ScienceDirect
Materials Today: Proceedings 18 (2019) 1978–1985 www.materialstoday.com/proceedings
2214-7853 © 2019 Elsevier Ltd. All rights reserved.
Selection and/or Peer-review under responsibility of INTERNATIONAL CONGRESS ON SEMICONDUCTOR MATERIALS AND DEVICES.
ICSMD-2017
Removal of Chromium(VI) from Aqueous Solution Using Chitosan
Doped with Carbon Nanotubes
Şerife Parlayıcı, Erol Pehlivan
*Department of Chemical Engineering, Selcuk University, Campus, 42079 Konya, Turkey
Abstract
This work investigates the adsorption of chromium ions on chitosan doped with multiwalled carbon nanotubes (Cht-MWCNT). The effects of initial pH, amount of Cht-MWCNT, temperature, contact time, and the initial concentration of Cr (VI) were investigated during the equilibrium and kinetic studies. The equilibrium time was found to be 60 min. Adsorption isotherms of Cr (VI) on Cht-MWCNT was determined and correlated with Langmuir, Freundlich, Scatchard and D-R isotherm equations. The adsorption data showed that adsorption of Cr (VI) was fitted by Langmuir isotherm model and pseudo-second-order kinetics model. Under optimum conditions, maximum adsorption capacity of Cr (VI) determined by Langmuir model were enhanced 26.14 mg/g. Thermodynamic parameters such as ΔG°, ΔH°, ΔS° were calculated and the interaction of Cr (VI) with Cht-MWCNT was found to be endothermic and spontaneous in nature.
© 2019 Elsevier Ltd. All rights reserved.
Selection and/or Peer-review under responsibility of INTERNATIONAL CONGRESS ON SEMICONDUCTOR MATERIALS AND DEVICES. Keywords: Chitosan; MWCNT; isotherm; kinetic; thermodynamic.
1. Introduction
Water pollution caused by draining of a large amount of hazardous waste into water stream without pre-treatment has increased the concern about treatment models and adsorbents for recent years. Different toxic materials poured into freshwater resources origins the health effect to drinking water as well as human life. Aquatic systems are especially very sensitive to metal toxicity possibly due to the food chain and their impacts to human. Cr (VI) is a highly toxic heavy metal, which is carcinogenic and mutagenic to living organisms, thus has been placed on the top of the priority
* Corresponding author. Tel.: +90.332.2232127; fax: +90.332.2410635. E-mail address: erolpehlivan@gmail.com
list of toxic pollutants by the U.S. EPA [1,2]. Cr (VI) is a harmful environmental pollutant. The maximum allowable limit by U.S. EPA is maximum acceptable Cr (VI) concentration of 50 mg/L in potable water [3].
Different techniques have been adopted for removal of heavy metals at low level from wastewater including precipitation, solid phase extraction, reverse osmosis, filtration, ion exchange, electrochemical treatment, and evaporation are reported previously [4,5]. Among these, metal adsorption method from the aqueous medium by using a suitable adsorbent has been widely used and is an attractive one for the removal of pollutants. For this target, a number of adsorbents have been employed for different types of pollutants.
Chitosan is widely used in a variety of areas, such as agriculture as a seed treatment and biopesticide, helping plants to fight off fungal infections. It can be used as a fining agent and is also used as a bleeding blocker and antibacterial agent in bandages. Chitosan is a linear polysaccharide consisted of indiscriminately distributed ƥ-(1-4)-linked D-glucosamine. The deacetylation of chitin, which is the structural element in the matrix of cell walls of fungi, results in the formation of chitosan. The amino group on the chitosan skeleton has a pKa value of 6.5, which results in a protonation in the acidic to the neutral environment with a charge density dependent on the pH and deacetylation percentage. This causes the chitosan to become hydrophilic, which makes it easier to integrate into beads, gels or thin films, and negatively charged surfaces [6]. The amine and hydroxyl groups in the chitosan are mostly suitable for adsorption of metal ions. Various studies have been carried out for chitosan as an adsorbent for the treatment of toxic metals from aqueous media [7,8]. On the other hand, different polymers were used along with chitosan to increase the thermal properties and surface area of chitosan by making a composite material. In this case, the perlite was selected as the copolymer as support. Carbon nanostructures with different morphologies, especially carbon nanotubes are assumed to be one of the major elements in nanotechnology. MWCNTs are preferred for their special physical and chemical properties. Due to their large specific surface area and uniform pore distribution, MWCNTs can be impressive candidates for potential applications of chromium removal from contaminated water reservoirs [9]. MWCNTs as solid materials have unique structures and properties. Chitosan can be used as a perfect
dispersing agent for MWCNT. It is known that the electron-donating -NH2 interacts strongly with MWCNT.
