https://doi.org/10.1007/s00276-020-02484-w
ORIGINAL ARTICLE
Evaluation of olfactory bulbus volume and olfactory sulcus depth
by 3 T MR
Neşat Çullu1 · İbrahim Önder Yeniçeri1 · Bünyamin Güney1 · Murat Yunus Özdemir1 · İlkay Koşar2
Received: 21 November 2019 / Accepted: 25 April 2020 / Published online: 7 May 2020 © Springer-Verlag France SAS, part of Springer Nature 2020
Abstract
Objective The aim of this study was to evaluate olfactory bulbus volume (OBV) and olfactory sulcus depth (OSD) according to age and sex with 3 T MRI in a healthy Turkish population.
Materials and methods In the current study, 200 patients who had cranial MRI were retrospectively evaluated. They were divided into the following groups to examine the effects of age: group 1: 18–30 years old; group 2: 31–40 years old; group 3: 41–50 years old; group 4: 51–60 years old; and group 5: >60 years old. OBV and OSD measurements were performed on coronal T2-weighted brain MR images. The mean right and left olfactory bulb volume and sulcus depths were used for evaluation.
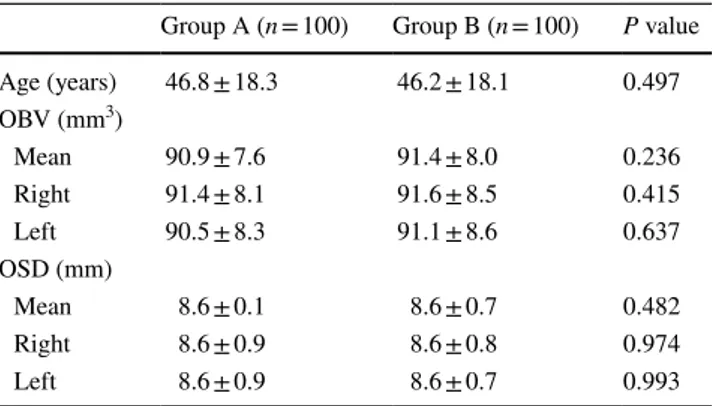
Results The mean age was 46.5 ± 18.1 (range 18-86) years. The mean OBV value of both sides was 91.17 ± 7.8 mm 3 in all patients. The mean OSD value of both sides was 8.62 ± 0.84 mm in all patients. There was no statistically significant difference in OBV and OSD between sexes (P < 0.236; P < 0.482). Group 5 (>60 years old) was found to have significantly lower OBV and OSD values than the other groups (all P < 0.001).
Conclusion The normal values of OBV and OSD should be established according to age to determine decreased OBV and OSD values.
Keywords Bulbus olfactorius · Olfactory sulci · MRI scans
Introduction
Olfactory bulbus (OB) is an ovoid-shaped anatomic struc-ture located in the cribriform plate [11]. OB has an impor-tant role in processing and evaluating the sense of smell [16]. Previous studies have demonstrated a close relationship between OB volume and olfactory functions [3]. In other words, there is a definite relationship between olfactory bulb volume and odor dysfunction in humans [12]. OBV measurements by MRI (magnetic resonance imaging) may provide clinical benefits in patients with olfactory function loss and help clinicians to follow the improvement of olfac-tory function [14]. On MRI, the detection rate of OB atro-phy has a high rate of differentiating normal patients from
dysfunctional patients [4]. In many studies, there is evidence that OBV and OSD measurements obtained on MRI images are reliable [7, 17].
