 823.77 lig15 320.00 32030.00 49310.00 45800.00 27700.00 43720.00 lig16 15.00 9820.00 8080.00 11510.00 22710.00 8310.00 lig18 120.00 349.84 472.66 369.78 619.01 171.04 lig19 170.00 472.13 703.23 612.95 1010.00 171.97 lig20 130.00 404.40 1040.00 374.36 1530.00 652.15
Table 4: AutoDock results of 16 prominent ligands on 5different nNOS crystal structures ADock
Log(nM) Experimental Values 1RS7 (nNOS) 3B3N (nNOS) 3B3O (nNOS) 3DQR (nNOS) 3N2R (nNOS)
lig1 2.88 2.51 2.21 2.29 2.18 1.78 lig2 2.08 2.32 2.96 2.98 3.01 2.42 ig2_a 2.00 1.51 0.78 1.23 2.16 1.03 lig3 2.11 2.59 2.64 3.00 2.61 2.40 lig4 1.58 2.76 2.52 2.33 2.62 2.00 lig6 2.08 3.33 2.79 3.14 2.32 2.78 lig7 2.00 1.99 2.35 1.73 2.14 1.58 lig8 1.70 1.95 2.43 2.76 2.93 2.13 lig10 2.78 3.10 4.12 3.51 2.97 3.45 lig12 1.76 2.76 3.16 3.26 3.33 2.88 lig14 1.41 3.85 2.56 2.42 3.67 2.92 lig15 2.51 4.51 4.69 4.66 4.44 4.64 lig16 1.18 3.99 3.91 4.06 4.36 3.92 lig18 2.08 2.54 2.67 2.57 2.79 2.23 lig19 2.23 2.67 2.85 2.79 3.00 2.24 lig20 2.11 2.61 3.02 2.57 3.18 2.81
Figure 11: nNOS AutoDock results chart of Table 5 denoting logarithmic behavior of inhibition constants
C-DOCK 1RS7
(nNOS) 3B3N (nNOS) 3B3O (NNOS) 3DQR (nNOS) 3N2R (nNOS)
lig1 22.79 28.89 14.35 26.83 x lig2 45.35 46.83 30.80 43.36 x lig2_a 33.18 37.78 16.23 38.53 x lig3 52.21 56.27 34.83 50.85 x lig4 50.07 48.54 44.65 49.47 x lig6 40.20 42.27 27.07 39.32 x lig7 38.00 33.76 19.77 31.47 x lig8 x x x x x lig10 35.37 36.26 23.00 36.38 35.44 lig12 52.17 65.09 43.72 57.10 66.06 lig14 30.96 30.73 29.28 18.12 27.24 lig15 18.33 23.61 9.36 18.44 23.41 lig16 23.29 27.35 16.59 22.94 24.61 lig18 43.70 44.61 28.78 39.19 45.09 lig19 43.19 44.88 30.86 41.09 44.35 lig20 51.10 54.67 39.04 53.54 53.40
Table 6: C-Dock results of 16 prominent ligands on 5different nNOS crystal structures
0.00 0.50 1.00 1.50 2.00 2.50 3.00 3.50 4.00 4.50 5.00
lig1 ig2_a lig4 lig7 lig10 lig14 lig16 lig19
EXP 1RS7(nNOS) 3B3N(nNOS) 3B3O(nNOS) 3DQR(nNOS) 3N2R(nNOS)
LIBDOCK 1RS7
(nNOS) 3B3N (nNOS) 3B3O (NNOS) 3DQR (nNOS) 3N2R (nNOS)
lig1 157.97 143.46 127.73 138.64 131.14 lig2 107.50 113.73 77.64 112.33 113.04 lig2_a 127.88 136.71 117.22 129.25 137.50 lig3 134.10 133.39 87.39 133.95 138.15 lig4 156.34 161.48 134.96 150.92 158.50 lig6 122.19 119.64 x 116.32 116.70 lig7 138.11 143.40 137.53 144.54 144.72 lig8 113.03 111.23 110.94 115.55 111.26 lig10 104.96 105.04 77.12 103.09 101.23 lig12 110.19 114.21 90.44 107.94 110.13 lig14 156.29 156.71 149.85 151.63 158.16 lig15 92.94 97.20 95.09 93.05 89.37 lig16 96.00 92.57 99.32 97.72 94.04 lig18 110.31 108.96 88.73 117.62 109.90 lig19 107.50 113.73 77.64 112.33 113.04 lig20 135.26 138.39 107.01 131.25 142.54
Table 7: LibDock results of 16 prominent ligands on 5different nNOS crystal structure
According to the docking result above, we have eliminated all others but the 1RS7 protein file, which satisfied the closest in slico results comparing to in vitro results. We proceed to the next step which is Lead Discovery by these nNOS enzymes.