Chitosan has many functional groups for adsorption of chromium. For that reason, this composite adsorbent has a stationary property as an adsorbent in the liquid medium. This indicates that MWCNT together with chitosan is advantageous in terms of adsorption capacity, as well as mechanical strength [10, 11].
This research reports the use of Cht-MWCNT composite as an adsorbent for Cr (VI) removal from aqueous media. Various adsorption parameters such as adsorbent amount, equilibrium time, pH and concentration of chromium at initial stage were applied and the morphological behavior before and after interactions was investigated
2. Experimental
2.1. Materials
Chitosan flakes (degree of deacetylation, DD=75-85%) and MWCNT (20-30%) was obtained from
Sigma-Aldrich. All other chemicals (K2Cr2O7, oxalic acid, NaOH and HCl etc.) were purchased from Merck Company.
Analytical grade chemicals were used without further purification in the experiments and doubly distilled water was used to prepare the required solutions. The stock Cr (VI) solution with a concentration of 1000 ppm was obtained by
dissolving K2Cr2O7 salt (Merck) in double distilled water.
2.2. Preparation of Cht-MWCNT
The composite was obtained by the method used previously by Hasan et al. (2008) [12]. MWCNT was first soaked into 0.2 M oxalic acid for 12 h to remove any acid soluble constituents. Acid-treated MWCNT was washed with distilled water and dried in an oven for 12 h. This was done to maintain a proper ratio of acid to chitosan in the mixture during the production. 5 g of chitosan flakes was dissolved in 500 mL of 0.2 mol/L oxalic acid solution to prepare a gel. MWCNT treated with oxalic acid was added to the gel. The mixture was stirred for 5 h while heating at (40–50 ºC) to obtain a homogeneous mixture. The spherical beads of chitosan-coated MWCNT were prepared by dropwise addition of the mixture into 0.7 M NaOH precipitation bath. The beads were washed with deionized water to a neutral pH and dried for the subsequent use (Fig. 1.).
Fig. 1. Preparation of Cht-MWCNT beads.
2.3. FTIR analysis of Cht-MWCNT
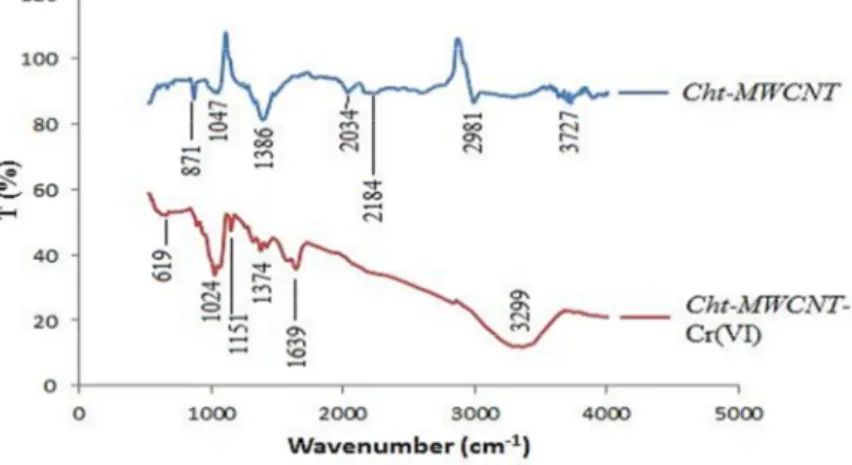
The surface chemical characteristics of Cht-MWCNT before and after Cr (VI) adsorption were determined by Fourier Transform Infrared Spectroscopy (FTIR), (ATR Bruker Vertex 70) and the spectrum is given in Fig. 2. As
seen in the FTIR spectrum; hydroxyl (-OH) group appeared at 3299 cm-1 [13]. CH
2 and CH3 group aliphatic C-H
stretching vibrations were determined at 2981 cm-1. -NH line and -NH
2 due to bending band appeared at 1639 cm-1
1374 cm-1[14]. These functional groups such as –NH
2 and –OH are involved in binding the Cr (VI) to Cht-MWCNT
[15, 16]. In the structure of chitosan, alcohol groups (COH) disappeared at 1386 cm-1 and –CO stretching vibration
in –COH appeared at 1047 cm-1. The peaks around 1000–1300 cm-1 presents C-O stretching in phenols, alcohols,
acids, ethers, and esters.
