Association of total serum antioxidant capacity with
the Tei index in echocardiography in patients with
microvascular angina
Alparslan Kilic
a, Mikail Yarlioglues
a, Ebru Akgul Ercan
b, Mustafa Duran
a,
Murat Ugurlu
b, Fatih Oksuz
a, Sedat Ozdemir
c, Alparslan Kurtul
a,
Muhammed Karadeniz
a, Sani Namik Murat
a, Sule Korkmaz
band
Selda Demirtas
cObjectives Cardiac syndrome X (CSX) is a condition characterized by exercise-induced chest pain that occurs considering a normal coronary angiogram. We aimed to investigate the total serum antioxidant capacity (TAC) and biventricular global functions using echocardiography in patients with CSX.
Patients and methods The study population included 55 patients with typical anginal symptoms and a positive exercise stress test, or ischemia in myocardial perfusion scintigraphy and normal coronary arteries detected angiographically, and 49 healthy volunteers with atypical chest pain and a negative stress test. TAC was assessed from blood samples. Transthoracic echocardiography was performed for the entire study population. The Tei index was calculated using the formula IVCT+ IVRT/ET.
Results TAC was found to be significantly lower in the CSX group compared with the control group (0.70 ± 0.37 vs. 1.5 ± 0.30, respectively,P < 0.001). The Tei index was significantly higher in patients with CSX than the control group (0.60 ± 0.18 vs. 0.42 ± 0.12, respectively,P < 0.001). There was a significant and inverse relationship between TAC and the Tei index (r = − 0.41, P < 0.001). When we divided the study population according to the normal range
of TAC into the decreased TAC group (< 1.30 mmol/l), the normal TAC group (1.30–1.77 mmol/l), and the increased TAC group (>1.77 mmol/l), it was found that the Tei index was higher in the decreased TAC group compared with the other groups (0.66 ± 0.18 vs. 0.49 ± 0.10 and
0.46 ± 0.13 mmol/l,P < 0.001, respectively). Conclusion Our study suggested that TAC was significantly decreased in CSX patients and decreased antioxidant levels were related to impaired Tei index in echocardiography in patients with microvascular angina. Coron Artery Dis 26:620–625 Copyright © 2015 Wolters Kluwer Health, Inc. All rights reserved.
Coronary Artery Disease2015, 26:620–625
Keywords: cardiac syndrome X, Tei index, total antioxidant capacity
aDepartment of Cardiology, Ankara Education and Research Hospital, bDepartment of Cardiology andcDepartment of Biochemistry, Ufuk University
School of Medicine, Ankara, Turkey
Correspondence to Alparslan Kilic, MD, Department of Cardiology, Ankara Education and Research Hospital, Ankara 06600, Turkey
Tel: + 90 554 569 8556; fax: + 90 312 363 3396; e-mail: dr-alp@hotmail.com
Received15 April 2015 Revised 3 July 2015 Accepted 13 July 2015
Introduction
Cardiac syndrome X (CSX), also called microvascular angina, is defined as patients with angina-like chest pain with detectable ischemia in various tests [such as exercise stress test, myocardial perfusion scintigraphy (MPS), or in SPECT] whose coronary arteries are detected to be normal. The pathophysiology of CSX is not clearly elu-cidated, but multiple parameters may impact micro-vascular angina and many other mechanisms exist to assess risk [1]. Coronary microvascular dysfunction, sys-temic inflammation, and arteriosclerosis of the small coronary vessel are possibly the principal causes of CSX [2,3]. These may be associated with an increased proin-flammatory cytokine, C-reactive protein (CRP), endothelin-1, oxidative stress, and decreased endogen-ous NO [2–9]. Free radicals, formed by oxidative stress,
react with phospholipids in cell membranes, and play an important role in endothelial dysfunction.
Total serum antioxidant capacity (TAC) is an oxidative stress marker, and decreased TAC reflects increased oxidative stress [10]. These data suggest that oxidant and antioxidant systems could play a role in the pathophy-siology of CSX. An index of myocardial performance (the Tei index), which can be measured by echocardiography, reflects the LV systolic and diastolic functions and is associated with survival in ischemic and nonischemic cardiac disease [11]. An increase in the Tei index is directly proportional to ventricular dysfunction.
In the present study, we aimed to investigate the TAC and biventricular global functions using echocardio-graphy in patients with CSX.
