Dyes and Pigments 174 (2020) 108019
Available online 5 November 2019
0143-7208/© 2019 Elsevier Ltd. All rights reserved.
Red and blue emitting fluorescent probe for cyanide and hypochlorite ions:
Biological sensing and environmental analysis
Sait Malkondu
a,*, Serkan Erdemir
b, Serdar Karakurt
baGiresun University, Faculty of Engineering, Department of Environmental Engineering, Giresun, 28200, Turkey bSelcuk University, Science Faculty, Department of Chemistry, Konya, 42250, Turkey
A R T I C L E I N F O Keywords: Hypochlorite Cyanide ICT Bioimaging Fluorescence Probe A B S T R A C T
Excessive levels of hypochlorite and cyanide ions are considered as the major threats towards human health and environment. Therefore, many researchers make considerable effort to develop small molecules for the quick, accurate and easy detection of biologically and environmentally important anions. Moreover, design of multiple analyte sensing sensors is still challenging issue due to broad application area. In the present study, we introduce a novel chemosensor (P) based on benzothiazole and diaminomaleonitrile units for the detection hypochlorite and cyanide ions in biological and environmental samples. The detection ability of P was examined towards a pool of analyte containing anions, cations and reactive oxygen species. P undergoes sharp turn-on fluorescence enhancement induced by hypochlorite or cyanide over other anions. The fluorescence response is attributed to oxidation of imine bond by hypochlorite or deprotonation of –NH2 and –OH functions by cyanide. The selectivity and sensitivity data demonstrated by P over other anions renders it an excellent candidate for hypochlorite and cyanide detection in biological and aqueous environments. Moreover, this is the first study that single probe has an ability of detecting both hypochlorite and cyanide anions through two distinct sensing modes.
1. Introduction
Hazardous and toxic chemicals are ubiquitous, mainly released by anthropogenic sources, and expose the environment and the living or-ganisms to serious risks. Therefore, reliable, fast and sensitive methods for the determination of dangerous chemicals are particularly needed. Hypochlorite anion (ClO ) is a strong oxidizer which is often used as water disinfectant and household bleach in our daily life. The human immune system produce minute quantities of hypochlorite, which serve as a powerful microbicide [1,2]. In somatic cells, enzyme myeloperox-idase catalyzes a reaction of formation of hypochlorite anion between chloride anion and hydrogen peroxide [3–5]. Hence, for normal cellular functions, it is crucial to maintain hypochlorite level within the physi-ological range. However, excessive hypochlorite exposure may result in tissue injury and various diseases including osteoarthritis, cancer, neu-rodegeneration and cardiovascular diseases [6–11].
Recently, notable research attempt has been dedicated to the development of novel techniques for cyanide sensing due to the continuing environmental impact resulted from by its large scale in-dustrial use as well as the high level toxicity of cyanide in physiological systems. Cyanide is a chemical precursor used in the production of
rubber, plastic, fertilizer, insecticide, pharmaceuticals and gold recovery [12]. Moreover, it is naturally found in certain seeds and fruit stones as almond, bitter and apricot [13] and also released from smoke which is formed through combustion of polymeric materials containing nitrogen [14]. However, It damages cellular respiration by prohibiting cyto-chrome c oxidase, a component of the electron transport chain. Impairment of respiration leads to cell death and suffocation and is a major concern in ecosystems in which concentrated cyanide accumu-lates [15,16]. Moreover, cyanide exposure influences directly the ner-vous, cardiovascular, and endocrine systems of humans [17]. Hence, it is urgently required to improve highly selective and sensitive methods for the detection of hypochlorite and cyanide for the advanced research of human diseases and the pathogenic pathway of them in living systems. Up to now, many techniques have been offered for measuring hy-pochlorite including iodometric titration [18], coulometry [19], potentiometric and amperometric measurements [20,21], and radiolysis [22], and also for cyanide such as voltammetric [23], titrimetric [24], potentiometric [25] and ion chromatography [26]. Despite, fluores-cence methods has recently come into prominence due to their proper characteristics such as simple operation, sensitivity, selectivity and biological potential. In particular, detection of fluorogenic compounds * Corresponding author.
E-mail address: smalkondu@gmail.com (S. Malkondu).
Contents lists available at ScienceDirect
Dyes and Pigments
journal homepage: http://www.elsevier.com/locate/dyepig
https://doi.org/10.1016/j.dyepig.2019.108019
which react toward hypochlorite and cyanide with visible and fast color conversion would provide an opportunity to monitor quickly biological and water samples.
Several chromogenic and fluorogenic sensor for hypochlorite [27–31] and cyanide [32–35] have been reported up to now. Some of them can sense cyanide [36,37] and hypochlorite [38,39] at micromolar levels. However, most of them have complicated synthetic procedures [40], work with quenching mechanism of fluorogenic center [41–45] or suffer from interference from other ions. Particularly, fluoride is in tendency to mask [46,47]. Therefore, searching for efficient chemo-sensors for sensing of hypochlorite and cyanide anions is still a chal-lenging issue. Moreover, detecting multiple analytes with a single probe gets great attention. To our knowledge, this is first report as a single molecule sensor detecting both hypochlorite and cyanide anions. Inspired by quenching effect of diaminomaleonitrile moiety on the
benzothiazole-salicylaldehyde moiety, a dual analyte detecting novel probe has been designed. The sensing pathway of the present probe for hypochlorite is the oxidation of the imine bond followed by the emer-gence of a significant blue emission. Cyanide is sensed through the deprotonation of –NH2 and –OH functions. The present study makes possible the detection of cyanide or hypochlorite in living cells and environmental samples through a single molecular probe P with different emission channels.