Many diseases are associated with a decrease in OBV such as postinfectious, posttraumatic, Parkinson’s disease, Alzheimer’s disease, idiopathic, and congenital diseases [14]. Olfactory sulcus depth (OSD) is another parameter in the assessment of olfactory dysfunction. Many diseases including Behcet’s, Alzheimer’s, Parkinson’s, major depres-sion/anxiety are associated with decreased olfactory sulcus depth and reduction of the sense of smell [1, 5, 8]. In the Paschen et al. study, there was no significant relationship between Parkinson’s disease (PD) and OBV values in the healthy control group. OBV was 42.1 ± 11.6 mm3 in the
patient group and 46.6 ± 12.3 mm3 in the healthy
con-trol group. Another important result of this study is that high-resolution 3 T MRI examination is not sufficient to identify OBV reduction in PD [13]. In the study of Wang et al., olfactory bulb volume and olfactory sulcus depth were significantly lower in patients with PD. OBV was 37.30 ± 10.23 mm3 in PD and 44.87 ± 11.84 mm3 in the * Neşat Çullu
nesatcullu@mu.edu.tr
1 Department of Radiology, Muğla Sıtkı Koçman University,
Faculty of Medicine, Muğla, Turkey
2 Department of Anatomy, Muğla Sıtkı Koçman University,
control group (P < 0.05) and OSD was 8.90 ± 1.42 mm vs. 9.67 ± 1.24 mm (P < 0.05) [17]. In the study of Doğan et al., Behçet’s disease was associated with decreased odor func-tion. OBV and OSD were decreased in Behçet’s disease [5]. In the study by Zhang et al., decreased olfactory function and OBV were observed in patients with allergic rhinitis. Hov-ewer, there was no correlation between OSD and olfactory function [20]. In the study of Rottstaedt et al., the OBV was lower in people over 50. However, in patients with impaired mental status such as major depression, it was emphasized that the decrease in OBV accelerated in relation to the dura-tion of the onset of the first mental state change [15]. In the study of Yu et al., it was found that OBV decreased in Alzheimer’s disease compared to the healthy control group. There was no significant difference in OSD between the two groups. OBV values were measured as 30.05 ± 5.08 mm3 in
AD and 36.46 ± 4.11 mm3 in control group (P < 0.01) [19].
In the normal population, olfactory bulbus volume and olfactory sulcus depth may vary according to age and gen-der. There is still no clear idea about normal values. The goal of this study is to evaluate olfactory bulbus volume and olfactory sulcus depth by 3 T MRI according to age and gender in healthy Turkish population.
Materials and methods
This retrospective study was performed in muğla sitki koçman university radiology department. This study was approved by the ethics committee of muğla sitki koçman university. Individuals with any disease (trauma, infection, tumor, congenital, psychiatric, endocrinological diseases, etc.) were excluded from the study. Patient files were taken as the basis for evaluation. Patients with diabetes mellitus, COPD, chronic drug use, smoking and alcohol use were excluded from the study. Patients with nonspecific symp-toms and without pathology who underwent MRI were included in the study. In general, patients had headache and vertigo. These patients were not diagnosed with any disease during file scans. In the current study, 200 patients who had cranial MRIs were retrospectively evaluated. Patients who had cranial MRIs in the first 6 months of 2019 were included in the study. Those with poor image quality were excluded from the study. The patients were 100 men and 100 women. They were divided into groups according to their age and gender: group 1: 18–30 years old; group 2: 31–40 years old; group 3: 41–50 years old; group 4: 51–60 years old; group 5: > 60 years old; group A consisting of 100 men; group B consisting of 100 women. This study was carried out accord-ing to the bases of the Declaration of Helsinki. OBV and OSD measurements were performed on coronal T2-weighted brain MR images. MR images were obtained with a 3 T scanner (Siemens Skyra, Berlin, Germany). Images were
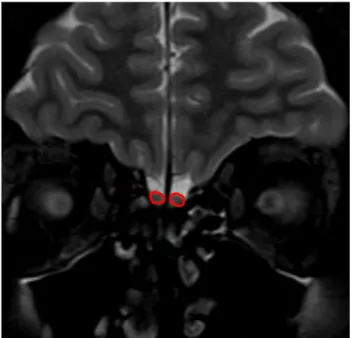
obtained with a protocol of 256 × 256 matrix and a 22-cm field of view, repetition time = 3500 ms (TR 3500 ms), echo time = 75 ms (TE 75 ms), number of excitations = 2 (NEX 2) and a 4-mm slice thickness. The volume and depth meas-urements were calculated by a radiologist who had 10-year experience and was blinded to the subjects. Images were transferred to the workstation singo.via (Siemens, Berlin, Germany). Measurements were made on this workstation. The mean right and left olfactory bulb volume and sulcus depths were used for evaluation (Figs. 1, 2). An electronic cursor was used for delineating the contours of OB manually. The surface of each slice area was calculated in mm2 and all
surfaces were added and multiplied by front–back length to obtain a volume in mm3. The mean of the three consecutive
measurements was taken into account. The observer estab-lished the minimum of the three consecutive measurements for measuring the MRI images. The intraobserver variability was determined as less than 5%.