Methods
Patients
The study population included 55 patients (patient group) with typical anginal symptoms (41 patients with effort angina, three patients with rest angina, and 11 patients with mix angina) and a positive exercise stress test (42 patients) or ischemia in MPS (13 patients) and normal coronary arteries (completely smooth without any coronary lesion including plaque formation in all patients) detected angiographically, and 49 healthy volunteers (control group) with atypical chest pain and a negative stress test between January 2013 and August 2013. The ethics committee of Ufuk University confirmed the study protocol and each participant provided written informed consent.
All patients underwent clinical and risk factor assess-ments. Baseline 12-lead ECG, M-mode and two-dimensional echocardiography, and provocative testing for coronary artery spasm with hyperventilation or ergo-novine were recorded.
Exclusion criteria
Participants with any of the following characteristic were excluded: uncontrolled hypertension (>160/100 mmHg), atrial fibrillation or left bundle branch block on ECG, valvular or congenital heart disease, infectious or inflammatory diseases, idiopathic hypertrophic and dila-ted cardiomyopathy, organic coronary artery disease, any regional wall motion abnormalities on resting echo-cardiogram, ejection fraction less than 50%, severe per-ipheral vascular disease, previous myocardial infarction, systemic disorders, and liver or renal insufficiency. In addition, we excluded patients who were receiving therapies including statin therapy and/or vitamin sup-plements, which may affect TAC levels.
The patient group and the control group were hospita-lized and coronary angiograms were performed in the patient group. Approximately 1 week before coronary angiography, transthoracic echocardiography was per-formed to calculate the Tei index and the exercise tol-erance test (ETT) was performed. TAC was measured for each patient.
Coronary angiography
Selective coronary angiography was performed using the femoral approach with standard catheters from conven-tional views. Nitroglycerin, adenosine, and calcium channel blocker were withheld for at least 1 week before the angiography. During coronary angiography, all patients underwent a hyperventilation test (which was performed by asking the patients to breathe quickly and deeply for 5 min) or an ergonovine test (a positive response to ergonovine spasm provocation testing is defined as transient occlusion more than 90% narrowing of a coronary artery with signs and symptoms of myo-cardial ischemia including angina/ST-segment changes
[12]) to exclude the potential of coronary artery vasos-pasm. All of the angiographic images were evaluated by two experienced cardiologists without knowledge of the ETT results.
Treadmill exercise stress testing
All participants underwent a symptom-limited ETT according to the Bruce protocol using a stress test appli-ance (TM-Pro 2000; Tepa, Ankara, Turkey). Before testing, all patients were instructed not to eat, drink, or smoke for 3 h before the test. All antianginal medications (β-blocker agents, nitrate, and calcium channel blockers) were discontinued at least 72 h before the study. The heart rate, blood pressure, and 12-lead ECG were recor-ded at baseline at a 3-min interval during and after the exercise, as well as at the onset of chest pain and/or sig-nificant ST-segment depression. In patients whose baseline 12-lead ECGs showed no ST-segment abnormalities, a horizontal or a downsloping ST-segment depression of more than 1 mm or an upsloping ST-segment depression of more than 2 mm 80 ms after the J point on the Stress ECG was defined to be positive for myocardial ischemia.
Myocardial perfusion scintigraphy
Imaging was performed using the thallium-201 stress redistribution protocol. Patients received ∼ 3.0–3.3 mCi of thallium-201 intravenously at peak stress. Exercise was continued for 60–90 s after radioisotope injection. Then, gated stress single photon emission computed tomo-graphy imaging began within 10 min after the time of injection. Finally, all patients underwent 3–4 h imaging for redistribution studies. All scans were collected in the gated mode, which enabled interpretation of wall motion and ejection fraction.