Fig. 2. FTIR analysis of Cht-MWCNT before and after Cr(VI) adsorption.
2.4. SEM analysis
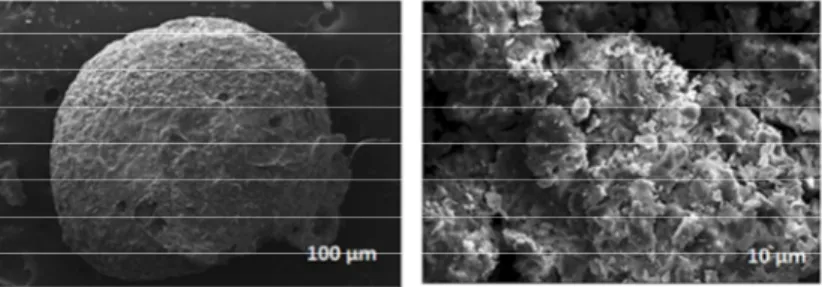
SEM is generally used to characterize an adsorbent by giving something knowledge about its surface topology as well as morphology and was used for determining the particle size and shape. A morphological change on the surface of Cts-MWCNT was observed through SEM images by applying 20 kV electron acceleration voltage (Fig. 3.). The shape of the capped particle can be described as spherical. The figure illustrates the surface texture and porous nature of Cts-MWCNT with holes and small openings on the surface, thereby increasing the contact area, which facilitates the pore diffusion during adsorption.
Fig. 3. SEM images showing the surface morphologies of the Cts-MWCNT beads.
2.5. Experimental methods
The effect of equilibrium parameters is usually applied to the adsorption of Cr (VI) to Cht-MWCNT and work well for estimating the adsorption capacity of the related adsorbant. The uptake capacity of Cht-MWCNT depends on the surface functionality of the applied adsorbent. Cht-MWCNT is a new adsorbent to be applied for taking away Cr (VI) in the diluted solution. A series of standard Cr (VI) solution by proper dilution of the stock solution was prepared for the uptake experiments.
Batch adsorption experiments were performed with the different amounts of Cht-MWCNT in a 100 mL polyethylene bottle with of various concentrations (with constant pH) on a rotary shaker at 150 rpm. The solution pH was adjusted to the required value with a strong acid and base solution. All experimental results were checked to remove the errors by blank tests in which no adsorbent was added into Cr (VI) solution. The samples were tested two times to ascertain the accuracy, reliability, and reproducibility of the information obtained from the
experimental results. The adsorption capacity at time t, qt (mg/g) was obtained as shown in Equation (1) [17]:
qt = [( Co – Ct ) V] / m (1)
where qt is adsorbed Cr (VI) (mg/g adsorbent) on Cht-MWCNT, m is the mass of Cht-MWCNT (g), V is volume
of Cr (VI) solution (l), Co is initial Cr (VI) concentration (mmol/l), and Ct is Cr (VI) concentration (mmol/l) at any
time.
3. Results and Discussion
3.1. Effect of contact time
The contact time was varied from 15 to 1500 min for the uptake of Cr (VI) by Cht-MWCNT. The effect of contact time on the uptake of Cr (VI) is shown in Fig. 4. The uptake of Cr (VI) increased during adsorption process and reached an optimum value at about 60 min. The uptake of Cr (VI) can be considered in two steps: Initially, the uptake rate is very high at the beginning of the reaction between Cr (VI) and Cht-MWCNT in a contact time of 60 minutes. Secondly, the adsorption rate continued at a slow pace until the equilibrium was reached.
