Original article
THE IMPORTANCE OF ACHILLEA SPECIES AND PHENOLIC
COMPOUNDS IN ACHILLEA SCHISCHKINII Sosn.
ACHILLEA TÜRLERĐNĐN ÖNEMĐ VE ACHILLEA SCHISCHKINII TÜRÜNÜN
FENOLĐK BĐLEŞĐKLERĐ
Elian KHAZNEH1, Gülçin SALTAN2*, Mehmet TEKĐN3,Özlem BAHADIR ACIKARA2,
Talha YEŞĐLOĞLU2, Serkan ÖZBĐLGĐN2
1
University of Veterinary and Pharmaceutical Sciences Brno, Faculty of Pharmacy, Department of Natural Drugs, Brno, CZECH REPUBLIC
2
Ankara University, Faculty of Pharmacy, Department of Pharmacognosy, 06100, Tandoğan-Ankara, TURKEY
3
Cumhuriyet University, Faculty of Science, Department of Biology, 58140, Sivas, TURKEY
ABSTRACT
Achillea species belonging to Asteraceae family have ethno pharmacological importance in folk medicine as remedies due to their various medicinal properties such as anti-inflammatory, analgesic, antispasmodic, hemostatic, wound healing, digestive and cholagogue. In present study, the phenolic composition of the extracts prepared using aerial parts of Achillea schischkinii Sosn. were investigated. The composition of the extracts were determined by HPLC on a SUPELCOSILTM ABZ+PLUS, 15 cm x 4.6 mm column. Water and water-ethanol extracts (25:75) of the aerial parts of plant materials were prepared and subjected to further extraction with ethyl acetate and chloroform respectively. Ethyl acetate and remaining water fractions of water extract as well as chloroform and remaining water fractions of water-ethanol extract (25:75) were characterized by HPLC using some standard compounds.
Key words: Achillea, Achillea schischkinii, Phenolic compounds, HPLC.
ÖZET
Asteraceae familyasına ait olan Achillea türleri halk arasında anti-enflamatuar, analjezik, antispazmodik, hemostatik, yara iyi edici, dijestif ve kolagog etkilerinden dolayı kullanımı nedeniyle etnofarmakolojik bir öneme sahiptir. Bu çalışmada Achillea schischkinii Sosn. türünün toprak üstü
kısımlarından hazırlanan ekstrelerin fenolik içeriği araştırılmıştır. Ekstrelerin içeriği SUPELCOSILTM ABZ+PLUS, 15 cm x 4.6 mm kolon üzerinde YBSK metodu kullanılarak araştırılmıştır. Bitkinin toprak üstü kısımlarından hazırlanan sulu ve sulu-etanollü (25:75) ekstrelerinden sıvı-sıvı ekstraksiyon ile sırasıyla etilasetatlı ve kloroformlu fraksiyonları hazırlanmıştır. Sulu ekstrenin etilasetatlı fraksiyonu ve kalan sulu fraksiyonu ile sulu-etanollü ekstrenin kloroformlu ve kalan sulu-etanollü fraksiyonlarının içeriği YBSK ile bazı standart bileşikler ile kıyaslanmak suretiyle aydınlatılmıştır.
Anahtar kelimeler: Achillea, Achillea schischkinii, Fenolik bileşikler, YBSK
* Correspondence:gulcin.saltan@pharmacy.ankara.edu.tr
INTRODUCTION
Achillea is one of the youngest evolutionary genera of the Asteraceae family, which is
distributed widely throughtout the world. More than 100 species have been recognized in this genus (1,2). The name of the genus originates from the ancient use as a wound-healing remedy by the Trojan hero Achilles. Achillea millefolium is the most known species from this genus. These plants are medicinal perennial herbs that are native to Europe and western Asia but are also found in Australia, New Zeland and North America (3,4). Aerial parts of different Achillea species are widely used in folk medicine for the preparation of herbal teas with antiphlogostic and spasmolytic activity and also they have been used an anti-inflammatory agent for treatment of rheumatic pain (5). Extracts from Achillea spp. have been extensively studied for their antimicrobial, antihypertensive and antihyperlipidemic effects. Furthermore antispasmodic, antidiabetic, antioxidant, antispermatogenic, antifertility and immunosuppressive activities have been reported (6).