3.1.2 Docking Results for eNOS
AutoDock
(nM) Experimental Values 1FOI (eNOS) 3DQS (eNOS) 3DQT (eNOS)
lig1 13300 822.72 661.69 579.00 lig2 314000 119.45 736.55 1.32 ig2_a 128000 32.75 11.48 1.93 lig3 200000 718.31 701.88 2.49 lig4 4200 360.21 291.57 1030.00 lig6 73000 180.07 4600.00 1.94 lig7 128000 26.76 174.50 0.17 lig8 105000 34.44 170.85 0.73 lig10 1200 54.07 1930.00 0.10 lig12 8500 26.72 2240.00 0.28 lig14 19000 1470.00 551.51 1940.00 lig15 9400 45790.00 78250.00 61780.00 lig16 30 7980.00 5490.00 7640.00 lig18 314000 16.72 624.81 1.97 lig19 191000 82.56 3070.00 0.55 lig20 2000000 173.48 1730.00 1.21
Table 8: AutoDock results of 16 prominent ligands on five different eNOS crystal structures
ADock
Log(nM) Experimental Values 1FOI (eNOS) 3DQS (eNOS) 3DQT (eNOS)
lig1 4.12 2.92 2.82 2.76 lig2 5.50 2.08 2.87 0.12 ig2_a 5.11 1.52 1.06 0.29 lig3 5.30 2.86 2.85 0.40 lig4 3.62 2.56 2.46 3.01 lig6 4.86 2.26 3.66 0.29 lig7 5.11 1.43 2.24 -0.77 lig8 5.02 1.54 2.23 -0.14 lig10 3.08 1.73 3.29 -1.02 lig12 3.93 1.43 3.35 -0.55 lig14 4.28 3.17 2.74 3.29 lig15 3.97 4.66 4.89 4.79 lig16 1.48 3.90 3.74 3.88 lig18 5.50 1.22 2.80 0.29 lig19 5.28 1.92 3.49 -0.26
Figure 12 : eNOS AutoDock results chart of Table 9. denoting logarithmic behavior of inhibition constants
C-DOCK 1FOI
(eNOS) 3DQS (eNOS) 3DQT (ENOS) 1NSI (iNOS)
lig1 19.93 21.21 -4.71 24.14 lig2 44.00 42.81 34.89 44.36 lig2_a 33.54 30.84 12.29 38.20 lig3 42.15 49.92 39.91 61.54 lig4 45.67 50.85 26.41 52.25 lig6 38.04 37.92 31.34 51.08 lig7 40.07 31.41 8.59 40.71 lig8 x x x x lig10 37.31 35.87 26.91 44.27 lig12 67.50 62.92 39.16 81.75 lig14 26.61 29.60 20.59 28.72 lig15 16.49 16.29 11.35 23.60 lig16 19.22 19.13 17.20 27.12 lig18 40.86 37.76 33.63 44.80 lig19 39.52 38.18 36.70 48.96 lig20 50.06 53.07 26.65 60.18
Table 10: C-Dock results of 16 prominent ligands on 5different eNOS crystal structures
-2.00 -1.00 0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00
lig1 ig2_a lig4 lig7 lig10 lig14 lig16 lig19
EXP 1FOI(eNOS) 3DQS(eNOS) 3DQT(eNOS)
LIBDOCK 1FOI
(eNOS) 3DQS (eNOS) 3DQT (ENOS) 1NSI (iNOS)
lig1 141.05 146.02 129.00 137.19 lig2 106.42 110.54 106.14 102.88 lig2_a 120.68 124.88 62.05 132.76 lig3 127.61 114.60 115.34 130.10 lig4 155.69 157.33 141.51 153.76 lig6 119.66 106.54 114.42 105.23 lig7 128.83 134.89 98.94 137.91 lig8 102.26 118.58 x 107.87 lig10 102.81 98.41 89.11 103.83 lig12 109.90 102.85 99.31 101.78 lig14 144.82 148.67 143.30 141.98 lig15 84.14 86.10 78.64 84.20 lig16 96.24 92.24 82.03 92.94 lig18 100.23 94.85 88.26 97.15 lig19 108.55 110.54 106.14 102.87 lig20 143.16 132.70 142.84 123.19