Statistical analysis
Statistical evaluation was done using the IBM SPSS version 20.0 software (IBM Corp, Armonk, NY, USA). Normal dis-tribution was checked with the Kolmogorov–Smirnov test. Descriptive data were shown as mean ± standard deviation. We did statistical comparison of right and left OBV values with paired t test. Independent-sample t test was used for evaluating the statistical differences between gender groups. One-way ANOVA test was used for evaluating the statistical
Fig. 1 Olfactory bulb volume measurement with magnetic resonance imaging. Coronal T2-weighted image shows an example measuring of olfactory bulb surface area
differences between age groups. Multiple comparisons were made with the Tukey test. P value of 0.05 was accepted as statistically significant.
Results
A total of 200 patients (100 men, 100 women) were included in the study. The intraobserver variability was determined at less than 5% for OBV and OSD. The mean age was 46.5 ± 18.1 (range 18–86) years. The OBV was 91.5 ± 8.3 (range 68–115.2) mm3 on the right and 90.8 ± 8.4 (range
72–115.2) mm3 on the left side. There was no statistical
dif-ference between right- and left-side OBV (P < 0.09). Right and left OBV for men are 91.4 ± 8.1, 90.5 ± 8.3 mm3,
respec-tively. Right and left OBV for the woman are 91.6 ± 8.5 and 91.1 ± 8.6 mm3, respectively. There was no
statisti-cal difference between the genders for right and left OBV (P = 0.415, P = 0.637, respectively). The mean OBV values on both side were 91.17 ± 7.8 mm3 in all patients. OSD
val-ues were 8.6 ± 0.86 mm on the right and 8.6 ± 0.84 mm on the left. The mean OSD on both sides varied from 6.35 to 10.6 (8.6 ± 0.8) mm in all patients. There was no statistical difference between right and left in terms of OSD. Right and left OSD for the men are 8.6 ± 0.9, 8.6 ± 0.9 mm, respec-tively. Right and left OBV for the women are 8.6 ± 0.8 and 8.6 ± 0.7 mm, respectively. There was no statistical differ-ence between the genders for right and left OSD (P = 0.974,
P = 0.993, respectively). The distribution of age, OBV and
OSD according to gender is given in Table 1. There was not
a statistically significant difference between the OBV and OSD in terms of gender (P = 0.236; P = 0.482).
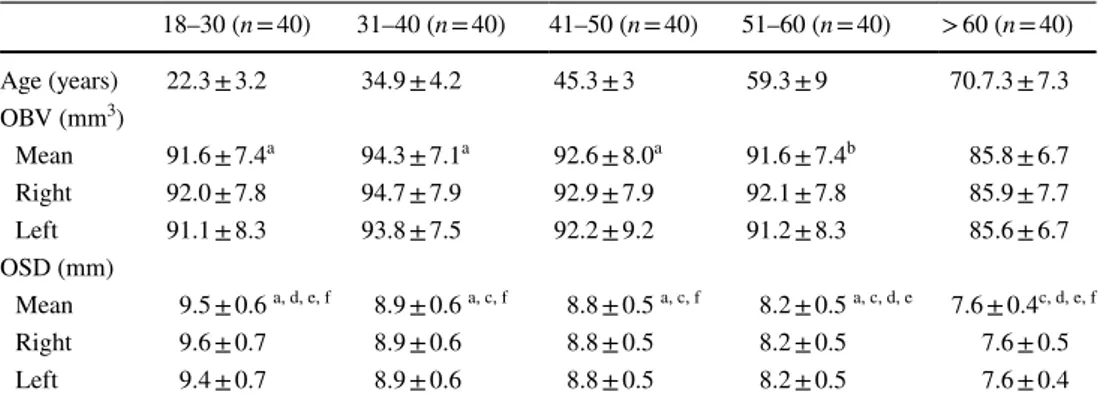
The distribution of mean OBV and OSD according to age groups is given in Table 2. There was a statistically signifi-cant difference in OSD and OBV values between the groups in the one-way ANOVA test (in both P < 0.001).
Discussion
There are two important results of our study. First, there was no significant difference between the OBV and OSD measurements in terms of gender in the normal population. Second, OBV and OSD values of individuals over 60 years of age were lower than other age groups. There was no dif-ference between the other age groups. Third, a decrease in OSD appeared to occur after the age of 30.