Achillea species have several components which essential oils, sesquiterpenes and phenolic
compounds such as flavonoids and phenolic acids (7). But phenolic compounds and flavonoids are the most important medicinal metabolites of Achillea species. Flavonoids have been reported to exert a wide range of biological activities including: anti-inflammatory, antibacterial, antiviral and anti-tumor effects (6).
Most of the species belonging to the Achillea genus contain flavonoids such as flavonols, flavones and their derivatives. In different species of Achillea, aglycons (apigenin, luteolin, quercetin), monoglycosides (mainly O-glucosides, C-glucosides, and O-glucuronides), diglycosides (O-diglucosides, C-diglucosides, O-rutinosides, 6-C-glucosyl-8-C-arabinosyl, 6-C-arabinosyl-8-C-glucosil, luteolin-6-C-apiofuranosyl-(1 → 2)-glucoside, 3-O-arabinosyl-(1 → 6)-glucoside), and methyl derivatives of flavonoids have been found previously (6).
There are about 42 species of this genus in Turkish flora and about 20 of them are endemic (8-9). Achillea schischkinii is an endemic species and widely distributed in Central and East Anatolia (9). In this study, phytochemical composition of the A. schischkinii aerial part extracts were investigated by HPLC using some phenolic acid and flavonoid standards such as, caffeic acid,
luteolin, luteolin glucoside, schaftoside, scutellarein dimethylether, vitexin, vitexin rhamnoside, in current study.
EXPERIMENTAL Plant Material
Plant material was collected from Sivas-Turkey. Identification of the plant was performed by Mehmet Tekin from Sivas Cumhuriyet University, Department of Biological Sciences. Voucher specimen was kept in the herbarium of Sivas Cumhuriyet University (Herbarium number 1330), Department of Biological Sciences.
Preparation of Extracts
Aerial parts and roots of the plant were separated, than dried and powdered. Aerial parts of the powdered plant material were weighed as 475.082 g. Exraction of the plant material was performed by using BANDELIN SONOREX DIGITEC ultrasonic bath with water and water:ethanol (25:75) respectively for 30 min and three times. After filtration each extract was combined to obtain water and water-ethanol exract. Water-ethanol extract was concentrated firstly under reduced pressure and low temperature (40-50 °C) on a rotary evaporator, than lyophilized to give 5.037 g (WEtOHE) crude extract. Water extract was also dried by freeze dryer to obtain 12.760 g (WE) crude extract.
Both lyophilized water and water/ethanol (25:75 v/v) extracts were subjected to further fractionation by liquid-liquid extraction. Water extract dissolved in water and water-ethanol extract was dissolved in water-ethanol mixture then transferred into the separatory funnel and extracted three times with the same portion of ethyl acetate and chloroform respectively. Chloroform and ethyl acetate phases were concentrated to dryness under reduced pressure and low temperature on a rotary evaporator. After this process remaining water and water-ethanol extracts were frozen and lyophilized to remove the water. Weight of dried residues as follow; water fraction residue: 8.435 g (RWP); ethyl acetate fraction residue: 0.44 g (EtOAcP); water-ethanol fraction residue: 2.302 g (RWEtOHP) and chloroform fraction residue: 0.35 g (CHCl3P).
HPLC Analysis
HPLC analyses were carried using HP Agilent 1100 series chromatograph with a quaternary pump (G1311A), autosampler (G1313A), column (G1316A), DAD detector (G1315B) on SUPELCOSILTM ABZ+PLUS, 15 cm x 4.6 mm, 3 µm column as stationary phase. Gradient system was used for elution with 1 mL/min. Mobile phase was made up acetonitrile and water mixture in 0th minute 10% acetonitrile (pump B) and 90% of water + 0.2% of formic acid (pump A) in 36th minute 100% acetonitrile. Column temperature was 40 ºC. 215, 230, 254, 280 and 350 nm were used for detection of the compounds.