Table 11: LibDock results of 16 prominent ligands on 5different eNOS crystal structures
According to the docking result above, we chose the 3DQS protein file, which yields the closest in slico results comparing to in vitro results. We proceed to the next step, which is Lead Discovery by this eNOS enzyme.
3.2 De Novo Results
3.2.1 Lead Discovery Results
From the AccelrysDS and ZINC libraries, we filtered out 6 molecules on 1RS7 nNOS, 2 molecules on 3DQS eNOS and, 2 molecules on 1NSI iNOS scaffolds from more than a million lead according to the Ludi scores. These 9 leads are going to be scaffolds in fragment-based lead discovery stage. De Novo best lead discovery results are shown in Table 12
ZINC Lib. Molecule Ludi Score
Structure ZINC Lib.
Molecule Ludi Score Structure P_0_3 zinc04859564 885 3RS7 - nNOS P_0_5 zinc42689701 779 3RS7 - nNOS P_0_5 zinc00056346 861 3RS7 - nNOS P_0_8 zinc71786250 832 3RS7 - nNOS P_0_9 zinc39941619 816 3RS7 - nNOS P_1_1 zinc35335410 741 3RS7 - nNOS P_0_9 0_8 0_7 zinc00081090 900 3DQS - eNOS P_1_1 zinc12546533 711 3DQS - eNOS P_0_9 0_8 0_7 zinc04649158 575 1NSI - iNOS P_1_1 zinc00151524 766 1NSI - iNOS
Table 12: De Novo Lead Discovery ( best ZINC molecules that will be used as a Lead in the Fragment based De Novo Stage)
3.2.2 De Novo Fragment Based Discovery Results
Each new leads are developed in fragments libraries and best 10 of evolved molecules are docked back to NOS enzymes. Total 100 evolved molecules are docked back to main three NOS isozymes. From the docking result in Table 14, we have selected the evolved 17 molecules (Table 13) whose AutoDock Ki values and CDock Ludi Scores reveal selectivity in binding. Best fragment based discovery results are given in Table 13
Ludi Score MWeight Structure Ludi Sc. MWeight Structure 1NSI_zinc00151524 Evo3 1266 542 Evo6 1246 584 Evo7 1230 548 Evo10 1222 541 1NSI_zinc04649158 Evo2 1054 529 Evo3 1053 574 Evo4 1048 514 1RS7_zinc39941619
Evo9 1017 508 Evo10 1017 523 1RS7_ zinc42689701 Evo5 1378 569 Evo6 1375 585 Evo10 1351 540 1RS7_zinc04859564 Evo5 1253 568 Evo6 1246 584 Evo10 1222 541 Evo_8 1444 524 3DQS_zinc00081090
3.3 ADMET Results
The ADMET behaviors of the selected evolved drug candidate molecules shown in Figure 13 states that five of them are not suitable for HIA. ADMET failing compounds are 1NSI_zinc00151524 evo6, 1NSI_zinc04649158 evo2, evo3 and evo4 and
1RS7_zinc04859564 evo9,evo10. The others, all fall into the middle of the area enclosed by ADMET boundary eclipses denoting they are better candidates to be a drug. In Figure 13, a few of the enzymes overlap in the same points denoted by blue color.