Doppler echocardiography
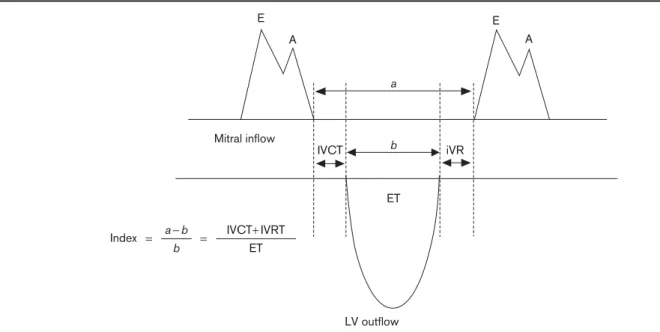
Doppler echocardiography was performed in all patients in the left lateral position using a commercially available echocardiograph (Vivid 7; GE Medical Systems Inc., Horten, Norway) with a 2.5-MHz ultrasound transducer. Left ventricular end-diastolic and end-systolic dimensions and wall thickness (ram) were obtained from parasternal long axis views, and the percent fractional shortening contraction and ejection fraction of the left ventricle (%) were subsequently calculated. All patients were assessed for global, regional left ventricular function, and diastolic function. All measurements were performed using the commercially available software program. Left and right ventricular diastolic function was assessed by pulsed wave Doppler of the transmitral flow. The sample volume was placed at the tips of the mitral leaflets and peak early (E) and atrial (A) velocities, the isovolumic contraction time (IVCT), the isovolumic relaxation time (IVRT), and the ejection time (ET) were measured. The E/A velocity ratio was calculated. The Tei index was calculated using the formula IVCT+ IVRT/ET (Fig. 1). The Tei index is
associated with survival in ischemic and nonischemic disease. A myocardial performance (the Tei index) value below 0.40 is considered normal. An increase in this value is directly proportional to ventricular dysfunction. The early and late diastolic annular velocities were measured at the lateral corner of the mitral annulus and tricuspid annulus by pulsed TDI.
Measurement of total serum antioxidant capacity TAC was determined using a novel automated measure-ment method, developed by Erel [13]. In this technique, the hydroxyl radical, which is the most potent biological radical, is produced. In the test, ferrous ion solution, present in Reagent 1, is mixed with hydrogen peroxide, which is present in Reagent 2. Sequentially produced radicals such as the brown-colored dianisidinyl radical cation, produced by the hydroxyl radical, are also potent radicals. Using this technique, the antioxidative effect of the sample against the potent free radical reactions, which is initiated by the produced hydroxyl radical, is measured. TAC levels were measured using the SYNCHRON LX System (Beckman Coulter, Fullerton, California, USA). The results are expressed as mmol Trolox equiv./l and 1.30–1.77 mmol/l is considered normal.
Statistical analysis
Statistical analysis was carried out using the SPSS 18.0 Statistical Package Program for Windows (SPSS Inc., Chicago, Illinois, USA). Quantitative variables were expressed as mean± SD or median and interquartile range. Continuous variables were analyzed for normal distribution using the Kolmogorov–Smirnov test and analyzed for
homogeneity using Levene tests. Comparisons of para-metric values between the two groups were performed using independent-samples Student’s t-tests. Comparisons of nonparametric values between the two groups were performed using Mann–Whitney U-tests. Comparisons of parametric values among three groups were performed using one-way analysis of variance. Categorical variables were compared using theχ2-test. The Pearson correlation test was performed. A two-tailed P less than 0.05 was considered statistically significant.
Results
The mean age and the number of male patients were similar in patients with CSX and the control group (52± 9 vs. 46± 10 years, P = 0.079 and 16 vs. 19, P = 0.262, respectively). The baseline demographic characteristics and classical risk factors of the two groups are summar-ized in Table 1. The two groups were similar with respect to the prevalence of diabetes mellitus, arterial hyper-tension, history of smoking, family history, age, sex, serum lipid level, CRP, and ESR.
As shown in Table 2, TAC was found to be significantly lower in the CSX group compared with the control group (0.70± 0.37 vs. 1.5 ± 0.30 mmol/l, respectively, P < 0.001). The Tei index was significantly higher in patients with CSX than the control group (0.60± 0.18 vs. 0.42 ± 0.12, respectively,P < 0.001). As shown in Fig. 2, there was a significant moderate and inverse relationship between TAC and the Tei index (r = − 0.41, P < 0.001). When we divided the study population according to the normal range of TAC into the decreased TAC group (< 1.30 mmol/l), the
Fig. 1 E A E A a b ET ET a− b Index b = = iVR IVCT IVCT+ IVRT Mitral inflow LV outflow
Schematic representation of the measurement of the Tei index. (a) Time interval from the end to the start of transmitral flow. (b) Left ventricular (LV) ejection time (ET). IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time.