min 0 5 10 15 20 25 30 35 mAU 0 20 40 60 80 100
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100331\10033106.D)
9
.0
0
9
Samples for HPLC analysis were prepared as follow; 10 mg water extract dissolved in 1 mL of deionized water. 10 mg water/ethanol (25:75 v/v) extract was dissolved in 1 mL of DMSO. 5 mg/mL solution of the ethyl acetate and chloroform as well as remaining water and water/ethanol (25:75) fraction were prepared by dissolving 5 mg of each in 1 mL DMSO. Then the samples were filtered (0.45 µm) before HPLC analysis. Injection volume was 10 µL.
RESULTS AND DISCUSSION
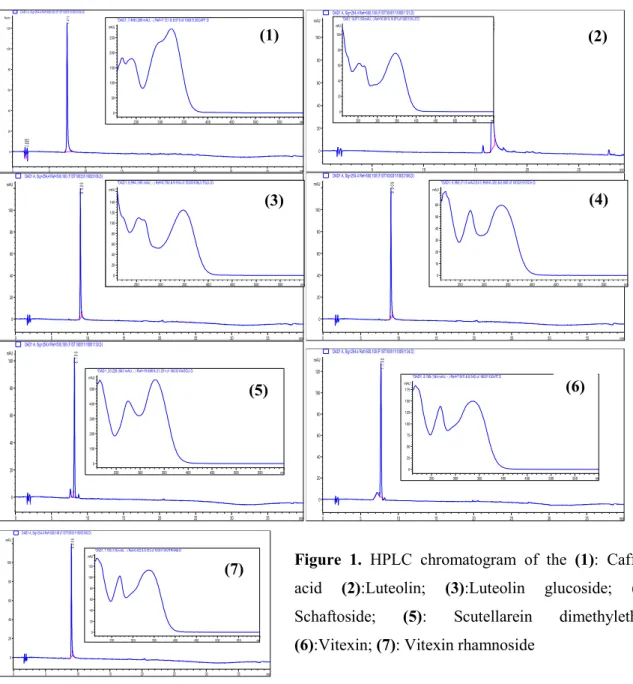
In current study, HPLC analysis of the water and water-ethanol extract of A. schischkinii have revealed that both extract were found to be rich in phenolic compounds. Chemical compositions of the extracts have been clarified by comparing retention times and UV spectra with authentic samples (Figure 1).
Figure 1. HPLC chromatogram of the (1): Caffeic
acid (2):Luteolin; (3):Luteolin glucoside; (4):
Schaftoside; (5): Scutellarein dimethylether;
(6):Vitexin; (7): Vitexin rhamnoside min 0 5 10 15 20 25 30 35 Norm. 0 20 40 60 80 100 120
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100331\10033120.D)
1 .6 98 1 .9 9 5 7 .4 7 2 min 5 10 15 20 25 mAU 0 20 40 60 80 100
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100511\10051131.D)
1 6 .5 9 1 min 0 5 10 15 20 25 30 35 mAU 0 20 40 60 80 100
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100331\10033106.D)
9 .0 0 9 min 0 5 10 15 20 25 30 35 mAU 0 20 40 60 80 100
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100331\10033106.D)
9 .0 0 9 min 0 5 10 15 20 25 30 35 mAU 0 20 40 60 80 100
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100511\10051130.D)
8 .1 9 0 min 0 5 10 15 20 25 30 35 mAU 0 20 40 60 80 100 120
DAD1 A, Sig=254,4 Ref=500,100 (F:\ST100511\10051134.D)
7 .7 0 0 nm 250 300 350 400 450 500 550 mAU 0 20 40 60 80 100 120 140
*DAD1, 8.994 (160 mAU, - ) Ref=8.700 & 9.914 of 10033106LUTGLU.D
(3) nm 250 300 350 400 450 500 550 mAU 0 10 20 30 40 50 60
*DAD1, 6.560 (71.6 mAU,Dn1) Ref=6.320 & 6.800 of 10033101SCH.D
nm 250 300 350 400 450 500 550 mAU 0 20 40 60 80 100 120 140
*DAD1, 8.994 (160 mAU, - ) Ref=8.700 & 9.914 of 10033106LUTGLU.D
nm 250 300 350 400 450 500 550 mAU 0 20 40 60 80 100
*DAD1, 16.671 (109 mAU, - ) Ref=16.391 & 19.071 of 10051131LUT.D
nm 250 300 350 400 450 500 550 mAU 0 50 100 150 200 250
