ORIGINAL ARTICLE
A Turn off-on Fluorescent Chemosensor for Sequential Determination
of Mercury and Biothiols
Şükriye Nihan Karuk Elmas1&Ibrahim Yilmaz1
Received: 2 August 2018 / Accepted: 15 October 2018 / Published online: 23 October 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018
Abstract
The selective and sensitive determination of biothiols in aqueous media has been great attention due to their important role in biological and pharmacological processes. We synthesized tryptophan functionalized perylene bisimide as a sensing chemosensor for mercury in aqueous solution. This complex between perylene dimide derivate (PDI/Trp) and mercury ions was evaluated and displayed to be turn on fluorescent chemosensor for the determination of biothiols in aqueous media. PDI/Trp showed fluorescence quenching in the presence of Hg2+and the fluorescence was recovered after addition of biological thiols (cysteine, homocysteine and glutathione). Therefore, PDI/Trp can be employed as a fluorescence probe for the sequencial recognization of Hg2+and biothiols in aqueous solution.
Keywords Perylene . Fluorescence . Molecular sensor . Mercury . Biothiols
Introduction
Researches based on the development of fluorescent sensors and their applications have been continued since the beginning of 1980. With the improvement of such sensors, fluorescent sensors have been used in a variety of fields from biochemical, biomedical and clinical studies to practical applications in en-vironmental pollution investigations [1]. The numbers of appli-cations and detectable species related to sensors are increasing day by day. Today, there are many fluorescent sensors in the market, and it is estimated that annual uses of the sensors for determination of anions, cations and neutral species in clinical analyzes and biological samples are about 1 million [2]. These sensors are considered to be the most effective molecular tools for visualization of target biomolecules in biological systems because of their unique properties such as simple, sensitive, practical, cheap, having very little process before analysis and high stability in living samples [3].
Mercury is one of the toxic heavy metals. Even low concen-tration of mercury, it has harmful effects on human health. Mercury compounds can cause allergic reactions or problems in brain and neurons [4]. The United States Environmental Protection Agency (EPA) has specified a maximum Hg2+ con-taminant level in drinking water at 0.002 mg.L−1(10 nmol.L−1), so measuring the trace of mercury is very important.
Biothiols such as cysteine, homocysteine and glutathione, play a key role in many cellular physiological processes. While high cysteine levels are associated with neurotoxicity [5], low levels of cysteine causes many diseases such as growth retardation, hair pigmentation, skin problems, liver damage, edema and goose weakness [6]. In addition, it is also known that increasing of homocysteine levels in blood plasma is a risk factor for cardiovascular diseases [7] and causes preg-nancy complications and increase the risk of osteoporosis [8]. Due to the important role of thiols in biological and phar-macological processes, studies on the development of methods for their determination has been increasing. For the analysis of thiols in biological samples, several techniques such as HPLC [9], electrochemical methods [10,11], mass spectroscopy [12], surface enhancement Raman spectroscopy [13], surface plasmon resonance spectroscopy [14], enzymatic methods [15], quantum dots [16], flow-injection analysis [10, 11], spectrophotometry [12] have been used. However, these classical methods are both very complex and expensive and also the processes are very time consuming [17]. Electronic supplementary material The online version of this article
(https://doi.org/10.1007/s10895-018-2320-6) contains supplementary material, which is available to authorized users.
* Ibrahim Yilmaz iyilmaz@kmu.edu.tr
1 Department of Chemistry, Kamil Ozdag Science Faculty,
Determination of compounds containing thiol groups, such as glutathione, cysteine and homocysteine in biological systems, especially in living cells and tissues, by methods that are sen-sitive and do not cause any complications will facilitate the diagnosis and treatment of many diseases. For this reason, the development of methods that can detect biological thiols ex-tremely important [18]. Among the reported techniques, the fluorescenceBturn-on^ method has great attention due to their high sensivity and applications for the biological imaging. Most fluoresence chemosensors were utilised for the detection of biothiol based on covalent interactions. In contrast, Bchemo-sensing ensemble^ organized non-covalent interac-tions during the determination of biological thiols. Thiophilic metal cations such as Hg2+or Cu2+are responsible for the formation of chemo-sensing ensemble platforms. Interactions between thiol groups and thiophilic metal cations can result in the dissociation of the ensemble.