normal TAC group (1.30–1.77 mmol/l), and the increased TAC group (>1.77 mmol/l), it was found that the Tei index was higher in the decreased TAC group compared with the other groups (0.66± 0.18 vs. 0.49 ± 0.10 and 0.46± 0.13 mmol/l, P < 0.001, respectively) (Fig. 3). The baseline echocardiographic measurements, conven-tional Doppler, and tissue Doppler findings are
presented in Table 3. In our study, the two groups were similar with respect to EF, mitral E velocity, mitral A velocity, mitral E/A, deceleration time, septal S′, right ventricule S, and right ventricule E, septal E′ and right ventricule E′/A′ were significantly lower in the CSX patients than in the control group. IVRT, septal E/E′, and right ventricule A′ in the patient group showed a statistically significantly higher degree compared with the control group.
Discussion
The main findings of this study were as follows: (a) TAC was significantly decreased in CSX patients, (b) this is the first study indicating that the Tei index was sig-nificantly higher in patients with CSX compared with the control group, and (c) there was a significant and inverse relationship between TAC and the Tei index.
Table 1 Comparison of the demographic and clinical characteristics of groups
Control Syndrome X P value Age (years) 46.50± 10.314 52.00± 9.593 0.08 Male [n (%)] 19 (38.77) 16 (29.09) 0.26 BMI (kg/m2) 29.43± 5.0 27.85± 3.8 0.74 Diabetes mellitus [n (%)] 10 (20.8) 12 (21.8) 0.90 Hypertension [n (%)] 20 (41.7) 28 (58.3) 0.34 Smoking [n (%)] 18 (37.5) 26 (47.3) 0.31 Family history of CAD [n (%)] 21 (38.2) 25 (52.1) 0.15 Fasting glucose (mg/dl) 101.9± 24.3 111.7± 41.2 0.16 Creatinine (mg/dl) 0.77± 0.17 0.74± 0.15 0.33 Hemoglobin (g/l) 13.6± 1.4 14.0± 1.6 0.27 Platelet count (×103/μl) 254.41± 62.07 249.29± 65.95 0.66 Total cholesterol (mg/dl) 202.81± 29.78 195.30± 47.02 0.34 LDL (mg/dl) 126.69± 26.71 115.90± 40.93 0.32 TG (mg/dl) 159.22± 74.27 169.44± 116.98 0.57 HDL (mg/dl) 44.63± 8.94 43.05± 8.66 0.35 CRP (mg/dl) 3.58± 2.88 3.04± 3.20 0.16 Data are mean± SD.
CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipo-protein; TG, triglycerides.
Table 2 Comparison of the Tei index and total serum antioxidant capacity levels of groups
Control Syndrome X P value Tei index 0.47± 0.13 0.60± 0.18 < 0.001 TAC (mmol/l) 1.57± 0.31 0.70± 0.37 < 0.001 TAC, total antioxidant capacity.
Fig. 2 P< 0.001 0.66± 0.18 1.20 0.80 1.00 0.60 0.40 0.20 Decreased TAC group Tei index Normal TAC group Increased TAC group 0.49± 0.10 0.46± 0.13
Correlation of total antioxidant capacity (TAC) with the Tei index.
Fig. 3 r= −0.41 P< 0.001 1.20 1.00 1.00 TAC (mmol/l) Tei index 1.50 2.00 2.50 3.00 0.80 0.60 0.40 0.20 0.00 0.50
Comparison of the Tei index among total antioxidant capacity (TAC) groups.
Table 3 Comparison of the echocardiographic findings between groups
Control Syndrome X P value EF (%) 61.50± 4.00 63.00± 6.50 0.184 Mitral E velocity (cm/s) 69.79± 10.11 72.76± 16.891 0.290 Mitral A velocity (cm/s) 69.08± 13.60 74.38± 24.01 0.180 Mitral E/A 1.04± 0.23 0.90± 0.47 0.443 DT (ms) 174.75± 35.26 167.01± 41.08 0.312 IVRT (ms) 75.50± 14.99 110.00± 18.16 < 0.001 Septal S′ (cm/s) 7.00± 0.89 7.00± 2.22 0.595 Septal E′ (cm/s) 10.00± 2.33 7.00± 2.51 < 0.001 Septal E/E′ (cm/s) 7.0± 1.91 9.1± 3.47 < 0.001 RV S′ 12.00± 2.7 12.00± 3.1 0.349 RV E′ 11.50± 2.8 11.00± 1.15 0.921 RV A′ 13.00± 3.62 17.00± 4.56 < 0.001 RV E′/A′ 0.94± 0.29 0.65± 0.21 0.003 TAPSE (cm) 2.15± 0.35 2.33±0.39 0.022 DT, duration time; IVRT, isovolumetric relaxation time; RV, right ventricule; TAPSE, tricuspid annular plane systolic excursion.