2. Experimental section
2.1. Materials and instruments
The chemical reagents were analytical grade and provided from Merck and Sigma Aldrich, and used without additional purification unless not specified otherwise. Fluorescence spectra were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer. UV–visible spectra were Fig. 1. Changes in absorption spectra of P (20.0 μM) upon addition of TBACN
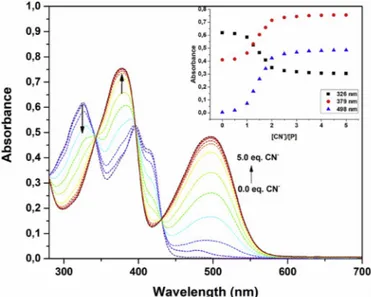
(0.0–5.0 equiv.) in a solution of DMSO/H2O (7/3, v/v). The inset plots the corresponding change of absorbance at 326, 379 and 498 nm versus ratio of [CN ]/[P].
Fig. 2. Changes of absorption spectra of P (20.0 μM) upon addition of NaClO (0.0–20.0 equv.) in a solution of DMSO/H2O (7/3, v/v). The inset plots the corresponding change of absorbance at 326 and 395 nm versus ratio of [ClO ]/[P].
Fig. 3. Emission spectra of the probe P (10.0 μM) in the presence of a pool of analyte (200.0 μM) in a solution of DMSO/H2O (7/3, v/v, 20 mM) (λex ¼361 nm).
Fig. 4. Emission titration spectra of the probe P (10.0 μM) in the presence of increasing concentration of CN (50.0 μM) The inset plots the corresponding change of emission at 604 nm versus ratio of [CN ]/[P].
Dyes and Pigments 174 (2020) 108019
measured on a PerkinElmer Lambda 25 spectrometer. NMR spectra were obtained by a Varian 400 MHz spectrometer in DMSO‑d6 or CDCl3 and chemical shifts (δ) were given in ppm units. The melting points were determined through a Stuart-SMP3 apparatus in a sealed capillary.
2.2. Synthesis
2.2.1. 4-(2-Benzothiazolyl)phenol (1)
A mixture of 4-hydroxybenzaldehyde (0.305 g, 2.5 mmol) and 2-ami-nothiophenol (0.33 g, 2.63 mmol) was prepared in ethanol (15 mL). Catalytic amount of amidosulfonic acid was added to the mixture. The mixture was stirred at rt for 2 h. The precipitated solid was filtered and washed with water. The crude product was recrystallized from ethanol. A pale yellow solid was obtained in a yield of 91% (0.52 g). M.p. 227–229 �C (228–229 �C [48]). 1H NMR (400 MHz, DMSO‑d
6) δ 10.25 (s, 1H, OH), 8.04 (d, J ¼ 8.0 Hz, 1H, Ar–H), 7.96 (d, J ¼ 8.0 Hz, 1H, Ar–H), 7.92 (dd, J ¼ 8.5, 1.6 Hz, 2H, Ar–H), 7.51–7.43 (m, 1H, Ar–H), 7.41–7.33 (m, 1H, Ar–H), 6.92 (dd, J ¼ 8.5, 1.6 Hz, 2H, Ar–H).
2.2.2. 5-(2-Benzothiazolyl)-2-hydroxybenzaldehyde (2)
The compound 1 was formylated through the Duff reaction. A mixture of 4-(2-benzothiazolyl)phenol (1) (0.50 g, 2.2 mmol), hexa-methylenetetramine (1.85 g, 13.2 mmol) and trifluoroacetic acid (20 mL) was refluxed for 12 h. After the resulting mixture was cooled to rt, water added to the mixture to precipitate the crude product. The solid washed with water well until pH of supernatant is neutral. Recrystalli-zation of the crude product from ethanol afford the product with the yield of 85% (0.48 g). M.p. 191–193 �C. 1H NMR (400 MHz, DMSO‑d
6) δ 11.43 (s, 1H, OH), 10.33 (s, 1H, CHO), 8.29 (s, 1H, ArH), 8.20 (d, 1H,
J ¼ 8.36 Hz, ArH), 8.09 (d, 1H, J ¼ 8.29 Hz, ArH), 8.00 (d, 1H, J ¼ 8.29 Hz, ArH), 7.51 (t, 1H, J ¼ 7.23 Hz, ArH), 7.41 (t, 1H, J ¼ 7.23 Hz, ArH), 7.17 (d, 1H, J ¼ 8.36 Hz, ArH).
Fig. 5. Emission titration spectra of the probe P (10.0 μM) in the presence of increasing concentration of ClO (200.0 μM) The inset plots the corresponding change of emission at 434 and 480 nm versus ratio of [ClO ]/[P].
Fig. 6. 1H NMR spectra of the probe P (0.144 M), 2 and P–CN molecules.
Scheme 1. Proposed interaction mechanism of P with ClO and CN ions.
2.2.3. 2-Amino-3-((5-(benzothiazol-2-yl)-2-hydroxybenzylidene)amino) maleonitrile (P)
The target probe P is an original compound. 5-(2-Benzothiazolyl)-2- hydroxybenzaldehyde (2) (0.42 g, 1.65 mmol) and diaminomaleonitrile (0.18 g, 1.73 mmol) were mixed with ethanol (20 mL). The resulting mixture was stirred at rt for 30 min. The yellow colored solid was precipitated. The solid washed with a mixture ethanol and water (1:1). Recrystallization of the crude product from ethanol gave a yellow colored crystal with a yield of 89% (0.51 g). M.p. 284–286 �C. 1H NMR (400 MHz, DMSO‑d6) δ 11.19 (s, 1H), 8.68–8.60 (m, 2H), 8.14–8.04 (m, 4H), 8.04–7.98 (m, 1H), 7.52 (t, J ¼ 7.7 Hz, 1H), 7.43 (t, J ¼ 7.7 Hz, 1H). 13C NMR (100 MHz, DMSO‑d6) δ 167.4, 161.0, 154.1, 152.3, 134.9, 132.2, 128.1, 127.1, 127.0, 125.6, 125.3, 122.9, 122.6, 122.3, 117.9, 115.0, 114.5, 103.8. 2.3. Spectral measurements
All spectral measurements were performed in a solution of DMSO/ H2O (7/3, v/v). Tetrabutylammonium salts of anions (I , Br , Cl , F , NO3, CN , NO2, N3, H2PO4, HSO4), perchlorate salts of cations (Agþ, Fe3þ, Hg2þ, Cu2þ) were used in the experiments. The solutions of ROS
analytes (NO�, H2O2, ClO , HO�, ROO�) were prepared in deionized water. Hypochlorite ion (ClO ) was obtained from NaOCl. Peroxyl radical (ROO�) was derived from dissociation of 2,2’-azobis [2- methylpropionamidine]dihydrochloride. H2O2 was used as hydrogen peroxide solution. The hydroxyl radical (HO�) was produced via Fenton reaction using H2O2 and Fe(NH4)2(SO4)2⋅6H2O. Nitric oxide (NO�) was obtained from the solution of S-Nitroso-N-acetylpenicillamine. For the determination of the possible binding stoichiometry between the probe
P and CN or ClO , Job’s plot method was used [49]. The detection limit was determined according to the earlier reported method. To determine the background noise σ, the emission spectrum of the probe P (20.0 μM) in a solution of DMSO/H2O (7/3, v/v) was measured for 20 times. The emission intensity at 480 nm for ClO and at 604 nm for CN were lin-early regressed, and the slopes of the curve were calculated. The detection limits were then determined from the equation of 3σ/slope.