Perylene diimide (PDI), the most widely used of perylene derivatives, is of interest due to its electron acceptor and donor properties. From a general point of view, perylene diimides have attracted much attention and found various area of usage be-cause of the advantage that they have high functional quantum yields [9,19], high molar absorption coefficient [20], high photo stability and thermal stability under visible light irradiation [21], high chemical stability [22,23]. Both symmetrical and asym-metric perylene diimide derivatives are extensively used in dye sensitized solar cells (29), organic light emitting diodes, liquid crystal displays, dye lasers [24], photocopiers [25], photovoltaic devices, charging of batteries, fluorescent paints, near IR paints, chemical oxidations, as photosensitizers in photodynamic ther-apy and pH sensitive chromogenic chemical sensors [26].
In this study, we report a tryptophan conjugated perylene diimide, which could sequentially detect Hg2+and biothiols. The chemosensor (PDI/Trp) recognize Hg2+by the fluores-cence quenching response. Sequential determination of mer-cury and biothiols by the PDI/Trp is reversible and repeatable over multiple cycles and the chemosensor (PDI/Trp) can be used repeatedly many times. In this context, a perylene diimide derivative with functional groups used in the thiol determinations, details were given in the experimental section, was synthesized and characterized. Biothiols responses of the probe was investigated and their characteristics such as pH effect, limit of detection, working range, response time, quan-tum yield, solvent effect, kinetic properties and selectivity against other ions and amino acids were determined.
Experimental
General
All chemical reagents and solvents were provided from Merck, Sigma–Aldrich, WVR, Alfa Aesar and utilised
without further purification. Ultrapure water was obtain-ed using a Milli-Q system (Millipore, Bobtain-edford, MA, USA). The pH of the solutions were adjusted by HEPES buffers (10 mM).
FT-IR and NMR spectra were recorded on a Perkin Elmer spectrum one FT-IR and a Varien Agilent 600-MHz nuclear magnetic resonance (NMR) spectrometer. Mass spectra was obtained with a Bruker microflex LT MALDI-TOF MS. The DMSO-d6 and the D2O were utilised as a deuterated solvent in NMR experiments. Fluorescence measurements were per-formed at room temperature at using on a Agilent Cary Eclipse Fluorescence Spectrophotometer.
Synthesis ofPDI/Trp
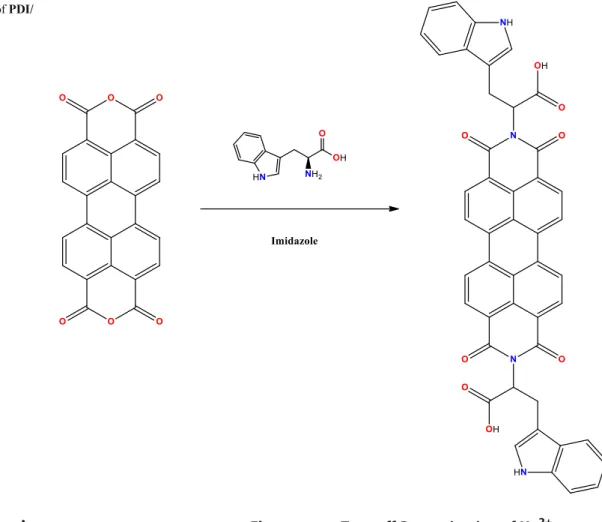
Tryptophan containing perylene bisanhydride derivative (PDI/Trp) was synthesized according to method in literature [27]. 3,4,9,10-perylenetetracarboxylic acid bisanhydride (250 mg, 0.64 mmol), tryptophan (391.1 mg, 1.915 mmol) was stirred in imidazole (1 g) heated at 120 °C for 24 h. The reaction mixture was cooled to 90 °C than water was added to the solution and filterated. Then, 2 M HCl was poured into the solution, the formed precipitate was filtrated and washed with excess distilled water than dried at 100 °C obtained to the PDI/Trp The procedure is shown in Scheme 1.1H NMR (600 MHz, DMSO-d6):δ = 7.81 (4H), 7.64 (4H), 7.16 (4H), 6.92 (2H), 6.03 (2H), 3.62 (4H).13C NMR (DMSO-d6):δ = 171.5, 162.5, 136.4, 133.3, 130, 128.2, 127.8, 124.2, 122.0, 121.4, 118.9, 111.8, 110.7, 54.0. IR (cm−1): 3382, 3235, 1726, 1691, 1651, 1590, 1572, 1451, 1433. MS–ESI (m/z) calcd for C46H28N4O8[M–H+] − 765.641.