Hummel et al., found a positive correlation between OBV and olfactory function performed in children aged 1–17 years. It was emphasized that OBV and olfactory func-tion increased with age [9]. In the study of Wang et al., in healthy subjects of middle (50–69 years = group 1) and old (> 70 years = group 2) age, left and both sides OB volumes were found to be 39.89 ± 8.7 mm3 and 81.70 ± 16. 8 mm3
in group 1 and 34.45 ± 10.4 mm3 and 72.10 ± 19.3 mm3 in
group two, respectively. There was a statistically signifi-cant difference between them (P < 0.05). OBV was more on the right side than on the left. The OBV was higher in women than in men [18]. In the study conducted by Hang et al., 100 healthy individuals with age range 20–70 years were evaluated for OBV and olfactory functions. Right, left and mean OBV values in males were 84.65 ± 7.11 mm3,
87.79 ± 7.57 mm3 and 86.14 ± 7.37 mm3, respectively. Right,
left and mean OBV values of women were 69.58 ± 4.72 mm3,
71.43 ± 5.29 mm3 and 70.22 ± 5.02 mm3, respectively. There
was no significant difference in right and left OBV in both men and women (P > 0.05). OBV was significantly lower in women compared to men (P < 0.01). As a result of this
Fig. 2 Olfactory sulcus depth measurement with magnetic resonance imaging. Coronal T2-weighted image shows an example measuring of olfactory sulcus depth
Table 1 The distribution of age, mean and both sides olfactory bulb volüme (OBV) and olfactory sulcus dept in according to sexes
Group A (n = 100) Group B (n = 100) P value
Age (years) 46.8 ± 18.3 46.2 ± 18.1 0.497 OBV (mm3) Mean 90.9 ± 7.6 91.4 ± 8.0 0.236 Right 91.4 ± 8.1 91.6 ± 8.5 0.415 Left 90.5 ± 8.3 91.1 ± 8.6 0.637 OSD (mm) Mean 8.6 ± 0.1 8.6 ± 0.7 0.482 Right 8.6 ± 0.9 8.6 ± 0.8 0.974 Left 8.6 ± 0.9 8.6 ± 0.7 0.993
study, OBV and olfactory function decreased with age [6]. The results of these studies show that different results are obtained in the same disease groups in different age and gender (Table 3).
In our study, no significant difference was found between the sexes in terms of OBV and OSD. There was no sig-nificant difference in OBV values between 18 and 60 years of age. OBV and OSD measurements were significantly reduced in patients over 60 years of age. For OSD, the high-est values were seen in people between 18 and 30 years old, and the lowest values were observed in people over 60 years old. A decrease in OSD appeared to occur after the age of 30. This decrease may be due to the decrease in vascular feeding of olfactory bulbus, degeneration, decrease in regeneration capacity, microtrauma and many other reasons with age. This reduction may be related to the decrease in the regenerative capacity of the olfactory sen-sory neurons and epithelium with aging and changes due to aging in the peripheral olfactory system [10]. Other factors include accumulation damage of olfactory epithelium from
environmental factors, decrease in mucosal metabolizing enzymes, a sensory decrease of receptor cells against smell-ing substances, changes in neurotransmitter and neuromodu-lator systems. Additionally, constructional and functional damage of the olfactory epithelium, olfactory bulb, central olfactory cortex and basic olfactory circuit may cause dete-rioration of olfactory sensation [2]. The other strength of our study is that the images were obtained with a 3 T MR scanner. Thus, we think that we can obtain more accurate results with cleaner and thin section images.
This study has some limitations. Our sample size was relatively small. Our study is retrospective. All information about a person’s curriculum vitae and medical histories were obtained through the hospital registration system. Another limitation is that interobserver variability was not evaluated.
In conclusion, there is still no consensus on the normal values of OBV and OSD. These values may change depend-ing on age. So, normal values should be determined accord-ing to the age for decreased OBV and OSD.