3.2. Effect of solution pH
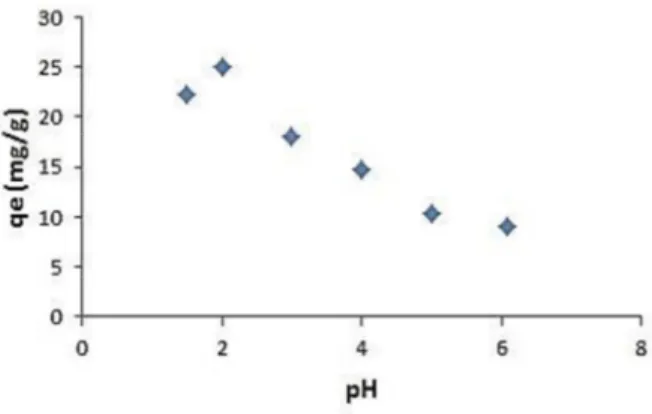
There are a lot affects for binding of Cr (VI) to Cht-MWCNT. These forces can be electrostatic interactions, complex formation, adsorption and ion exchange mechanisms and they are effective during the process. Fig. 5 represents the effect of the initial pH on the uptake of the Cr (VI) by the Cht-MWCNT. In order to find the optimum pH for maximum uptake efficiency, the experiments were carried out in the pH range 2.0-6.0. It was found that the protonation of the surface decreases at pH value over 4.0. Adsorption accelerated at pH 3.0-4.0 and then reached a maximum value when approaching pH 2 Optimum pH value is considered as 2.0. The composite protonated in the acidic environment and electrostatic interactions were created between composite surface and Cr (VI). For this reason, the adsorption existed more in the acidic conditions compared to neutral cases.
Fig. 5. Effect of pH on the uptake of Cr (VI) using the Cht-MWCNT.
3.3. Adsorption isotherms
The adsorption capacity of the MWCNT can be calculated from the equilibrium studies. Cr (VI) and Cht-MWCNT have performed an adsorption isotherm. This isotherm is usually the relationship between the quantity adsorbed and that staying in the solution phase at a certain temperature at equilibrium conditions. The uptake of Cr (VI) ions was observed at different initial chromium concentrations at pH 2.0 with the optimum agitation speed and
period. These isotherms relates Cr (VI) uptake per unit weight of adsorbent (qe) to the equilibrium Cr (VI)
concentration in the bulk fluid phase (Ce) (Fig. 6.).
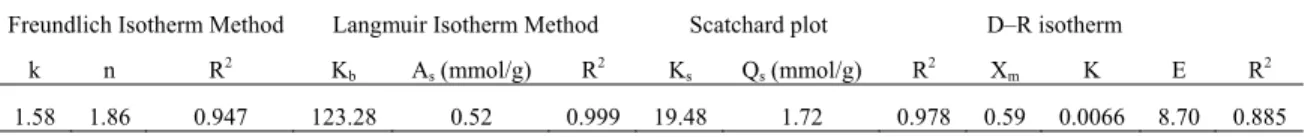
Table 1. Adsorption isotherm parameters.
Freundlich Isotherm Method Langmuir Isotherm Method Scatchard plot D–R isotherm
k n R2 K
b As (mmol/g) R2 Ks Qs (mmol/g) R2 Xm K E R2
1.58 1.86 0.947 123.28 0.52 0.999 19.48 1.72 0.978 0.59 0.0066 8.70 0.885
Freundlich, Langmuir, Scatchard and D-R adsorption isotherms parameter are given in Table 1. k and n constant values were calculated to define how the equilibrium fit Freundlich equation. n values were determined as 1.86 for Cr (VI). If this value is between 1 and 10 then Freundlich isotherms can be selected for adsorption. For
Dubinin-Radushkevich (D-R) isotherm, Xm, K and E values were calculated and given in Table 1. Adsorption energy (Ead)
were determined 8.7 kJ/mol. Scatchard curve was plotted and Qs, Ks constant values were obtained and is given
Table 1. The linear plots of Ceq/q vs Ceq show that the uptake followed the Langmuir isotherm model. The Langmuir
model fitted well in the pH range of 1.5-2.0. The Langmuir equation was more applicable than the Freundlich equation although both described the uptake data adequately. According to Langmuir isotherm, the correlation coefficients are calculated as 0.99 and the maximum uptake capacities are found as 26.14 mg Cr (VI) per g of
Cht-MWCNT. Table 2 presents the comparison of adsorption capacity (qm; mg/g) of Cth-MWCNT for Cr (VI) ions with
that of various sorbents reported in the literature [18-22]. The experimental data of the present investigations are comparable with the reported values. The results indicated that the prepared Cts-MWCNT has a great potential for the uptake of Cr (VI) ions from the aqueous solution.