*DAD1, 7.468 (280 mAU, - ) Ref=7.121 & 8.575 of 10033120CAFF.D
(3) (4) (1) (2) nm 250 300 350 400 450 500 550 mAU 0 100 200 300 400 500
*DAD1, 20.228 (563 mAU, - ) Ref=19.988 & 21.201 of 10033104SCU.D
(5) nm 250 300 350 400 450 500 550 mAU 0 25 50 75 100 125 150 175
*DAD1, 8.169 (184 mAU, - ) Ref=7.915 & 8.542 of 10051130VIT.D
(6) nm 250 300 350 400 450 500 550 mAU 0 20 40 60 80 100 120
*DAD1, 7.759 (135 mAU, - ) Ref=6.492 & 8.572 of 10051134VITRHAM.D
min 0 5 10 15 20 25 30 35 Norm. 0 25 50 75 100 125 150 175 200
DAD1 A, Sig=254,4 Ref=900,100 (ACHILLEA1_2_13\AC130201 2013-02-01 14-12-33\AC130201000006.D)
1 .2 4 2 1 .3 4 2 1 .4 7 3 1 .7 5 9 1 .8 5 3 1.9 7 1 2 .2 3 5 2 .4 8 7 2 .6 1 0 2 .8 1 4 3 .0 3 3 3 .5 3 3 3 .7 0 1 3 .9 6 3 4 .0 5 5 4 .1 8 4 4 .3 0 0 4 .4 3 7 4 .5 7 4 4 .6 8 6 4 .8 2 1 5 .0 6 8 5 .3 2 1 5 .4 8 2 5 .8 7 6 6 .1 3 4 6 .2 8 5 6 .5 1 1 6 .6 0 4 6 .7 3 8 6 .9 7 5 7 .1 6 4 7 .4 3 3 7 .5 6 8 7 .7 9 5 8 .0 3 8 8 .2 0 9 8 .3 5 4 8 .7 4 2 8 .8 9 6 9 .0 8 5 9 .2 9 0 9 .5 5 6 9 .8 2 4 1 0 .1 9 6 1 0 .4 5 7 1 1 .2 1 4 1 1 .5 5 1 1 2 .0 6 5 1 2 .4 4 9 1 7 .1 5 8 min 0 5 10 15 20 25 30 35 Norm. 0 50 100 150 200 250 300
DAD1 A, Sig=254,4 Ref=900,100 (ACHILLEA1_2_13\AC130201 2013-02-01 14-12-33\AC130201000007.D)
1 .2 1 0 1 .2 9 5 1 .4 8 1 1 .7 3 3 1 .8 4 6 2 .0 1 0 2 .1 3 1 2 .2 6 6 2 .6 6 9 2 .8 3 3 3 .0 3 2 3 .4 4 6 4.6 7 0 5 .3 4 9 5 .8 6 9 6 .1 51 6.5 01 6.6 0 2 6 .7 3 8 6 .9 7 5 7 .1 8 5 7.4 4 2 7.5 4 3 7 .7 8 6 8 .0 3 1 8 .2 0 6 8 .7 4 1 9 .0 3 9 9 .1 5 7 9 .2 52 9.5 5 2 9 .9 2 2 1 0 .1 9 7 1 0 .3 3 4 1 0 .4 8 6 1 0 .7 0 2 1 0 .9 6 8 1 1 .2 1 5 1 1 .4 3 9 1 1 .5 6 3 1 1 .8 7 2 1 2 .0 6 7 1 2 .4 4 4 1 2 .7 5 7 1 2 .9 4 1 1 3 .1 3 6 1 3 .5 1 7 1 3 .7 1 5 1 3 .8 1 5 1 3 .9 7 1 1 4 .3 8 3 1 4 .6 2 2 1 5 .1 0 9 1 5 .3 4 7 1 5 .5 6 5 1 5 .7 3 6 1 6 .0 3 4 1 6 .4 0 2 1 6 .6 6 0 1 7 .1 5 1 1 7 .3 8 2 1 7 .7 3 4 1 7 .9 7 6 1 8 .1 7 4 1 8 .6 0 9 1 9 .2 3 6 1 9 .8 8 3 2 0 .4 2 1 2 2 .0 5 7 2 2 .4 6 3 2 3 .0 4 0 2 3 .7 0 9 2 4 .4 1 3 2 4 .7 1 8 2 5 .2 1 3 2 5 .7 2 1
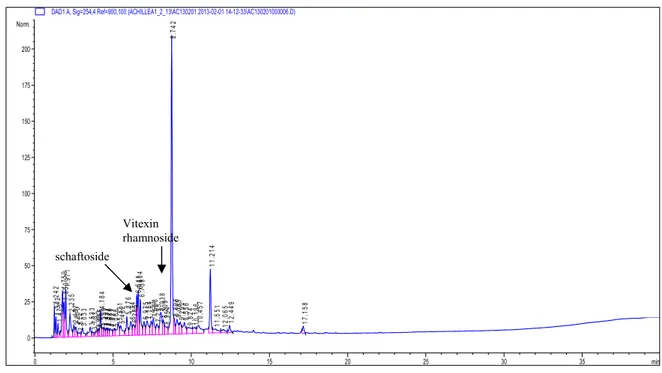
Vitexin, caffeic acid, scutellarein dimethylether (Figure 2) and luteolin were determined in water-ethanol extract while schaftoside and vitexin rhamnoside (Figure 3) were detected in water extract as given in Table 1.
Figure 2. HPLC Chromatogram of A. schischkinii water extract
Figure 3. HPLC Chromatogram of A. schischkinii water-ethanol extract
schaftoside Vitexin rhamnoside Scutellarein dimethylether luteolin vitexin caffeic acid