Table 2 The distribution of age, mean and and both sides olfactory bulb volume (OBV) and olfactory sulcus dept in according to age groups
Data are n of participants, mean ± SD
a P < .001 compared with > 60-year group (One-way ANOVA–Tukey test)
b P < .01 compared with > 60-year group (One-way ANOVA–Tukey test)
c P < .001 compared with 18–30-year group (One-way ANOVA–Tukey test)
d P < .001 compared with 31–40-year group (One-way ANOVA–Tukey test)
e P < .001 compared with 41–50-year group (One-way ANOVA–Tukey test)
f P < .001 compared with 51–60-year group (One-way ANOVA–Tukey test)
18–30 (n = 40) 31–40 (n = 40) 41–50 (n = 40) 51–60 (n = 40) > 60 (n = 40) Age (years) 22.3 ± 3.2 34.9 ± 4.2 45.3 ± 3 59.3 ± 9 70.7.3 ± 7.3 OBV (mm3) Mean 91.6 ± 7.4a 94.3 ± 7.1a 92.6 ± 8.0a 91.6 ± 7.4b 85.8 ± 6.7 Right 92.0 ± 7.8 94.7 ± 7.9 92.9 ± 7.9 92.1 ± 7.8 85.9 ± 7.7 Left 91.1 ± 8.3 93.8 ± 7.5 92.2 ± 9.2 91.2 ± 8.3 85.6 ± 6.7 OSD (mm) Mean 9.5 ± 0.6 a, d, e, f 8.9 ± 0.6 a, c, f 8.8 ± 0.5 a, c, f 8.2 ± 0.5 a, c, d, e 7.6 ± 0.4c, d, e, f Right 9.6 ± 0.7 8.9 ± 0.6 8.8 ± 0.5 8.2 ± 0.5 7.6 ± 0.5 Left 9.4 ± 0.7 8.9 ± 0.6 8.8 ± 0.5 8.2 ± 0.5 7.6 ± 0.4
Table 3 Com par ison of pr evious s tudies in ter ms of OB V and OSD İPD idiopat hic P ar kinson ’s disease, PD P ar kinson ’s disease, BD Behçe t’s disease, AD Alzheimer ’s disease, AR aller gic rhinitis, MD ment al disor ders, C contr ol, M male, F Famale, R Right, L Lef t *S tatis tical significance Firs t aut hor Year Countr y Diagnosis N Ag e (y ears ± SD) Olf act or y tes t Magne tic field (Tesla) OB V (mm 3) OSD (mm) P W ang J 2009 Chinese Healt
hy middle and oldag
ed persons 95 Gr oup 1. 50–69 y ears Gr oup 2. > 70 y ears No 1.5 T Gr oup 1. 81.7 ± 16.8 Gr oup 2. 72. 1 ± 19.3 * < 0.05 W ang J 2011 Japanese PD 29 T&T 3 T PD:37.3 ± 10.2 C:44.87 ± 11.8 PD:8.9 ± 1.4 C:9.67 ± 1.2 * < 0.05 Hummel T 2011 Fr ance Childr en and adolescent 87 8 ± 5.5(1–17) Sniffin ’ S tic ks 1.5 T M: (21–98) R:68 L:71 F: (24–121) R:66 L:65 * < 0.05 Zhang Q 2014 Chinese AR 100 No t mentioned T&T 1.5 T AR:29.6 ± 4.1 C:48.8 ± 7.1 * < 0.01 Pasc hen L 2015 Ger man y İPD 52 64.4 ± 8.7 Sniffin ’ S tic ks 3 T PD:42.1 ± 11.6C:46.6 ± 12.3 > 0.10 Yu H 2015 Chinese AD 50 No t mentioned T&T 1.5 T AD: 30.1 ± 5.1 C:36.4 ± 4.1 * < 0.01 Hang W 2015 Chinese Healt hy individuals 100 42.6 ± 4.8 (20–70) T&T 1.5 T 86.1 ± 7.4 * < 0.01 Doğan A 2018 Tu rk ey BD 27 43.5 ± 7.9 No 1.5 T BD:34.1 ± 11.4 C:45.3 ± 4.9 BD:6.53 ± 0.89 C:7.03 ± 0.64 * < 0.05 Ro tts taedt F 2018 Ger man y MD 73 40.4 ± 12.1 No 3 T MD: 64.2 ± 18.5 C:74.2 ± 17.5 * < 0.01 Our s tudy 2020 Tu rk ey Healt hy individuals 200 46.5 ± 18.1 (18–86) No 3 T 91.17 ± 7.8 (68–115.2) 8,6 ± 0,84 (6.3–10.6) * < 0.001
Author contributions NC: Protocol/project development, manuscript writing, İÖY: Source search, BG: Data collection and processin g, MYÖ: Data collection and processing, İK: Data analysis.