Table 2. Cr (VI) removal using of low-cost adsorbents.
Adsorbent Adsorption capacities (mg/g) Reference
Sago waste activated carbon 5.78 [18]
Polyaniline with rice husk 4.739 [19]
Multi-walled carbon nanotubes 2.48 [20]
Bael fruit shell activated carbon 17.27 [21]
Avocado kernel seeds 10.08 [22]
Cht-MWCNT 26.14 Present work
3.4. Effect of adsorbent dose on Cr (VI)
Fig. 7. Effect of adsorbent dose on the uptake of Cr (VI).
The selected doses of adsorbent were 1.0, 2.0, 3.0, 4.0 and 5.0 g/L while the concentration of Cr (VI) was held constant throughout the uptake equilibrium and the results were compiled in Fig. 7. The results in Fig. 7 showed that the retention of Cr (VI) increased with the increase of the adsorbent dose up to 3.0 g/L and after that, it decreased. It is readily understood that the number of available uptake sites increased with the increase of the adsorbent dose. But, beyond a certain value of the dose amount, the uptake efficiency of Cr (VI) ion decreased. The decrease in
uptake density was due to the fact that some of the uptake sites stayed unsaturated during the uptake process. As the adsorbent dose increased, the number of available sites on the adsorbent surface increased, and this caused in an increase in uptake percentage.
3.5. Adsorption kinetics
Batch tests were performed to determine the kinetics of adsorption. The pseudo-first-order kinetic model is generally valid to initial state of the adsorption process. It describes the rate of change of adsorbate concentration with the time that is directly proportional to the difference in a saturation concentration and the adsorbent capacity in Eq. (1):
= (1 − ) (1)
The adsorption rate constant (k1) can be evaluated experimentally by fitting experimental data in Eq. (2):
= ( − )
⁄ (2)
In order to find the adsorption rate constant at room temperature, the experimental data were fitted to pseudo-second-order isotherm. This model is more probably to foretell the kinetic behavior of adsorption with chemical adsorption being the rate controlling step. The linear form of pseudo-second order rate expression is (Eq. 3) [23]:
1⁄ = (1⁄ )+( ⁄ ) (3)
Table 3. Kinetics parameters for the adsorption of Cr (VI). First-order Second-order
k1 qe R2 k2 qe R2
0.00092 65.89 0.847 0.0388 25.77 0.996
The correlation coefficients for the second-order kinetic model are greater than 0.995 (Table 3). Also, the
calculated qe values agree very well with the experimental data. These indicated that the adsorption system belongs
to the second-order kinetic model.
3.6. Thermodynamic modeling
Adsorption of Cr (VI) onto Cht-MWCNT was definitely carried out different temperatures (25 °C, 35 °C, 45 °C and 55 °C) for thermodynamic analysis. The standard Gibbs free energy is calculated from the following equation (4) [24]:
where T is the solution temperature (K), and R the gas constant (J/mol.K). The thermodynamic parameters including ΔH°, ΔS° and ΔG° for the adsorption of Cr (VI) onto the Cht-MWCNT samples were calculated from the plotted data and the results were given in Table 4. The reaction was endothermic in nature with increasing temperature and the adsorption of Cr VI) increased. This means that there was a tendency or a mobility of Cr (VI) from solution phase to the adsorbent. ΔG° was negative and adsorption tended to be spontaneous.
Table 4. Thermodynamic parameters.