“1NSI_zinc00151524_ Evo3,Evo7, Evo10 “, “1RS7_ zinc42689701_Evo5, Evo 6, Evo 10”, “1RS7_zinc04859564_Evo5, Evo 6, Evo 10” and “3DQS_zinc00081090_Evo8” are all have outstanding ADMET results.
3.4 2D & 3D Results of De Novo Evolved Ligands
60
AutoDocks (All Ki values in nm) LibDocks Scores
Ligands 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1NSI_zinc00151524_evo1 63.96 4.6 10.53 0.4 0.1 2.3 13.9 52.96 154.76 132.26 1NSI_zinc00151524_evo2 75.17 4.25 3.64 1.2 0.1 0.9 17.7 57.65 125.02 116.01 1NSI_zinc00151524_evo3 94.75 2.72 28.97 0.1 0.0 10.7 34.8 x 117.27 76.02 1NSI_zinc00151524_evo4 72.36 3.96 14.53 0.3 0.1 3.7 18.3 x 87.81 69.17 1NSI_zinc00151524_evo5 103.38 59.15 365.18 0.2 0.6 6.2 1.7 x 147.39 119.60 1NSI_zinc00151524_evo6 585 12.9 332.05 0.0 0.0 25.7 45.3 x 127.14 113.65 1NSI_zinc00151524_evo7 345.43 6.28 716.13 0.0 0.0 114.0 55.0 x 113.52 73.49 1NSI_zinc00151524_evo8 974.44 71.26 364.63 0.2 0.1 5.1 13.7 x 96.61 92.16 1NSI_zinc00151524_evo9 368.15 59.37 426.26 0.1 0.2 7.2 6.2 x 113.86 89.53 1NSI_zinc00151524_evo10 123.06 3.49 194.76 0.0 0.0 55.8 35.3 x 127.49 88.97 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1NSI_zinc04649158_evo1 9700 3.76 11.66 0.3 0.0 3.1 2579.8 x 135.41 123.30
1NSI_zinc04649158_evo2 95.52 0.44088 124.2 0.0 0.0 281.7 216.7 x 138.34 123.71 1NSI_zinc04649158_evo3 93.95 0.53878 131.48 0.0 0.0 244.0 174.4 x x 139.59 1NSI_zinc04649158_evo4 93.74 0.35568 152.75 0.0 0.0 429.5 263.6 x 134.58 121.28 1NSI_zinc04649158_evo5 7.2 0.46604 2.18 0.2 0.1 4.7 15.4 x 144.58 124.41 1NSI_zinc04649158_evo6 13.34 0.45125 1.24 0.4 0.0 2.7 29.6 x 148.28 131.03 1NSI_zinc04649158_evo7 11.6 0.41526 1.49 0.3 0.0 3.6 27.9 x 149.52 145.30 1NSI_zinc04649158_evo8 7.67 0.76733 2.15 0.4 0.1 2.8 10.0 x 146.15 134.86 1NSI_zinc04649158_evo9 7.95 0.46691 1.77 0.3 0.1 3.8 17.0 x x 66.50 1NSI_zinc04649158_evo10 42.58 103.38 626.49 0.2 2.4 6.1 0.4 x 125.61 56.74 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1RS7_zinc00056346_evo1 11 1.5 14.75 0.1 0.1 9.8 7.3 x 168.68 120.99 1RS7_zinc00056346_evo2 3.08 2.35 14.47 0.2 0.8 6.2 1.3 x 165.51 117.68 1RS7_zinc00056346_evo3 5.26 2.02 5.17 0.4 0.4 2.6 2.6 x 164.49 117.41 1RS7_zinc00056346_evo4 2.87 1.88 10.56 0.2 0.7 5.6 1.5 x 163.26 112.87 1RS7_zinc00056346_evo5 5.01 2.98 19.65 0.2 0.6 6.6 1.7 x 162.35 116.39