2.4. Cell proliferation and bio-imaging studies
Human colon cell line DLD-1 was obtained from ATCC (American Type Culture Collection) and was cultured in RPMI-1640 medium sup-plemented with 10% FBS, 2 mM L-glutamine, and 100 units mL-1
Fig. 7. The frontier molecular orbitals (HOMO and LUMO) of 2, P and P–CN at excited state (S1) geometries from the TD-DFT calculations (top), molecular ge-ometries of 2, P and P–CN with dihedral angles between the phenol ring and the diaminomaleonitrile moiety (bottom).
Dyes and Pigments 174 (2020) 108019
Penicillin G, and 100 μg mL-1 streptomycin at 37 �C in a humidified incubator containing 5% CO2. Following the incubation of DLD-1 cells for 24 h, 1 � 104 cells were transferred into 96-well plate to examine the cytotoxic potential of the probe P. The cell viability of DLD-1 was tested with different concentration of the probe P ranging from 0 to 250 μM by using Alamar Blue as an indicator. The obtained data were transformed to a sigmoidal plot that was used to calculate IC50 values of the probe P by using GraphPad Prism 5.0.
To determine cellular localization, 1 � 105 of DLD-1 cells were seeded into glass-bottom dark 24-well plates (Corning, USA) and incu-bated at 37 �C for 24 h. Then the medium was removed and cells were washed with 10 mM of PBS for three times. The cells were pretreated with 10 μM of the probe P and incubated at 37 �C for 45 min. Then the probe P was removed and the cells were washed with 10 mM of PBS for three times to remove excess P. The cells were treated with an equal volume of CN or ClO and incubated at 37 �C for 45 min. The excess CN or ClO was removed and the cells were washed. The color for-mation was monitored by using fluorescence microscope (Bio-Rad, USA).
3. Results and discussions
3.1. Design and synthesis
The probe P was easily obtained in three steps (Scheme S1). The preparative synthetic route to P was outlined in Scheme 1. Compound 1 was prepared from 4-hyxdroxy benzaldehyde and 2-aminothiophenol in 85% yield. Then, 1 has been converted to aldehyde derivative (2) via a straightforward formylation stage using trifluoracetic acid and hexa-methylenetetramine. Finally, probe P was synthesized from the condensation reaction of 2 with diaminomaleonitrile. The molecular structures of all prepared compounds were fully verified by spectro-scopic techniques (Figs. S2–9). It was aimed to develop a dual analyte detecting probe for biological sensing and environmental analysis of cyanide and hypochlorite. The probe P was constituted from a benzo-thiazole, phenol and a diaminomaleonitrile moieties (Scheme 1). The probe P was nonemissive due to the excited state C––N isomerization and internal charge transfer. Hypochlorite triggered the oxidation of C––N bond to release corresponding aldehyde derivative (2) with blue emitting signal. As to cyanide, red emitting signal was obtained due to deprotonation of the probe P. Hypochlorite (ClO ) reacts rapidly with free cyanide and oxidize to finally carbon dioxide in aqueous solution [50]. Therefore, it was considered to be that cyanide concomitant could not be a problem on the detection of hypochlorite.
3.2. UV–vis response of the probe P towards cyanide and hypochlorite
In order to reveal the capability of chromogenic detection of P, UV–vis spectrum of P (20.0 μM) in the absence and presence of various anions (I , Br , Cl , F , NO3, CN , NO2, N3, H2PO4, HSO4), cations (Agþ, Fe3þ, Hg2þ, Cu2þ) and ROS (NO⋅, H
2O2, ClO , HO⋅, ROO⋅) were measured in a mixed solvent of DMSO/H2O (7/3, v/v). As seen in Fig. 1, the probe P exhibited two main absorption band at 326 and 395 nm with a shoulder at 413 nm arising due to n- π* and π- π* transitions of C––C and C––N. Therefore, UV–vis. spectra of the free probe P could not coincide with the visible region. Upon addition of CN (5.0 equiv.) to a solution of the probe P, a distinct color change from colorless to red was monitored. However, other competing anions and cations could not make a difference in absorption spectra except of hypochlorite. Detailed investigation of the probe P in the presence of CN was performed through the progressive addition of CN to colorless solution of the probe P. Cyanide addition led to a decrease in an absorption band at 326 nm while an increase in an absorbance at 379 nm, together with the emergence of a new band at 498 nm which was referred to the CN induced deprotonation of amino and hydroxyl functions in the struc-tures of P [51,52]. The equilibrium between the probe P and the cor-responding deprotonated species is justified by the presence of three clear isosbestic points at 343, 396 and 431 nm. From these results, it was understood from that CN could be successfully monitored by naked eye through the probe P.