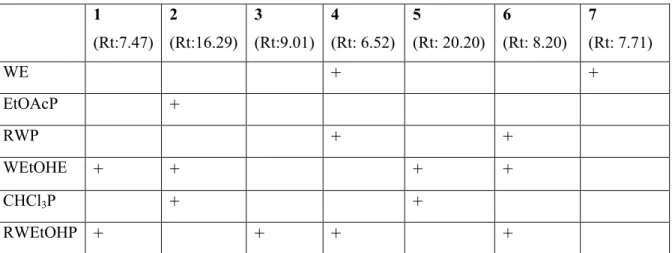
While luteolin was detected in ethyl acetate part vitexin and schaftoside were determined in the remaining water part of water extract. According to these results luteolin a flavonoid aglycone can be separated from vitexin and schaftoside by liquid-liquid extraction of total water extract. In chloroform fraction of water-ethanol extract luteolin and scutellarein dimethylether were found to be major components while schaftoside, caffeic acid, vitexin and luteolin glucoside were detected in remaining water-ethanol extract. All results were given Table 1 with their retention times.
Table 1. Phenolic composition of A. schischkinii extracts 1 (Rt:7.47) 2 (Rt:16.29) 3 (Rt:9.01) 4 (Rt: 6.52) 5 (Rt: 20.20) 6 (Rt: 8.20) 7 (Rt: 7.71) WE + + EtOAcP + RWP + + WEtOHE + + + + CHCl3P + + RWEtOHP + + + +
1: Caffeic acid; 2:Luteolin; 3:Luteolin glucoside; 4: Schaftoside; 5: Scutellarein dimethylether; 6:Vitexin; 7: Vitexin rhamnoside; Rt: Retention times
Achillea species have ethnopharmacological importance for their usage in treatment of digestive problems, liver and gall-bladder conditions, menstrual irregularities, cramps, fever, wound healing. Internal usage for loss of appetite and dyspeptic ailments (gastric catarrh, spastic discomfort), externally usage in form of sitz bath or as a compress against skin inflammation, slow healing wounds, bacterial or fungal infections were approved by Commission E. In the last decades, pharmacological studies became intensive, although human clinical investigations are still rare. Recent findings have confirmed several traditional uses. The largest number of data accumulated for antioxidant and antiinflammatory effects. There are positive results on the analgesic, anti-ulcer, choleretic, hepatoprotective and wound healing activities. First results on other interesting therapeutical areas, antihypertensive, antidiabetic, antitumor, antispermatogenic activities, need confirmation. A. millefolium can be used also as an insect repellent. Contact dermatitis as adverse effect may be connected to sesquiterpenes. The diversity and complexity of the effective compounds of yarrow species explains the broad spectrum of their activity. According to the literature the pharmacological effects are mainly due to the essential oil, proazulenes and other sesquiterpene lactones, dicaffeoylquinic acids and flavonoids (11).