1RS7_zinc00056346_evo8 6.23 2.13 7.34 0.3 0.3 3.4 2.9 118.98 160.35 135.28 1RS7_zinc00056346_evo9 3.26 1.79 8.57 0.2 0.5 4.8 1.8 x 149.67 131.39 1RS7_zinc00056346_evo10 3.79 8.72 19.46 0.4 2.3 2.2 0.4 129.20 151.20 132.38 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1RS7_zinc39941619_Evo_1 1.79 3.49 2.15 1.6 1.9 0.6 0.5 x 137.56 143.36 1RS7_zinc39941619_Evo_2 0.10248 17.91 41.04 0.4 174.8 2.3 0.0 x 148.49 136.36 1RS7_zinc39941619_Evo_3 0.11523 2.62 4.28 0.6 22.7 1.6 0.0 x 115.78 126.48 1RS7_zinc39941619_Evo_4 4120 10.69 97.63 0.1 0.0 9.1 385.4 x 114.35 126.55 1RS7_zinc39941619_Evo_5 4120 11.3 91.7 0.1 0.0 8.1 364.6 x 135.61 135.03 1RS7_zinc39941619_Evo_6 4280 9.94 118.73 0.1 0.0 11.9 430.6 x 126.20 98.39 1RS7_zinc39941619_Evo_7 4110 9.86 101.08 0.1 0.0 10.3 416.8 x 153.60 108.24 1RS7_zinc39941619_Evo_8 58220 100.05 869 0.1 0.0 8.7 581.9 x x 116.98 1RS7_zinc39941619_Evo_9 112860 137.65 10260 0.0 0.0 74.5 819.9 x 154.09 96.58 1RS7_zinc39941619_Evo_10 71710 146.13 68660 0.0 0.0 469.9 490.7 x 139.95 82.28 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1RS7_zinc42689701_Evo_1 576.36 33.89 81.55 0.4 0.1 2.4 17.0 141.15 128.66 119.72
1RS7_zinc42689701_Evo_2 2520 30.32 2080 0.0 0.0 68.6 83.1 x 120.72 114.35 1RS7_zinc42689701_Evo_3 2510 34.47 2050 0.0 0.0 59.5 72.8 124.78 133.69 116.86 1RS7_zinc42689701_Evo_4 383.81 40.32 306.67 0.1 0.1 7.6 9.5 122.84 133.56 111.65 1RS7_zinc42689701_Evo_5 354.01 16.65 252.47 0.1 0.0 15.2 21.3 x 141.21 139.58 1RS7_zinc42689701_Evo_6 404.46 12.27 251.22 0.0 0.0 20.5 33.0 111.83 123.16 103.43 1RS7_zinc42689701_Evo_7 379.74 17.75 339.9 0.1 0.0 19.1 21.4 117.57 137.11 137.56 1RS7_zinc42689701_Evo_8 379.6 45.6 1200 0.0 0.1 26.3 8.3 114.06 156.78 114.43 1RS7_zinc42689701_Evo_9 376.07 26.01 253.89 0.1 0.1 9.8 14.5 125.62 136.83 133.33 1RS7_zinc42689701_Evo_10 381.72 16.51 1090 0.0 0.0 66.0 23.1 129.56 129.44 123.86 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1RS7_zinc71786250_Evo_1 13.13 21.69 4.69 4.6 1.7 0.2 0.6 160.19 159.18 157.18 1RS7_zinc71786250_Evo_2 15.82 10.59 10.1 1.0 0.7 1.0 1.5 145.38 150.09 148.59 1RS7_zinc71786250_Evo_3 23.19 3.98 3.36 1.2 0.2 0.8 5.8 151.68 146.56 140.86 1RS7_zinc71786250_Evo_4 18.26 16.14 2.29 7.0 0.9 0.1 1.1 150.40 148.34 144.16 1RS7_zinc71786250_Evo_5 15.73 22.27 4.97 4.5 1.4 0.2 0.7 145.47 153.41 156.57