Spectral Studies
The stock solution of PDI/Trp (10 mmol) was dissolved in DMSO. For the final concentration of PDI/Trp (50μM), the stock solution was diluted with a buffer (pH = 7.4, 0,01 M HEPES). The volume of PDI/Trp solutions were 3.0 mL in the fluorescence experiments (λex= 312 nm,λem= 445 nm). The fluorescence measurements of PDI/Trp were recorded in the presence of various amounts of cations and amino acids.
1
H NMR Titrations
For the NMR titration experiment, the chemosensor of PDI/ Trp dissolved in DMSO-d6 then gradual concentrations of Hg(NO3)2.H2O dissolved in D2O solutions were added to the solution of PDI/Trp and shaked for a minute. 1H NMR spectra of PDI/Trp complexes were recorded at room temperature.
Results and Discussion
General
The chemosensor of PDI/Trp was synthesized according to previous literature and characterized by NMR, FT-IR and mass spectra (Fig. S1-S4).
Fluorescence Turn-off Determination of Hg
2+The spectral response of PDI/Trp was evaluated by fluores-cence spectroscopy (λex= 312 nm,λem= 455 nm). Initially, PDI/Trp (50 μM) in HEPES:DMSO (95:5, v/v) was evaluat-ed toward 2 equiv. of metal ions (Ag+, K+, Fe3+, Fe2+Co2+, Zn2+, Cd2+, Pb2+, Ca2+, Cr3+, Mg2+, Cu2+, Mn2+, Hg2+, Ba2+ Scheme 1 The synthesis of PDI/
Trp
Fig. 1 Fluorescence study of PDI/Trp solution (50 μM) in presence of various metal cations (2 eq.)
and Al3+). It is shown, PDI/Trp displays the fluorescence quenching of emission band at 445 nm in the presence of Hg2+(Fig. 1). Except of two metals (Hg2+ and Cu2+), the PDI/Trp displays no significant changes of the fluorescence behaviour in the presence of other metal ions. Such a fluores-cence quenching response of PDI/Trp was probably carried out to the strong interaction between Hg2+ions and amino acids moeities in PDI/Trp as a result of charge transfer pro-cess of the variation of electronic structure of PDI/Trp [28].
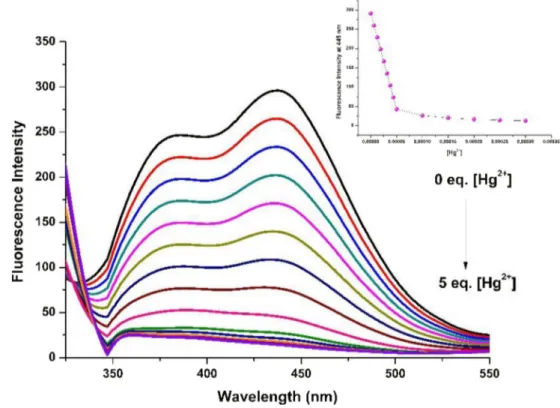
The fluorescence sensing behaviour of PDI/Trp with Hg2+ was investigated using titration experiments (Fig.2). The fluo-rescence intensity of PDI/Trp at 445 nm gradually decreased and quenched by the addition of 2 equivalent of Hg2+ions. The binding constant of PDI/Trp with Hg2+was determined 8,2 × 103M−1according to Benesi Hildebrand equation (Fig. S5). The detection limit (LOD) of PDI/Trp for the determi-nation of Hg2+was calculated from a plot of the concentration of mercury ions versus fluorescence intensity at 445 nm (Fig. Fig. 2 Fluorescence titration
spectra of PDI/Trp (50μM) with the various amount of Hg2+ion
Fig. 3 Competition studies of PDI/Trp with Hg2+
in the presence of various metal ions at 455 nm in HEPES/DMSO (95:5, v/v)
S6). The detection limit of PDI/Trp for Hg2+was 6 nm based on equation LOD = 3σ/s which calculated from linear regres-sion curve.