The term syndrome X or microvascular angina was coined by Kemp [14] 41 years ago. CSX refers to patients with angina-like symptoms, abnormalities on stress test-ing performed with or without perfusion studies, and normal epicardial coronary arteries on coronary angio-graphy. Although patients with CSX generally have an excellent prognosis, at least in terms of life-threatening cardiac events, the quality of life is impaired because of the high recurrence rate of angina pectoris [15]. However, previous studies incorporating assessment of endothelial function and MPS indicated that subsets of patients may be at a higher risk of serious cardiovascular events in CSX patients with totally normal coronary artery disease [16,17]. Halcoxet al. [16] reported that coronary vascular resistance with acetylcholine predicted outcome, including event-free survival from cardiovascular death, acute myocardial infarction, unstable angina pectoris, and acute ischemic stroke, in a subset of 171 individuals with normal coronary arteries. Fragassoet al. [17] reported that patients with inducible myocardial hypoperfusion at MPS had a higher incidence of the primary endpoints includ-ing all-cause mortality and hospitalizations for cardiac causes among patients with syndrome X.
The pathogenesis of CSX is uncertain. Many theories have been put forward to explain this, including small vessel abnormalities [18], cardiomyopathy [19], coronary artery spasm [20], metabolic abnormalities [21], impaired coronary flow reserve [21], oxyhemoglobin dissociation defects [22], psychosomatic factors [23], altered pain perception [7], increased sympathetic drive [21], mis-interpretation of the coronary angiograms, and endothe-lial dysfunction [24]. According to the studies so far, CSX may be associated with an increased proinflammatory cytokine, CRP, endothelin-1, oxidative stress, and decreased endogenous NO [2–9]. Endothelial dysfunc-tion is believed to play an important role in the etiol-ogy [24].
We showed the relationship between CSX and decreas-ing TAC. The underlydecreas-ing mechanism of this association may be explained as follows: free radicals that are highly active compounds are produced in an organism con-tinuously. These free radicals formed are neutralized by the antioxidant systems of the organism. There is a bal-ance of oxidant and antioxidant systems in the organism. If this balance is disrupted in favor of oxidizing agents, oxidative stress occurs. Increased oxidative stress plays an important role in the incidence of atherosclerosis [25]. Free radicals formed by oxidative stress react with phospholipids in cell membranes and play an important role in endothelial dysfunction. This suggests that the deterioration in antioxidant systems may play an impor-tant role in the etiology of CSX. Measurement of TAC can provide more valuable information than measure-ment of antioxidants alone because the TAC reflects the total activity of substances with antioxidant properties found in serum and provides a more accurate approach.
As we mentioned above, coronary artery spasm may play a role in the pathogenesis of CSX. Investigators reported that chronic oxidant stress plays an important role in coronary artery spasm related to thiol oxidation and rho-kinase signaling [26]. Thus, coronary artery spasm may have contributed toward the relationship between the reduction of TAC and CSX.
Increased oxidative stress plays a role in the onset of atherosclerotic cardiovascular disease [8,27–29]. Previous studies showed endothelial dysfunction and systematic inflammation in patients with CSX. In a study by Lekakis et al. [30], flow-mediated dilatation was significantly lower in patients with CSX and those with coronary heart disease in comparison with healthy control participants, indicating impaired endothelium-dependent vasodilatation. Increased oxidative stress has been linked to impaired endothelial dysfunction in atherosclerosis and may play a role in the pathogenesis of CSX [30]. We report that a decrease in TAC may exacerbate the onset of CSX by increasing oxidative stress.