∆So ∆Ho ∆Go (J/mol-1)
(J/K-1mol-1) J/mol-1 T=298.15 K T=308.15 K T=318.15 K T=328.15 K R2
30.87 0.092 -9048.49 -9511.49 -9974.49 -10437.5 0.972
4. Conclusions
The mechanism of Cr (VI) adsorption on the Cht-MWCNT was discussed. The isotherm equilibrium data were correlated well to the Langmuir model. Maximum removal of Cr (VI) on Cht-MWCNT adsorbent was found at pH 2.0 and the maximum monolayer adsorption capacity of Cht-MWCNT was calculated as 26.14 mg/g. Kinetics studies proved that the dynamic behavior of Cr (VI) adsorption could be described by pseudo-second-order kinetic model. In conclusion, results obtained suggest that Cht-MWCNT has a high potential as an adsorbent in the removal Cr (VI) from wastewater and the application of Cht-MWCNT as adsorbent is suitable because of its low production cost.
References
[1] V.K. Gupta, M. Gupta, S. Sharma, Water Res. 35 (2001) 1125–1134.
[2] D.D. Maksin, A.B. Nastasovic, A.D. Milutinovic-Nikolic, L.T. Surucic, Z.P. Sandic, R.V. Hercigonja, A.E. Onjia, J. Hazard. Mater. 209–210 (2012) 99–110.
[3] X. Sun, L. Yang,Q. Li, J. Zhao, X. Li, X. Wang, H. Liu, Chem Eng J, 241 (2014) 175-183. [4] C.E. Barrera-Diaz, V. Lugo-Lugo, B. Bilyeu, J. Hazard. Mater. 223–224 (2012) 1–12. [5] M. Owlad, M.K. Aroua, W.A.W. Daud, S. Baroutian, Water Air Soil Poll. 200 (2009) 59–77. [6] E. Khor, Elsevier. (2001) 83–107.
[7] F. Sayılkan, F.B. Emre, Turk. J. Chem. 40 (2016) 28–37.
[8] S. Hasan, K.Aburi, T.K. Ghosh, D.S. Viswanath, V.M. Boddu, E.D. Smith, Sep. Sci. Technol. 38 (2003) 3775–3793. [9] Ş. Parlayici, V. Eskizeybek, A. Avcı, E. Pehlivan, J. Nano. Chem., 5(3) (2015) 255-263.
[10] L.Y. Yan, Y.F. Poon, M.B. Chan-Park, Y. Chen, Q. Zhang, J. Phys. Chem. C. 112 (20) (2008) 7579–7587. [11] S. Chatterjee, M.W. Lee, S.H.Woo, Biores. Techn. 101 (6) (2010) 1800-1806.
[12] S. Hasan, T. K. Ghosha, D. S. Viswanath, V. M. Boddu, J. Hazar. Mater. 152 (2008) 826–837. [13] S. Zhang, M. Zeng, W. Xu, J. Li, J. Li, J. Xu, X. Wang, Dalton Trans. 42 (2013) 7854–7858. [14] K. Swayampakulaa, V. M. Boddub, S. K. Nadavala, K. Abburia, J. Hazar. Mater. 170 (2009) 680–689. [15] W.Kaminski, W. Modrzejewska, Sci. Technol., 32(16) (1997) 2659-2668.
[16] V.W.D. Chui, K.W. Mok, , C.Y. Ng B.P. Luong, K.K. Ma, Environ. Int., 22(4) (1996) 463–468. [17] T.Altun, E. Pehlivan. CLEAN–Soil, Air, Water, 35.6 (2007) 601-606.
[18] N. Vennilamini, K. Kadirvelu, Y. Sameena, S. Patabhi, Adsorpt. Sci. Technol., 23(2) 2005 145–160. [19] F. Kanwal, R. Rehman, T. Mahmud, J. Anwar, R. Ilyas, J. Chilean Chem. Soci. 57 (2012) 1058-1063. [20] X. Jung, C. Heo, J. Han, J. Her, N. Lee, S.J. Oh, J.J. Ryu, Y. Yoon, Sep. Purif. Techn. 106 (2013) 63–71. [21] J. Anandkumar, B. Mandal, J. Hazar. Mater. 168 (2010) 633‒640.
[22] E. Mekonnen, M. Yitbarek, T.R. Soreta, , S. Afr. J. Chem. 68 (2015) 45–52.
[23] N. T. Tavengwa, L. Chimuka, L. Tichagwa, Des. Water Treat. 57 (2016) 16843–16854. [24] Ş. Parlayıcı, E. Pehlivan, Pow. Tech. 317 (2017) 23–30.