The binding stoichiometry between PDI/Trp and Hg2+ was investigated through Job’s method and shown in fig. S7. The molar fraction of Hg2+was plotted the emission intensity at 445 nm of PDI/Trp. Maximum value of the graph was found to be 0.66, indicating that 1:2 stoichiometry.
For more information of the binding mechanism between PDI/Trp and Hg2+
, the FT-IR studies (Fig. S8) were per-formed to prove the interaction of Hg2+with imidazole nitro-gen of PDI/Trp. The bands of PDI/Trp at 3382 and 3235 cm −1indicate to the phenolic -OH and -NH groups vibrations. In the IR spectra of PDI/Trp-Hg, these two bands disappear that indicate the coordination of -NH and -OH with Hg2+[29]. The band at 1587 cm−1 that is indicated to the imidazole NH
bonding vibration of PDI/Trp has shifted to lower frequency (1590 cm−1) as a result of complexation [30]. There is no significant changes at wavenumbers of the bands at 1724, 1689 and 1651 cm−1in the complex which shows that carbon-yl groups of PDI/Trp doesn’t involved in complexation. In addition,1H NMR titration studies were performed. Fig. S9 displays the spectral changes of PDI/Trp upon addition of 0.0–2.0 eq. of Hg2+
. Upon addition of Hg2+ions to the PDI/ Trp solution, the phenolic -OH signal at δ 10.57 ppm disap-peared because of binding of Hg2+ions to hydroxyl group of PDI/Trp. The -NH signal of PDI/Trp could not be detected in NMR spectra. PDI/Trp as a selective mercury sensor, the competition experiments were evaluated by measurements of the emission spectra of PDI/Trp (1 eq.) with Hg2+(2 eq.) in the presence of other metal cations (2 eq.). The sensor PDI/ Trp was selected Hg2+
and displayed fluorescence turn-off responses in the presence of other metal ions (Fig.3).
Fluorescence Turn-on Sensing of Biothiols
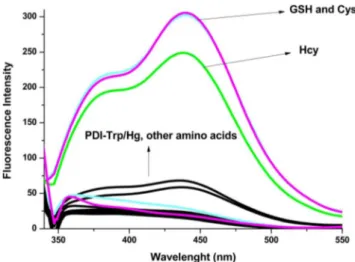
To investigate the selectivity of PDI/Trp-Hg towards various amino acids (Ala, Cys, Glu, Gly, Hcy, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Trp, Val, Thr, Asp and GSH) were performed. Among all amino acids, Cys, Hcy and GSH resulted in en-hancement of fluorescence intensity of the chemosensor. They could caused to recovered the intensity of fluorescence emis-sion of PDI/Trp (Fig. 4). Instead of biothiols (Cys, Hcy, GSH), other amino acids weren’t exerted any enhancement of fluorescence intensity of PDI/Trp-Hg (Fig.5). Upon addi-tion thiol species to the soluaddi-tion of PDI/Trp-Hg, the emission intensity of sensor restored at initial level as a results of the dissociation of PDI/Trp-Hg). The reason for this Hg-S Fig. 4 Fluorescence spectra of PDI/Trp-Hg (50μM) with the addition of
amino acids
Fig. 5 Fluorescence changes ratio (I/Io) of PDI/Trp-Hg2+(50μM)
interaction is much stronger than Hg-N interaction [31,32]. Also, the reappearance of the characteristic emission peak at 455 nm shows us, there is no chemical interaction between PDI/Trp and thiol containing amino acids.