Chromogenic behavior of probe P was also investigated in company with several reactive oxygen species (ROS), only addition of ClO prompted considerable decrease in the absorbance of the probe P without any wavelength shifts. In the presence of ClO , both absorption maxima of the probe P at 326 and 394 nm with a shoulder at 412 were step by step diminished and a band at 394 nm with a shoulder at 412 nearly disappeared (Fig. 2), originating from the ClO triggered oxida-tion of C––N bond [53,54] into corresponding aldehyde derivative (2) and diaminomaleonitrile moiety. Meanwhile, no significant solution color change was observed. Unfortunately, the probe P could not be convenient for a “naked-eye” probe for ClO .
3.3. Fluorescence response of the probe P towards cyanide and hypochlorite
To examine the sensing ability, the fluorescence response of P toward Fig. 8. (a) Design of the single-input Buffer gate, (b) circuit scheme
representation. Table 1
Determination of CN and ClO in the spiked tap water samples by the present method.
Samplesa Added CN and
ClO (μM) Found CN and ClO (μM) Recovery (%) RSD (n ¼ 3) S1 2.0 1.93 (CN ) 96.5 1.8 1.87 (ClO ) 93.5 2.3 S2 4.0 4.12 (CN ) 103.0 2.1 3.89 (ClO ) 97.2 1.4 S3 6.0 6.07 (CN ) 101.1 1.8 6.21 (ClO ) 103.5 2.2 aS1–S2 are known concentrations of CN and ClO solution samples.
a pool of analyte including various anions (I , Br , Cl , F , NO3, CN , NO2, N3, H2PO4, HSO4), cations (Agþ, Fe3þ, Hg2þ, Cu2þ) and ROS (NO⋅, H2O2, ClO , HO⋅, ROO⋅) was investigated in DMSO/H2O (7/3, v/ v) (Fig. 3). Free probe P showed a very low emission upon exited at 361 nm possibly due to a radiation less deactivation by fast isomeriza-tion of imine bond. The probe P shows dramatic “turn on” response toward cyanide and hypochlorite ions over other tested analytes. After exposure to cyanide, a distinct red-emitting “turn-on” response in emission spectra of P was monitored (Fig. 4). Increasing concentration of cyanide induced the development of a weak emission band at 482 nm and a strong emission band centered at 604 nm. These emission bands reached a maximum with a concentration of CN of 50.0 μM. In other words, that point corresponds that a molar concentration ratio of [CN ]/[P] is 2:1, indicating probe P and CN forms a 2:1 of complex. The Job’s plot method from the emission data also confirms the a 2:1 of binding stoichiometry (Fig. S9). The complexation process was pro-ceeded between CN and two protons belongs to hydroxyl and amino group. Consequently, colorless fluorescence of the free probe P under UV light turns to a fascinating red color which could be monitored only for CN (Fig. S10). However, other anions demonstrated no obvious emission enhancement except hypochlorite. The emission intensity enhancement ratio of I/I0 for CN was found to be about 124 folds which was remarkably higher than those of the reported many studies earlier [51,55,56]. Benesi-Hildebrand plot based on fluorescence titration data was consistent with a binding ratio of 1:2 (P:CN ) and the association constant was found to be 10.30 M-2 (Log Ka) (Fig. S11). The calibration curve demonstrated a good linear range at micromolar levels consid-ering an emission band at 604 nm with a calculated limit of detection (LOD) of 1.32 μM (Fig. S12), which is less than the levels of CN in drinking water suggested by the World Health Organization [57].
Emission response of probe P in company with various ROS was examined (Fig. 5). Addition of ClO to a solution of probe P caused an intense emission at 480 nm with a moderate shoulder at 434 nm was
developed, simultaneously. In contrast, other tested ROS had negligible effect on the emission of the probe P. Process was resulted in an obvious blue-emitting “off-on” signal, which could be monitored easily by naked eye under UV light. The phenomenon was referred to the enhancing of ICT process due to the conversion of the acceptor structure followed by the breakage of the imine bond to release the corresponding aldehyde derivative (2) and diaminomaleonitrile moiety (Scheme 1). It was found to be an emission intensity increase ratio (I/I0) was 112 which is considerably high compared to those of the reported many papers earlier [58,59]. In the emission titration results, gradual intensity development at 480 and 434 nm was noticed with the increasing amount of ClO up to 200.0 μM. The Job’s plot method from the emission data indicated the a 1:1 of interaction stoichiometry (Fig. S13). The probe P showed good linearity (Fig. S14) and a limit of detection of 0.136 μM. Stability con-stant for ClO were calculated as 5.57 M (Log Ka) from the
Benesi-Hildebrand plot based on fluorescence titration data (Fig. S15). Fluorescence quantum yields were calculated to previously reported method [60]. Quantum yield of the probe P (ΦP ¼2.76 � 10 3) reached to 8.68 � 10 2 for cyanide and 0.257 for hypochlorite (Fig. S16). The results indicated that quantum yield of P was increased ~32-fold for cyanide and ~93-fold for hypochlorite. Hence, these results show that the present probe P has the great potential of detection quantitatively unknown concentrations of ClO .
The response time is a key parameter for a probe in practical appli-cations. As can be seen from Fig. S17, around 97% for ClO and 96% for CN of the total emission enhancement taken place within just 1 min. At the same time, their emissions were reached to equilibrium for both analytes. Therefore, the probe P displayed excellent response times with respect to the previously reported many probe [61–64].
Considerable effort has been paid to develop reversible probes due to the reusable nature of a chemosensor is essential for sensing applica-tions. Therefore, we determined that the cyanide-triggered turn-on response was completely reversible with some cations such as Agþand
Dyes and Pigments 174 (2020) 108019 Hg2þ. The addition of 2.0 eq. of Agþinduced the reemergence of an
emission band at 604 nm. The emission intensity variation demonstrated obviously a cycle by alternate addition of Agþand CN (Fig. S18). The reversible deprotonation cycle could be repeated several times and still remained the ability of cyanide sensing. Compared with other reported probes including diaminomaleonitrile unit as receptor unit for fluores-cence hypochlorite or cyanide detection, the present method shows a comparable response due to its promising properties low detection limit, high selectivity and sensitivity, rapid response and two distinct sensing modes in bioimaging and environmental analysis [40–47,65–69].