Compliance with ethical standards
Conflict of interest The authors declare that they have no confict of interest.
References
1. Asal N, Muluk NB, Inal M, Şahan MH, Doğan A, Buturak SV (2018) Olfactory bulbus volume and olfactory sulcus depth in psychotic patients and patients with anxiety disorder/depression. Eur Arch Otorhinolaryngol 275:3017–3024
2. Attems J, Walker L, Jellinger KA (2015) Olfaction and aging: a mini-review. Gerontology 61:485–490
3. Buschhüter D, Smitka M, Psuchmann S et al (2008) Correlation between olfactory bulb volume and olfactory function. Neuroim-age 42:498–502
4. Chung MS, Choi WR, Jeong HY, Lee JH, Kim JH (2018) MRI maging-based evaluations of olfactory bulb atrophy in patients with olfactory dysfunction. Am J Neuroradiol 39:532–537 5. Doğan A, Muluk NB, Asal N, Şahan MH, Inal M, Gündüz Ö,
Arıkan OK (2018) Olfactory bulb volume and olfactory sul-cus depth in patients with Behçet’s diseas. J Laryngol Otol 132:1088–1092
6. Hang W, Liu G, Han T, Zhang J, Zhang Q (2015) A correlation study on olfactory bulb volumes with ages and olfactory function in healthy adults. Chinese J Otorhinolaryngol Head Neck Surg 50:744–748
7. Held P, Seitz J, Fründ R, Nitz WR, Haffke T, Hees H, Bonkowsky V (2000) MRI detection of olfactory bulb and tract. J Neuroradiol 27:112–118
8. Hummel T, Urbig A, Huart C, Duprez T, Rombaux P (2015) Volume of olfactory bulb and depth of olfactory sulcus in 378 consecutive patients with olfactory loss. J Neurol 262:1046–1051 9. Hummel T, Smitka M, Puschmann S, Gerber JC, Schaal B,
Buschhüter D (2011) Correlation between olfactory bulb volume
and olfactory function in children and adolescents. Exp Brain Res 214:285–291
10. Kass MD, Czarnecki LA, McGann JP (2018) Stable olfactory sen-sory neuron in vivo physiology during normal aging. Neurobiol Aging 69:33–37
11. Leboucq N, Champfleur N, Champfleur S, Bonafé A (2013) The olfactory systems. Diagn Int Imaging 94:985–991
12. Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T (2005) Reduced olfactory bulb volume in post-trau-matic and post-infectious olfactory dysfunction. Neuro Report 16:475–478
13. Paschen L, Schmidt N, Wolff S, Cnyrim C, van Eimeren T, Zeuner KE, Deuschl G, Witt K (2015) The olfactory bulb vol-ume in patients with idiopathic Parkinson’s disease. Eur J Neurol 22:1068–1073
14. Rombaux Ph, Duprez T, Hummel T (2009) Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 47:3–9
15. Rottstaedt F, Weidner K, Hummel T, Croy I (2018) Pre-aging of the olfactory bulb in major depression with high comorbidity of mental disorders. Front Aging Neurosci 10:354
16. Seubert J, Freiherr J, Frasnelli J, Hummel T, Lundström JN (2013) Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cereb Cortex 23:2448–2456
17. Wang JH, You JF, Liu DF, Ni DF, Zhang ZX, Guan J (2011) Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with parkinson disease. Am J Neuroradiol 32:677–681
18. Wang J, You H, Zhang ZX, Guan J, Chen XM, Liu JF, Yang DH, Shang YY, Zhu YY, Zou QJ, Gao ZQ, Ni DF (2009) MRI features of olfactory bulb volume in healthy middle and oldaged persons. Chinese J Otorhinolaryngol Head Neck Surg 44:1006–1009 19. Yu H, Hang W, Zhang J, Liu G (2015) Olfactory function in
patients with Alzheimer’ disease. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 29:444–447
20. Zhang Q, Liu G, Hang W (2014) Olfactory bulb volume and depth of olfactory sulcus in patients with allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 28:1956–1960
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.