To evaluate the sensing behaviours of thiol containing ami-no acids by PDI/Trp-Hg, titration experiments were conduct-ed. Emission spectra of PDI/Trp-Hg sensor gradually in-creased with the enhancement of the concentration of GSH, Hcy and Cys (Fig.6, S10). The notable enhancement of the emission intensity at 445 nm can also been seen by the naked
eye under UV irridation. Recovery of the characteristic emis-sion peak at 445 nm of PDI/Trp proved that there are no chemical interactions between PDI/Trp-Hg and GSH, Hcy, Cys.
The binding constants for PDI/Trp-Hg with biothiols at pH 7.4 were calculated from the Benesi-Hildebrand equation (Fig. S11, 7.2 × 103M−1for Cys, 2.6 × 103M−1for Hcy and 4.1 × 103M−1for GSH). Based on titration studies, detection limits of PDI/Trp-Hg for thiol containing amino acids were calculated to be 1.5 nM for Cys, 0.83 nM for Hcy and 1.1 nM for GSH on basis of 3 s/K (Fig. S12). It is important to detect abnormal level of the biological thiols (Cys, Hcy, GSH) for the early detection of cancer cells. The obtained result shows that the PDI/Trp-Hg has the potential applications for the diagnose of some types of diseases.
The response time of sensor studies are also very crucial for the practical application or real time monitoring. Therefore, the response time for the interactions between PDI/Trp-Hg and thiol containing amino acids were investigated (Fig. S13). Fluorescence intensities of PDI/Trp-Hg reached stable values within 30 s following the addition of 2.0 eq. thiol-containing amino acids to the solutions.
As a results, the chemosensor PDI/Trp can respond revers-ible and allow for the identification of various amounts of biological thiols. We carried out the reversible experiments of PDI/Trp by the alternate addition of Hg2+ and thiol-containing amino acids (Fig. 7, fig. S14). This reversibility could be studied by the addition of Hg2+to Cys, Hcy, GSH Fig. 6 Fluorescence titration spectra of PDI/Trp (50μM) with various
amount of (a)Cys (0.0–4.0 equiv.) in DMSO/HEPES (v/v, 5/95)
Fig. 7 The reversibility of PDI/ Trp with Cys and Hg2+
containing PDI/Trp-Hg solutions. When thiol containing amino acids were added to the solutions again, it was observed that the emission intensity of solutions at 445 nm were in-creased. This reversible studies could be repeated several times with good recovery. The reversibility of PDI/Trp is remarkable behaviour for practical applications.
Conclusion
In summary, we have developed a turn off-on fluorescence sensor for sequential detection of Hg2+and biological thiols at physiological pH. As a result of the coordination of the PDI/Trp probe with Hg2+
, fluorescence quenching has oc-curred. The complex quenched of fluorescence with Hg2+ was dissociated due to strong interaction of sulfur with mer-cury in the presence of biological thiols and the characteristic fluorescence emission of PDI/Trp was recovered. In addition, the sensor has been tested for reproducibility against Hg2+and biothiols, and the results show that the sensor works very successfully reused. This developed fluorescence sensor has been promising in practical applications, since it can easily detect biological thiols in mixed matrices containing other amino acids.
Acknowledgments The manuscript is part of the Ph.D. thesis ofŞükriye Nihan KARUK ELMAS.