3.4. Selectivity studies
To evaluate the selectivity of the probe P, competition studies were conducted in the presence of a pool of analyte. It can be seen clearly from Fig. S19, emission intensity of the probe P was enhanced even in the presence of a combination of cyanide and other competing analytes. Therefore, the presence of competing analytes could not make consid-erable interference on the detection of CN . Only HSO4 demonstrated slight interference (<14%) due to the acidic character of HSO4. Simi-larly, hypochlorite sensing system was not significantly influenced by a pool analyte including anions, cations and ROS (Fig. S20). Only exis-tence of some competing analytes could make a slight interference up to maximum of <12%. Because sodium hypochlorite is often used to treat cyanide in waste waters [70], hypochlorite easily oxidize cyanide. Therefore, competition experiments indicated that emission response of the probe P toward cyanide or hypochlorite was not interfered by other competing analytes. Besides, the addition of the probe P to a solution containing both CN (2.0 equiv.) and ClO (1.0 equiv.) triggered the emergence of purple emission (Fig. S21). Then, the purple color was rapidly turned to blue within 1 min. It can be inferred from the results that the oxidation reaction by ClO is fastest reaction in such a mixture.
3.5. 1H NMR studies
In order to acquire an insight into the proposed interaction pathway of the probe P toward cyanide or hypochlorite, 1H NMR experiments for probe P were conducted with or without cyanide and hypochlorite (Fig. 6). Addition of 2.0 equivalents of CN to a solution of the probe P was resulted in remarkable changes in the 1H NMR spectra. Signals belong to hydroxyl (Ha) and amino protons (Hc) observed at 11.15 and 8.07 ppm, respectively was disappeared. Meanwhile, aromatic proton signals were shifted upfield. These results were consistent with the deprotonation mechanism by cyanide ions. Similarly, addition of 2.0 equivalents of hypochlorite to a solution of the probe P resulted in sig-nificant spectral changes. The disappearance of signals belongs to dia-minomaleonitrile (Hc) at 8.07 ppm, imine (Hb) at 8.64 ppm and phenolic OH protons (Ha) at 11.15 ppm and also appearance of a new signal at 10.37 ppm were indicators that the probe P oxidized with hypochlorite to release free aldehyde derivative. The rest of signals belong to aro-matic protons were upfield shifted. Therefore, these findings are consistent with the proposed oxidation and deprotonation mechanisms.
3.6. TD/DFT calculations
Theoretical calculations were used to clarify variations in the pho-tophysical behaviors of P, 2 and P–CN using the density functional theory with B3LYP using 6-31G (d,p) basis sets in Gaussian 16.0 soft-ware package. All geometries were optimized in the excited states. As can be seen from contour plots of frontier orbitals (Fig. 7a), electron distribution of HOMO is dispersed on the entire structure while LUMO is mainly localized on phenol ring and diaminomaleonitrile unit, pointing out an ICT character for both P and 2. In addition, leaving of dia-minomaleonitrile moiety through oxidation of P by hypochlorite leads to the raising of energy gap (ΔE) of P from 3.000 to 3.986 eV. These results suggested that a blue emission was originated from the larger
energy gap of 2. In order to clarify the effect of steric hindrance in-teractions, the optimized geometries of P and 2 were analyzed (Fig. 7b). However, the planar structure of P associated with diaminomaleonitrile moiety [dihedral angle ¼ 0�(C
6, C5, C17, N18)] was remained unchanged compared to 2.
In the case of P and P–CN, the electron distribution in HOMO for
P–CN is solely populated on the diaminomaleonitrile moiety while that
of P are distributed on the conjugation system. However, LUMO for
P–CN is merely localized over the P–CN except the diaminomaleonitrile
moiety. Thus, potentiality for ICT between diaminomaleonitrile to benzothiazole moiety is rational to understand. Moreover, significant decrease between the HOMO–LUMO energy difference is 0.613 eV for
P–CN, indicating corroboration well with the decreased ICT and the
enhanced emission. Narrowing of energy gap resulted in a red emitting emission. P–CN was almost planar, with a dihedral angle of 1.168� associated with diaminomaleonitrile moiety. However, a dihedral angle within diaminomaleonitrile moiety of P is remarkably changed from 179.9�to 92.7�(C
17, C18, C18, N19) upon the deprotonation.
3.7. Logic gate application
Research interested in molecular logic is a fast-developing interdis-ciplinary area, which has attracted many scientist’s attention. Taking into account the emission of the free probe P and it interacting with CN and ClO , the variations are evaluated as digital outputs. A single-input Buffer gate is easily designed (Fig. 8). The emission outputs (I480 or I604) lower than the predetermined threshold point are converted to binary “0”, conversely the outputs higher than threshold are correspond to binary “1”. The inputs are CN and ClO , and their outputs are read out at λ604 and λ480, respectively. In the absence of CN and ClO , which is regarded as input signal “0”, the probe P shows no emission and output signal “0”. But, with addition of CN (input signal “1”), the strong emission at 604 nm emerges together with distinct red emitting emission color and the output is a digital “1”. Disparately, in the presence of ClO (input signal “1”), blue emitting emission color is observed at 480 nm and the output is a digital “1”. Thus, the operation of the present mo-lecular logic is well consistent with Buffer gates.
3.8. Environmental analysis
The feasibility of the present fluorescent method for the detection of CN or ClO was checked in tap water samples. To mimic real- environment measurements, the samples were spiked with known con-centrations of CN or ClO . After CN or ClO spiked water samples were treated with a solution of the probe P, their emission intensities were measured. The results obtained by standard addition method were given in Table 1. Recoveries of CN and ClO were between 93.5 and 103.5% with the lower relative standard deviation (RSD) values than 2.3. Therefore, the results demonstrated that the present method could accurately and precisely work to detect CN or ClO in water samples.