Clinical studies have reported left ventricular diastolic dysfunction in patients with CSX [31–33]. Fragasso et al. [31] reported the similarity of the symptoms and elec-trocardiographic and ventricular filling abnormalities found in patients with CSX and in those with CAD and they suggest that ischemia is involved in both groups. Nelson et al. [32] reported that diastolic function is impaired in women in patients with CSX, as assessed by cardiac magnetic resonance tissue tagging. In our study, we evaluated left ventricular systolic and diastolic func-tions with left ventricular ejection fraction using Simpson methods, conventional Doppler, tissue Doppler echo-cardiography, and the Tei index. To our knowledge, there are no studies using all of these parameters together to evaluate the systolic and diastolic biventricular func-tions. The myocardial performance index, also called the Tei index, is a Doppler-based parameter that reflects left ventricular systolic and diastolic functions [34]. Poulsen et al. [35] also reported that it is more sensitive than ejection fraction in predicting LV systolic dysfunction. In our study, we found that the Tei index was significantly higher in patients with CSX than that in the control group and we also detected biventricular diastolic dysfunction in patients with CSX. Ejection fraction was similar in both groups. Thus, the difference in the Tei index between the two groups may be because of differences in the diastolic functions of patients with CSX and the control group. We showed biventricular diastolic dys-function in patients with CSX.
To the best of our knowledge, this is the first study to show that there was a significant and inverse relationship between TAC and the Tei index. It could be speculated that increased oxidative stress may lead to myocardial ischemia episodes in CSX patients and recurrent ische-mia leads to impairment in left ventricular diastolic
function, which is more sensitive than systolic function to ischemia. This may play a role in the etiopathogenesis of the disease. As CSX patients usually develop myocardial ischemia episodes, this ischemia would increase the levels of free radicals and oxidative stress, hence decreasing the levels of antioxidant substances to neu-tralize it, and therefore decreasing the available TAC when new radicals are included in the measuring technique.
In conclusion, comprehensive studies are needed to detect the exact mechanism of CSX and to determine the effect of antioxidant therapy in patients with CSX. Decreased antioxidant levels (hence the increase in oxi-dative stress) may be associated with myocardial ischemia and may play a role in the etiopathogenesis of the disease.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
1 Ahmed B. New insights into the pathophysiology, classification, and diagnosis of coronary microvascular dysfunction. Coron Artery Dis 2014; 25:439–449.
2 Kaski JC. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (cardiac syndrome X). Circulation 2004; 109:568–572.
3 Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol 1991; 17:499–506.
4 On YK, Park R, Hyon MS, Kim SK, Kwon YJ. Are low total serum antioxidant status and elevated levels of C-reactive protein and monocyte chemotactic protein-1 associated with cardiac syndrome X? Circ J 2005; 69:1212–1217. 5 Luo C, Li Y, Liu D, Hu C, Du Z. The association of brachial flow-mediated
dilation and high-sensitivity C-reactive protein levels with Duke treadmill score in patients with suspected microvascular angina. Exp Clin Cardiol 2012; 17:197–201.
6 Cosín-Sales J, Pizzi C, Brown S, Kaski JC. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. J Am Coll Cardiol 2003; 41:1468–1474. 7 Crea F, Lanza GA. Angina pectoris and normal coronary arteries: cardiac
syndrome X. Heart 2004; 90:457–463.
8 Gur M, Yildiz A, Demirbag R, Yilmaz R, Aslan M, Ozdogru I, Erel O. Paraoxonase and arylesterase activities in patients with cardiac syndrome X, and their relationship with oxidative stress markers. Coron Artery Dis 2007; 18:89–95.
9 Kocak E, Yesildağ O, Yazici M, Demircan S, Birinci A, Sagkan O, et al. Kardiyak Sendrom X’li Hastalarda Istirahat ve Egzersiz Sonrasmda Plazma Endotelin-1 Düzeyleri [Endothelin levels in patients with cardiac syndrome X at rest and exercise]. Türk Kardiyol Dern Ars [Arch Turk Soc Cardiol] 2002; 30:671–674.
10 Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol 1994; 234:279–293.
11 Lakoumentas JA, Panou FK, Kotseroglou VK, Aggeli KI, Harbis PK. The Tei index of myocardial performance: applications in cardiology. Hellenic J Cardiol 2005; 46:52–58.
12 JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J 2010; 74:1745–1762.
13 Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004; 37:112–119. 14 Kemp HG Jr. Left ventricular function in patients with the anginal syndrome
and normal coronary arteriograms. Am J Cardiol 1973; 32:375–376.