References
1. Bakker E, Bühlmann P, Pretsch E (1997) Carrier-based ion-selec-tive electrodes and bulk Optodes. 1. General characteristics. Chem Rev 97:3083–3132
2. Oehme I, Wolfbeis OS (1997) Optical sensors for determination of heavy metal ions. Mikrochim Acta 126:177–192.https://doi.org/ 10.1007/BF01242319
3. Liu Y, Lv X, Liu J, Sun YQ, Guo W (2015) Construction of a selective fluorescent probe for GSH based on a Chloro-functionalized Coumarin-enone dye platform. Chem - A Eur J n/a-n/a 21:4747–4754.https://doi.org/10.1002/chem.201406004 4. Firooz AR, Ensafi AA, Karimi K, Khalifeh R (2013) Specific
sens-ing of mercury(II) ions by an optical sensor based on a recently synthesized ionophore. Sensors Actuators B Chem 185:84–90. https://doi.org/10.1016/j.snb.2013.04.108
5. Wang XF, Cynader MS (2001) Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci 21:3322–3331
6. Shahrokhian S (2001) Lead Phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal Chem 73:5972– 5978.https://doi.org/10.1021/ac010541m
7. As PH, Factor R, Dementiaalzheimer FOR (2002) Plasma Homocysteine As a Risk Factor for Dementia and Alzheimer’ S Disease 346:476–483
8. Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM (2004) Facts and recommendations about total homocysteine
determinations: an expert opinion. Clin Chem 50:3–32.https:// doi.org/10.1373/clinchem.2003.021634
9. Dubey RK, Efimov A, Lemmetyinen H (2011) 1,7-and 1,6-regioisomers of diphenoxy and dipyrrolidinyl substituted perylene diimides: synthesis, separation, characterization, and comparison of electrochemical and optical properties. Chem Mater 23:778–788. https://doi.org/10.1021/cm1018647
10. Miao P, Liu L, Nie Y, Li G (2009) An electrochemical sensing strategy for ultrasensitive detection of glutathione by using two gold electrodes and two complementary oligonucleotides. Biosens Bioelectron 24:3347–3351.https://doi.org/10.1016/j.bios.2009.04. 041
11. Pacsial-Ong EJ, McCariey RL, Wang W, Strongin RM (2006) Electrochemical detection of glutathione using redox indicators. Anal Chem 78:7577–7581.https://doi.org/10.1021/ac061451q 12. Huang Y-F, Chang H-T (2007) Analysis of adenosine triphosphate
and glutathione through gold nanoparticles assisted laser desorption/ionization mass spectrometry. Anal Chem 79:4852– 4859.https://doi.org/10.1021/ac070023x
13. Huang GG, Hossain MK, Han XX, Ozaki Y (2009) A novel re-versed reporting agent method for surface-enhanced Raman scat-tering; highly sensitive detection of glutathione in aqueous solu-tions. Analyst 134:2468–2474.https://doi.org/10.1039/b914976g 14. Wu C, Xu QH (2009) Stable and functionable mesoporous
silica-coated gold nanorods as sensitive localized surface plasmon reso-nance (LSPR) nanosensors. Langmuir 25:9441–9446.https://doi. org/10.1021/la900646n
15. Timur S, Odaci D, Dincer A, Zihnioglu F, Telefoncu A (2008) Biosensing approach for glutathione detection using glutathione reductase and sulfhydryl oxidase bienzymatic system. Talanta 74: 1492–1497.https://doi.org/10.1016/j.talanta.2007.09.026 16. Zhang Y, Li Y, Yan XP (2009) Photoactivated CdTe/CdSe quantum
dots as a near infrared fluorescent probe for detecting biothiols in biological fluids. Anal Chem 81:5001–5007.https://doi.org/10. 1021/ac900394e
17. Wei M, Yin P, Shen Y, Zhang L, Deng J, Xue S, Li H, Guo B, Zhang Y, Yao S (2013) A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells. Chem Commun (Camb) 49:4640–4642. https://doi.org/10.1039/ c3cc39045d