3.9. Bioimaging
To further explore the ability of the probe P in live cell fluorescence visualizing, DLD-1 in Human colon cells was employed. Cell viability studies showed that the probe P was inhibited proliferation of DLD- 1 cells as a dose-dependent manner (Figs. S22–a). Treatment of DLD- 1 cell with 10 μM of the probe P just inhibited 3% of cell proliferation (p > 0.5). The IC50 value of the probe P on DLD-1 cell was calculated after 48 h treatment of the cells and IC50 was calculated as 176.2 μM (Figs. S22–b). Therefore, for the bioimaging studies, as a safe dose 10 μM of the probe P was used, which was nontoxic against DLD-1. They treated with 10 μM the probe P itself as a control demonstrated no emission (Fig. 9). After the addition of 10 μM CN to DLD-1 cells pre-treated with the probe P and incubated for 45 min more, an excellent red emission could be observed. Besides, ClO treatment resulted in a S. Malkondu et al.
remarkable blue emission, showing that the probe P has well cell penetrability and ability to visualize intracellular CN or ClO . There-fore, the results revealed that the probe P has great potential for imaging of CN or ClO in living cells.
4. Conclusion
A novel of fluorescence probe for the detection of cyanide and hy-pochlorite ions has been successfully constructed on the basis of the ICT mechanism. The probe P is comprised of a benzothiazole fluorophore and diaminomaleonitrile recognition unit which has ability of oxi-dization with hypochlorite and also deprotonation with cyanide. Other tested analytes could not interfere with the detection results. The pres-ence of cyanide was resulted in the emergpres-ence of remarkable red emission while hypochlorite led to the distinct blue emission. Probe P demonstrated excellent fluorescence “off-on” responds to cyanide at 604 nm with 124-fold emission enhancement within 1 min or to hypo-chlorite at 480 nm with 112-fold increase. Studies on biological imaging demonstrated that the present probe P as a new diagnostic tool can selectively recognize cyanide or hypochlorite in intracellular media. Moreover, the probe P has been employed for observing the level of cyanide or hypochlorite in tap water samples with good recoveries. The fluorescence behavior and ICT mechanism have been found that P could show excellent sensing properties such as wide linear range, rapid response, low LOD, good selectivity and sensitivity. The promising re-sults will make a great contribution to biomedical and environmental scientists studying hypochlorite and cyanide in biological and environ-mental systems.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi. org/10.1016/j.dyepig.2019.108019.
References
[1] Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol 2001;14(10): 1453–64.
[2] Prokopowicz ZM, Arce F, Biedron R, Chiang CL-L, Ciszek M, Katz DR, et al. Hypochlorous acid: a natural adjuvant that facilitates antigen processing, cross- priming, and the induction of adaptive immunity. J Immunol 2010;184(2):824–35. [3] Wang L, Long L, Zhou L, Wu Y, Zhang C, Han Z, et al. A ratiometric fluorescent
probe for highly selective and sensitive detection of hypochlorite based on the oxidation of N-alkylpyridinium. RSC Adv 2014;4(103):59535–40.
[4] Liu S-R, Vedamalai M, Wu S-P. Hypochlorous acid turn-on boron dipyrromethene probe based on oxidation of methyl phenyl sulfide. Anal Chim Acta 2013;800:71–6. [5] Zhou J, Li L, Shi W, Gao X, Li X, Ma H. HOCl can appear in the mitochondria of macrophages during bacterial infection as revealed by a sensitive mitochondrial- targeting fluorescent probe. Chem Sci 2015;6(8):4884–8.
[6] Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 2002;3(5):420–5.
[7] Wu SM, Pizzo SV. α2-Macroglobulin from rheumatoid arthritis synovial fluid: functional analysis defines a role for oxidation in inflammation. Arch Biochem Biophys 2001;391(1):119–26.
[8] Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 2004;24(7): 1309–14.
[9] Pattison DI, Davies MJ. Evidence for rapid inter-and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry 2006;45(26): 8152–62.
[10] Steinbeck MJ, Nesti LJ, Sharkey PF, Parvizi J. Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease. J Orthop Res 2007; 25(9):1128–35.
[11] Yap YW, Whiteman M, Cheung NS. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal 2007;19(2):219–28.
[12] Johnson CA. The fate of cyanide in leach wastes at gold mines: an environmental perspective. Appl Geochem 2015;57:194–205.
[13] Vetter J. Plant cyanogenic glycosides. Toxicon 2000;38(1):11–36.
[14] Anseeuw K, Delvau N, Burillo-Putze G, De Iaco F, Geldner G, Holmstr€om P, et al. Cyanide poisoning by fire smoke inhalation: a European expert consensus. Eur J Emerg Med 2013;20(1):2–9.
[15] Hall AH, Isom GE, Rockwood GA. Toxicology of cyanides and cyanogens. Wiley Online Library; 2015.
[16] Jensen P, Wilson M, Aasa R, Malmstr€om BG. Cyanide inhibition of cytochrome c oxidase. A rapid-freeze epr investigation. Biochem J 1984;224(3):829–37. [17] Warburg O. Inhibition of the action of prussic acid in living cells. Hoppe-Seyler’s Z
für Physiol Chem 1911;76:331–46.
[18] Association APH, Clesceri LS, Greenberg AE, Trussell RR, Franson MAH. M�etodos normalizados para el an�alisis de aguas potables y residuales. Díaz De Santos; 1992. [19] Lieu VT, Kalbus GE. Analysis of hypochlorite in commercial liquid bleaches by
coulometric titration. J Chem Educ 1975;52(5):335.
[20] Thiagarajan S, Wu Z-Y, Chen S-M. Amperometric determination of sodium hypochlorite at poly MnTAPP-nano Au film modified electrode. J Electroanal Chem 2011;661(2):322–8.