Achillea species have been reported to contain various compounds such as sesquiterpenes,
diterpenes, flavonoids, lignans, essential oil and triterpenes [12]. According to the literature survey steroids (β-sitosterol, β-sitosteryl-3-glucoside), sesquiterpenoids (8β-hydroxyachillin, schischkinin A, schischkinic acid methyl ester), flavonoids (salvigenin, artemetin, 6-hydroxyluteolin 6,7,3’,4’-trimethyl ether, 6-hydroxyluteolin 6,3’,4’-6,7,3’,4’-trimethyl ether) and coumarines (scopoletin, scoparon) have been isolated from A. schischkinii [11,12]. Our results have revealed that aerial parts of A.
schischkinii collected from Sivas-Turkey contain flavonoids such as luteolin, luteolin glucoside,
vitexin, vitexin rhamnoside, scutellarein dimethylether, schaftoside, and caffeic acid as phenolic acids. These compounds have been identified in A. schischkinii for the first time. Further studies on investigation of flavonoid contents of A. schischkinii are ongoing.
REFERENCES
1. Goli SAH, Rahimmalek M, Sayed Tabatabaei BE, Characteristics and fatty acid profile of yarrow (Achillea tenuifolia) seed oil, International Journal of Agricultural and Biological Engineering 10, 355– 357, 2008.
2. Rahimmalek M, Sayed Tabatabaei BE, Etemadi N, Goli SAH, Arzani A, Zeinali H, Essential oil variation among and within six Achillea species transferred from different ecological regions in Iran to the field conditions, Industrial Crops and Products 29, 348–355, 2009.
3. Gudaityte O, Venskutonis PR, Chemotypes of Achillea millefolium transferred from 14 different locations in Lithuania to the controlled environment, Biochemical Systematics and Ecology 35, 582-592, 2007.
4. Re49chinger KH, (Ed.) Flora Iranica, Wien, No. 158, p. 49-71, 1963. 5. Wichtl M. Herbal Drugs and Phytopharmaceuticals, Stuttgart, 1994.
6. Tuberoso C, Montoro P, Piacente S, Corona G, Deiana M, Dessi MA, Pizza C, Cabras P, Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All, Journal of Pharmaceutical and Biomedical Analysis 50, 440-448, 2009.
7. Benedek B, Koop B, Achillea millefolium L. s.l. revisited: recent findings confirm the traditional use, Wien. Medizinische Wochenschrift 157, 312–314, 2007.
8. Baytop T, Therapy with Medicinal Plants in Turkey: Today and in Past, Đstanbul, p. 176–177, 1999. 9. Davis PH, (Ed.) Flora of Turkey and the East Aegean Islands, Edinburgh, 5, p. 224–252, 1972.
10. Nemeth E, Bernath J, Biological Activities of Yarrow Species (Achillea spp.), Current Pharmaceutical Design 14, 3151-3167, 2008.
11. Ulubelen A, Öksüz S, Tuzlacı E, Flavonoids and Coumarins from Achillea schischkinii, Planta Medica 53, 507, 1989.
12. Ulubelen A, Öksüz S, Tuzlacı E, New Sesquiterpenoids from Achillea schischkinii, Planta Medica 54, 473, 1988.
Received: 16.02.2013