1RS7_zinc71786250_Evo_8 27.06 16.31 3.72 4.4 0.6 0.2 1.7 143.33 158.86 157.15 1RS7_zinc71786250_Evo_9 7.12 19.15 11.27 1.7 2.7 0.6 0.4 137.34 133.86 131.94 1RS7_zinc71786250_Evo_10 17.94 2.39 2.95 0.8 0.1 1.2 7.5 123.28 136.83 134.22 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1RS7_zinc04859564_Evo_1 35.58 10.82 15.28 0.7 0.3 1.4 3.3 52.96 154.76 132.26 1RS7_zinc04859564_Evo_2 50.23 13.32 12.61 1.1 0.3 0.9 3.8 57.65 125.02 116.01 1RS7_zinc04859564_Evo_3 74.15 1.88 4.13 0.5 0.0 2.2 39.4 x 117.27 76.02 1RS7_zinc04859564_Evo_4 52.12 2.95 14.5 0.2 0.1 4.9 17.7 x 87.81 69.17 1RS7_zinc04859564_Evo_5 1370 36.71 311.62 0.1 0.0 8.5 37.3 x 147.39 119.60 1RS7_zinc04859564_Evo_6 1710 6.59 694.18 0.0 0.0 105.3 259.5 x 127.14 113.65 1RS7_zinc04859564_Evo_7 98.97 129.63 352.75 0.4 1.3 2.7 0.8 x 113.52 73.49 1RS7_zinc04859564_Evo_8 488.61 64.56 302.03 0.2 0.1 4.7 7.6 x 96.61 92.16 1RS7_zinc04859564_Evo_9 323.62 60.7 414.2 0.1 0.2 6.8 5.3 x 113.86 89.53 1RS7_zinc04859564_Evo_10 182.26 3.33 165.83 0.0 0.0 49.8 54.7 x 127.49 88.97 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 1RS7_zinc35335410_Evo_1 1030000 97060 56830 1.7 0.1 0.6 10.6 x 173.79 147.74
1RS7_zinc35335410_Evo_2 1040000 96370 57090 1.7 0.1 0.6 10.8 x 151.97 143.97 1RS7_zinc35335410_Evo_3 1040000 101770 56850 1.8 0.1 0.6 10.2 x 145.95 125.37 2RS7_zinc35335410_Evo_4 1040000 96650 56900 1.7 0.1 0.6 10.8 x x 141.47 1RS7_zinc35335410_Evo_5 1040000 96340 56810 1.7 0.1 0.6 10.8 x 110.73 149.42 1RS7_zinc35335410_Evo_6 1040000 96560 56890 1.7 0.1 0.6 10.8 x 116.45 144.99 1RS7_zinc35335410_Evo_7 1040000 96240 56900 1.7 0.1 0.6 10.8 x 92.07 145.05 0RS7_zinc35335410_Evo_8 1050000 96810 56950 1.7 0.1 0.6 10.8 x x 162.24 1RS7_zinc35335410_Evo_9 1040000 96390 56890 1.7 0.1 0.6 10.8 x 158.43 x 2RS7_zinc35335410_Evo_10 1040000 96360 56870 1.7 0.1 0.6 10.8 x x 128.56 AutoDocks 1NSI iNOS 1RS7 nNOS 3DQS
eNOS n/e n/i e/n i/n
1NSI iNOS 1RS7 nNOS 3DQS eNOS 3DQS_zinc00081090_Evo_1 160.73 6.03 144.67 0.0 0.0 24.0 26.7 92.59 131.34 129.05 3DQS_zinc00081090_Evo_2 222.35 35.41 240.09 0.1 0.2 6.8 6.3 112.52 136.54 137.78 3DQS_zinc00081090_Evo_3 107.08 35.8 151.36 0.2 0.3 4.2 3.0 94.17 146.11 137.53 3DQS_zinc00081090_Evo_4 264.51 2.57 5.96 0.4 0.0 2.3 102.9 95.68 146.36 122.29 3DQS_zinc00081090_Evo_5 190.24 2.73 30.4 0.1 0.0 11.1 69.7 117.74 141.49 153.81