[21] Soldatkin A, Gorchkov D, Martelet C, Jaffrezic-Renault N. New enzyme potentiometric sensor for hypochlorite species detection. Sens Actuators B Chem 1997;43(1–3):99–104.
[22] Quinn RC, Martucci HF, Miller SR, Bryson CE, Grunthaner FJ, Grunthaner PJ. Perchlorate radiolysis on Mars and the origin of martian soil reactivity. Astrobiology 2013;13(6):515–20.
[23] Shan D, Mousty C, Cosnier S. Subnanomolar cyanide detection at polyphenol oxidase/clay biosensors. Anal Chem 2004;76(1):178–83.
[24] Suzuki T, Hioki A, Kurahashi M. Development of a method for estimating an accurate equivalence point in nickel titration of cyanide ions. Anal Chim Acta 2003;476(1):159–65.
[25] Blaedal W, Easty D, Anderson L, Farrell T. Potentiometric determination of cyanide with an ion selective electrode. Application to cyanogenic glycosides in Sudan grasses. Anal Chem 1971;43(7):890–4.
[26] Destano�glu O, Gümüs¸ Yılmaz G, Apak R. Selective determination of free cyanide in environmental water matrices by ion chromatography with suppressed conductivity detection. J Liq Chrom Relat Tech 2015;38(16):1537–45. [27] Long L, Zhang D, Li X, Zhang J, Zhang C, Zhou L. A fluorescence ratiometric sensor
for hypochlorite based on a novel dual-fluorophore response approach. Anal Chim Acta 2013;775:100–5.
[28] Chang C, Wang F, Qiang J, Zhang Z, Chen Y, Zhang W, et al. Benzothiazole-based fluorescent sensor for hypochlorite detection and its application for biological imaging. Sens Actuators B Chem 2017;243:22–8.
[29] Algi MP. A fluorescent hypochlorite probe Built on 1, 10-phenanthroline scaffold and its ion recognition Features. J Fluoresc 2016;26(2):487–96.
[30] Algi MP. A highly selective dual channel hypochlorite probe based on fluorescein and 1, 10-phenanthroline. Tetrahedron 2016;72(12):1558–65.
[31] Wu D, Chen L, Xu Q, Chen X, Yoon J. Design principles, sensing mechanisms, and applications of highly specific fluorescent probes for HOCl/OCl–. Accounts Chem Res 2019;52(8):2158–68.
[32] Yahaya I, Seferoglu Z. Fluorescence dyes for determination of cyanide. Photochemistry and photophysics: fundamentals to applications. 2018. p. 179. [33] Ma J, Dasgupta PK. Recent developments in cyanide detection: a review. Anal
Chim Acta 2010;673(2):117–25.
[34] Ekmekci Z, Yilmaz MD, Akkaya EU. A monostyryl-boradiazaindacene (BODIPY) derivative as colorimetric and fluorescent probe for cyanide ions. Org Lett 2008;10 (3):461–4.
[35] Erdemir S, Malkondu S. On-site and low-cost detection of cyanide by simple colorimetric and fluorogenic sensors: smartphone and test strip applications. Talanta 2019:120278.
[36] Liu Z, Wang X, Yang Z, He W. Rational design of a dual chemosensor for cyanide anion sensing based on dicyanovinyl-substituted benzofurazan. J Org Chem 2011; 76(24):10286–90.
[37] Peng M-J, Guo Y, Yang X-F, Suzenet F, Li J, Li C-W, et al. Coumarin–hemicyanine conjugates as novel reaction-based sensors for cyanide detection: convenient synthesis and ict mechanism. RSC Adv 2014;4(37):19077–85.
[38] Yan Y-H, Ma H-L, Miao J-Y, Zhao B-X, Lin Z-M. A ratiometric fluorescence probe based on a novel recognition mechanism for monitoring endogenous hypochlorite in living cells. Anal Chim Acta 2019;1064:87–93.
[39] Shi W-J, Huang Y, Liu W, Xu D, Chen S-T, Liu F, et al. A BODIPY-based “OFF-ON” fluorescent probe for fast and selective detection of hypochlorite in living cells. Dyes Pigments 2019;170:107566.
[40] Li S, Huang D, Wan J, Yan S, Jiang J, Xiao H. A two-photon fluorescent probe derived from spirobifluorene for fast sensing of hypochlorite and mercury ions. Sens Actuators B Chem 2018;275:101–9.
[41] Li L, Zan M, Qie X, Miao P, Yue J, Chang Z, et al. A highly selective fluorescent probe for cyanide ion and its detection mechanism from theoretical calculations. Talanta 2018;185:1–6.
[42] Shojaeifard Z, Hemmateenejad B, Shamsipur M. Efficient on–off ratiometric fluorescence probe for cyanide ion based on perturbation of the interaction between gold nanoclusters and a copper (II)-phthalocyanine complex. ACS Appl Mater Interfaces 2016;8(24):15177–86.
[43] Shahid M, Ali R, Razi SS, Srivastava P, Gupta RC, Dwivedi SK, et al. Design and synthesis of some imidazolyl derivatives: photophysical studies and application in detection of anions. Open Che J 2015;2(1).
Dyes and Pigments 174 (2020) 108019 [44] Hwang SM, Kim A, Kim C. A simple hydrazine-based probe bearing anthracene
moiety for the highly selective detection of hypochlorite. Inorg Chem Commun 2019;101:1–5.
[45] Starzak K, Matwijczuk A, Creaven B, Matwijczuk A, Wybraniec S, Karcz D. Fluorescence quenching-based mechanism for determination of hypochlorite by coumarin-derived sensors. Int J Mol Sci 2019;20(2):281.
[46] Mahapatra AK, Manna SK, Pramanik B, Maiti K, Mondal S, Ali SS, et al. Colorimetric and ratiometric fluorescent chemodosimeter for selective sensing of fluoride and cyanide ions: tuning selectivity in proton transfer and C–Si bond cleavage. RSC Adv 2015;5(14):10716–22.