18. Jiang X, Yu Y, Chen J, Zhao M, Chen H, Song X, Matzuk AJ, Carroll SL, Tan X, Sizovs A, Cheng N, Wang MC, Wang J (2015) Quantitative imaging of glutathione in live cells using a reversible reaction-based Ratiometric fluorescent probe. ACS Chem Biol 10:864–874.https://doi.org/10.1021/cb500986w 19. Dinalp H, Akar Z, Zafer C, Li S (2011) Effect of side chain
substit-uents on the electron injection abilities of unsymmetrical perylene diimide dyes. Dyes Pigments 91:182–191.https://doi.org/10.1016/ j.dyepig.2011.03.022
20. Refiker H, Icil H (2011) Amphiphilic and chiral unsymmetrical perylene dye for solid-state dye-sensitized solar cells. Turkish J Chem 35:847–859.https://doi.org/10.3906/kim-1107-39 21. Langhals H (2004) Color chemistry. Synthesis, properties and
ap-plications of organic dyes and pigments. 3rd revised edition. By Heinrich Zollinger. Angew Chemie Int Ed 43:5291–5292.https:// doi.org/10.1002/anie.200385122
22. Würthner F (2004) Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem Commun (Camb):1564–1579.https://doi.org/10.1039/b401630k 23. Huang L, Zhu F, Liu C, Wang H, Geng Y, Yan D (2010)
Heteroepitaxy growth high performance films of perylene diimide derivatives. Org Electron physics, Mater Appl 11:195–201.https:// doi.org/10.1016/j.orgel.2009.10.014
24. Jin Y, Hua J, Wu W, Ma X, Meng F (2008) Synthesis, characteri-zation and photovoltaic properties of two novel near-infrared ab-sorbing perylene dyes containing benzo[e]indole for dye-sensitized
solar cells. Synth Met 158:64–71. https://doi.org/10.1016/j. synthmet.2007.12.005
25. Sapagovas VJ, Gaidelis V, Kovalevskij V, Undzenas a. (2006) 3,4, 9,10-Perylenetetracarboxylic acid derivatives and their photophysical properties. Dyes Pigments 71:178–187 . doi: https://doi.org/10.1016/j.dyepig.2005.06.012
26. Sukul PK, Santra DC, Singh PK, Maji SK, Malik S (2015) Water soluble perylene bisimide and its turn off/on fluorescence are used to detect cysteine and homocysteine. New J Chem 39:5084–5087. https://doi.org/10.1039/C5NJ00608B
27. Farooqi MJ, Penick MA, Burch J, Negrete GR, Brancaleon L (2016) Characterization of novel perylene diimides containing aro-matic amino acid side chains. Spectrochim Acta Part AMolecular Biomol Spectrosc 153:124–131. https://doi.org/10.1016/j.saa. 2015.08.013
28. Oliveira E, Costa SPG, Raposo MMM, Faza ON, Lodeiro C (2011) Inorganica Chimica Acta synthesis , characterization , fluorescence and computational studies of new cu 2 + , Ni 2 + and hg 2 + complexes with emissive thienylbenzoxazolyl-alanine ligands.
Inorganica Chim Acta 366:154–160.https://doi.org/10.1016/j.ica. 2010.10.025
29. Cukurovali A, Kirbag S (2006) Spectroscopic characterization and biological activity of salicylaldehyde thiazolyl hydrazone ligands and their metal complexes. Transit Met Chem 31:207–213.https:// doi.org/10.1007/s11243-005-6353-8
30. Ranjbar M, Malakooti E, Sheshmani S (2013) Synthesis and char-acterization of mercury (II) complexes containing 2,9-dimethyl-1, 10-phenanthroline by sonochemical method. J Chem 2013:1–6. https://doi.org/10.1155/2013/560983
31. Ruan Y-B, Li A-F, Zhao J-S, Shen JS, Jiang YB (2010) Specific Hg2+−mediated perylene bisimide aggregation for highly sensitive detection of cysteine. Chem Commun 46:4938–4940.https://doi. org/10.1039/c0cc00630k
32. Sukul PK, Santra DC, Singh PK, Maji SK, Malik S (2015) Water soluble perylene bisimide and its turn off/on fluorescence are used to detect cysteine and homocysteine. New J Chem 39:5084–5087. https://doi.org/10.1039/C5NJ00608B