15 Vermeltfoort IA, Teule GJ, van Dijk AB, Muntinga HJ, Raijmakers PG. Long-term prognosis of patients with cardiac syndrome X: a review. Neth Heart J 2012; 20:365–371.
16 Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002; 106:653–658.
17 Fragasso G, Lauretta L, Busnardo E, Cera M, Godino C, Colombo A, et al. Prognostic role of stress/rest myocardial perfusion scintigraphy in patients with cardiac syndrome X. Int J Cardiol 2014; 173:467–471.
18 Mosseri M, Yarom R, Gotsman MS, Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation 1986; 74:964–972.
19 Pasternac A, Bourassa MG. Pathogenesis of chest pain in patients with cardiomyopathies and normal coronary arteries. Int J Cardiol 1983; 3:273–280.
20 Boden WE, Bough EW, Korr KS, Benham I, Gheorghiade M, Caputi A, et al. Exercise induced coronary spasm with ST segment depression and normal coronary arteriography. Am J Cardiol 1981; 48:193–197.
21 Cannon RO III, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation 1992; 85:883–892.
22 Elliot RS, Bratt G. The paradox of myocardial ischaemia and necrosis in young women with normal coronary arteriograms: relation to abnormal haemoglobin-oxygen dissociation. Am J Cardiol 1969; 23:633–638. 23 Bass C, Wade C. Chest pain with normal coronary arteries: a comparative
study of psychiatric and social morbidity. Psychol Med 1984; 14:51–61. 24 Quyyumi AA, Cannon RO III, Panza JA, Diodati JG, Epstein SE. Endothelial
dysfunction in patients with chest pain and normal coronary arteries. Circulation 1992; 86:1864–1871.
25 Lear SA, Sarna LK, Siow TJ, Mancini GB, Siow YL. O K. Oxidative stress is associated with visceral adipose tissue and subclinical atherosclerosis in a healthy multi-ethnic population. Appl Physiol Nutr Metab 2012;
37:1164–1170.
26 Hoshino Y, Yamada S, Saitoh S, Machii H, Mizukami H, Miyata M, et al. Age-related oxidant stress with senescence marker protein-30 deficiency plays a pivotal role in coronary artery spasm. Coron Artery Dis 2013; 24:110–118. 27 Demirbag R, Rabus B, Sezen Y, Taşekin A, Kalayci S, Balkanay M. The
plasma and tissue oxidative status in patients with coronary artery disease. Turkish J Thorac Cardiovasc Surg J 2010; 18:79–82.
28 Sezen Y, Bas M, Polat M, Yildiz A, Buyukhatipoglu H, Kucukdurmaz Z, et al. The relationship between oxidative stress and coronary artery ectasia. Cardiol J 2010; 17:488–494.
29 Walter MF, Jacob RF, Bjork RE, Jeffers B, Buch J, Mizuno Y, Mason RP. PREVENT Investigators. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: the PREVENT study. J Am Coll Cardiol 2008; 51:1196–1202.
30 Lekakis JP, Papamichael CM, Vemmos CN, Voutsas AA,
Stamatelopoulos SF, Moulopoulos SD. Peripheral vascular endothelial dysfunction in patients with angina pectoris and normal coronary arteriograms. J Am Coll Cardiol 1998; 31:541–546.
31 Fragasso G, Chierchia SL, Pizzetti G, Rossetti E, Carlino M, Gerosa S, et al. Impaired left ventricular filling dynamics in patients with angina and angiographically normal coronary arteries: effect of beta adrenergic blockade. Heart 1997; 77:32–39.
32 Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging 2014; 7:510–516.
33 Moreno R, García-Fernández MA, Moreno M, Puerta P, Bermejo J, Ortega A, et al. Regional diastolic function in microvascular angina studied by pulsed-wave doppler tissue imaging. Echocardiography 1999; 16:239–244. 34 Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index
of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function– a study in normals and dilated cardiomyopathy. J Cardiol 1995; 26:357–366.
35 Poulsen SH, Jensen SE, Nielsen JC, Møller JE, Egstrup K. Serial changes and prognostic implications of a Doppler-derived index of combined left ventricular systolic and diastolic myocardial performance in acute myocardial infarction. Am J Cardiol 2000; 85:19–25.