[47] Lim B, Lee J. A peptoid-based fluorescent sensor for cyanide detection. Molecules 2016;21(3):339.
[48] Wang Y-T, Jin KJ, Myers LR, Glover SA, Novak M. Hydrolysis and photolysis of 4- Acetoxy-4-(benzothiazol-2-yl)-2, 5-cyclohexadien-1-one, a model anti-tumor quinol ester. J Org Chem 2009;74(12):4463–71.
[49] Valeur B. Molecular fluorescence. Digital encyclopedia of applied physics. 2003. p. 477–531.
[50] Teo W, Tan T. Hypochlorite oxidation of cyanate under mildly alkaline conditions. Water Res 1987;21(6):677–82.
[51] Chemchem M, Yahaya I, Aydıner B, Sefero�glu N, Doluca O, Merabet N, et al. A novel and synthetically facile coumarin-thiophene-derived Schiff base for selective fluorescent detection of cyanide anions in aqueous solution: synthesis, anion interactions, theoretical study and DNA-binding properties. Tetrahedron 2018;74(48):6897–906.
[52] Barare B, Yıldız M, Ünver H, Aslan K. Characterization and use of (E)-2-[(6- methoxybenzo [d] thiazol-2-ylimino) methyl] phenol as an anion sensor and a DNA-binding agent. Tetrahedron Lett 2016;57(5):537–42.
[53] Han J, Li Y, Wang Y, Bao X, Wang L, Ren L, et al. A water-soluble fluorescent probe for monitoring hypochlorite in water and in living cells. Sens Actuators B Chem 2018;273:778–83.
[54] Wu W-L, Zhao Z-M, Dai X, Su L, Zhao B-X. A fast-response colorimetric and fluorescent probe for hypochlorite and its real applications in biological imaging. Sens Actuators B Chem 2016;232:390–5.
[55] Zhou C, Sun M, Yan C, Yang Q, Li Y, Song Y. A new colorimetric and fluorescent chemodosimeter for fast detection of cyanide. Sens Actuators B Chem 2014;203: 382–7.
[56] Sun M, Wang S, Yang Q, Fei X, Li Y, Li Y. A new colorimetric fluorescent sensor for ratiometric detection of cyanide in solution, test strips, and in cells. RSC Adv 2014; 4(16):8295–9.
[57] Organization WH. Guidelines for drinking-water quality. World Health Organization; 1993.
[58] Shangguan M, Jiang X, Lu Z, Zou W, Chen Y, Xu P, et al. A coumarin-based fluorescent probe for hypochlorite ion detection in environmental water samples and living cells. Talanta 2019;202:303–7.
[59] Guo J, Zhang Z, Kuai Z, Wang R, Yang Q, Shan Y, et al. A new turn-on fluorescent probe towards hypochlorite in living cells. Analytical Methods 2017;9(5):864–70. [60] Malkondu S, Erdemir S. A triphenylamine based multi-analyte chemosensor for
Hg2þ and Cu2þ ions in MeCN/H2O. Tetrahedron 2014;70(35):5494–8. [61] Li Y, Tang Y, Gao M, Wang Y, Han J, Xia J, et al. A sensitive BODIPY-based
fluorescent probe suitable for hypochlorite detection in living cells. J Photochem Photobiol A Chem 2018;352:65–72.
[62] Long L, Wu Y, Wang L, Gong A, Hu F, Zhang C. A fluorescent probe for hypochlorite based on the modulation of the unique rotation of the N–N single bond in acetohydrazide. Chem Commun 2015;51(52):10435–8.
[63] Zang L, Wei D, Wang S, Jiang S. A phenolic Schiff base for highly selective sensing of fluoride and cyanide via different channels. Tetrahedron 2012;68(2):636–41. [64] Lin Q, Cai Y, Li Q, Shi B-B, Yao H, Zhang Y-M, et al. Fluorescent “turn-on” detecting
CN by nucleophilic addition induced schiff-base hydrolysis. Spectrochim Acta A Mol Biomol Spectrosc 2015;141:113–8.
[65] Wang Y, Xia J, Han J, Bao X, Li Y, Tang X, et al. A fast-responsive fluorescent probe based on BODIPY dye for sensitive detection of hypochlorite and its application in real water samples. Talanta 2016;161:847–53.
[66] Das S, Aich K, Patra L, Ghoshal K, Gharami S, Bhattacharyya M, et al. Development of a new fluorescence ratiometric switch for endogenous hypochlorite detection in monocytes of diabetic subjects by dye release method. Tetrahedron Lett 2018;59 (12):1130–5.
[67] Chen B, Fu H, Lv Y, Li X, Han Y. An oxidative cyclization reaction based fluorescent “Turn-On” probe for highly selective and rapid detection of hypochlorous acid. Tetrahedron Lett 2018;59(12):1116–20.
[68] Feng Y, Li S, Li D, Wang Q, Ning P, Chen M, et al. Rational design of a diaminomaleonitrile-based mitochondria–targeted two-photon fluorescent probe for hypochlorite in vivo: solvent-independent and high selectivity over Cu2þ. Sens Actuators B Chem 2018;254:282–90.
[69] Kim MJ, Manivannan R, Kim IJ, Son Y-A. A colorimetric and fluorometric chemosensor for the selective detection of cyanide ion in both the aqueous and solid phase. Sens Actuators B Chem 2017;253:942–8.
[70] Khodadad A, Teimoury P, Abdolahi M, Samiee A. Detoxification of cyanide in a gold processing plant tailings water using calcium and sodium hypochlorite. Mine Water Environ 2008;27(1):52–5.
![Fig. 5. Emission titration spectra of the probe P (10.0 μ M) in the presence of increasing concentration of ClO (200.0 μ M) The inset plots the corresponding change of emission at 434 and 480 nm versus ratio of [ClO ]/[P]](https://thumb-eu.123doks.com/thumbv2/9libnet/4969413.100529/3.892.59.430.84.378/emission-titration-spectra-presence-increasing-concentration-corresponding-emission.webp)