J Food Process Preserv. 2019;43:e13885. wileyonlinelibrary.com/journal/jfpp
|
1 of 9 https://doi.org/10.1111/jfpp.13885© 2019 Wiley Periodicals, Inc.
1 | INTRODUCTION
The pomegranate (Punica granatum) fruit can be used for juice, jam, and Jellies production (Fernandes et al., 2015; Goula & Adamapoulos, 2012). The pomegranate fruits are rich in phytochemical compounds (Elfalleh et al., 2011; Jing et al., 2012; Liu, Xu, Gong, He, & Gao, 2012; Matthaus & Özcan, 2016; Mousavinejad, Emam‐Djomeh, & Rezaei, 2009; Prakash & Prakash, 2011). Most parts such as peel, aril, seed of pomegranate fruit have strong antioxidant activity (Elfalleh et al., 2011; Negi & Jayaprakasha, 2003; Singh, Murthy, & Jayaprakasha, 2002). Rowayshed, Salama, Abul‐Fadl, Akila‐Hamza, & Mohamed, (2013) determined 27.92% and 0.25% total phenol
in pomegranate peel and seed powders. In addition, pomegranate fruit peel powder contained 44.19 mg/100 g ellagic acid, 868.40 catechin, 125.80 gallic, 12.50 resocenol, 4.17 protocatechol, 9.02 parahydroxy benzoic acid, 242.70 phenol, 3.91 vanilline, 60.46 caffeic acid, 5.89 ferulic acid, 17.64 p‐coumaric acids (Rowayshed et al., 2013). Arils dried in mechanical cabinet drier contained more total anthocyanin (33.08 mg/100 g) due to less photooxidation of the anthocyanins of arils (Bhat, Thakur, & Jindal, 2014). In previous study, juice of Ostokhani Tabas, Malase Ashkezar, Malase Ardestan, and Sooleghan pomegranates contained 9,304, 8,129, 7,923, and 7,438 mg/L total phenol (Mousavinejad et al., 2009). Pomegranate contains polyphenols, alkaloids, and vitamins with potent free
Received: 12 August 2018
|
Revised: 12 December 2018|
Accepted: 27 December 2018 DOI: 10.1111/jfpp.13885O R I G I N A L A R T I C L E
Effect of oven drying on antioxidant activity, phenolic
compounds, fatty acid composition and tocopherol contents
of pomegranate aril and oils
Mehmet Musa Özcan
1| Fahad Aljuhaimi
2| Nurhan Uslu
1| Isam A. Mohamed Ahmed
2|
Magdi A. Osman
2| Mustafa A. Gassem
2| Hesham A. A. Salih
2 1Faculty of Agricultural, Department ofFood Engineering, Selcuk University, Konya, Turkey
2Department of Food Science & Nutrition, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
Correspondence
Mehmet Musa Özcan, Faculty of Agricultural, Department of Food Engineering, Selcuk University, Konya 42079, Turkey.
Email: mozcan@selcuk.edu.tr Funding information King Saud University
Abstract
The antioxidant activity of Hicaznar and 33 N 16Keben varieties changed between 32.213% and 68.492% and between 47.885% and 85.195%, respectively (p < 0.05). Gallic acid, 3,4‐dihydroxybenzoic acid, (+)‐catechin, 1,2‐dihydroxybenzene, and isor‐ hamnetin were the key phenolic compounds of Hicaznar and 33 N 16Keben arils. While oil contents of Hicaznar aril change between 6.78% (control) and 9.71% (20 hr), oil contents of 33 N 16Keben aril varied between 7.19% (control) and 10.96% (con‐ trol). Punicic was the predominant fatty acid in two pomegranate cultivar oils. While punicic acid contents of Hicaznar aril oil vary between 75.23 (20 hr) and 75.85% (con‐ trol), punicic acid contents of 33 N 16Keben oil changed between 73.81 (20 hr) and 74.79% (control). ɣ‐tocopherol contents of Hicaznar aril oil are determined between 224.86 (20 hr) and 227.84 mg/100 g (control), ɣ‐tocopherol contents of 33 N 16Keben aril oil changed between 284.36 (20 hr) and 289.44 mg/100 g (control).
Practical applications
Pomegranate is one of the most important fruits. For a long time the preservation is very difficult as fresh. The dried fruit should be rehydrated and its usefulness should be investigated. This form can be used as compost or fruit juice by rehydrating the dried product further. The change in the bioactive properties of the post‐drying product will also be demonstrated.
2 of 9
|
ÖZCAN etAl.radical scavenging properties (Kulkarni & Aradhya, 2005; Matthaus & Özcan, 2016). Extracts of pomegranate fruit peels and arils have inhibitory activity against bacteria, with the highest inhibition zones on ethanol extracts (26.3–34.0 mm inhibition zones for peels and 19.7–24.3 mm inhibition zones for arils) (Nuamsetti, Dechayuenyong, & Tantipaibulvut, 2012). The seed oil of pomegranate contain conju‐ gated fatty acid (punicic acid), which is showed a potential to prevent cancer and artheroschlerosis (Soetjipto, Pradipta, & Timotius, 2010). Pomegranate kernel oil is rich in some tocopherols that prevent lipid oxidation and show antioxidant properties (Jing et al., 2012). Freeze‐drying, convective drying (50°C, 60°C, or 70°C), vacuum mi‐ crowave drying (240, 360, or 480 W) and a combined convection pre‐drying and vacuum microwave drying were evaluated in the pomegranate drying process (Calin‐Sanchez et al., 2013). Drying can result in structural, chemical, and phytochemical changes that can affect quality properties of foods (texture, color, and nutritional values) (Attanasio, Cianquanta, & Matteo, 2004). The main aim of drying food products is to preserve and prolong shelf life of various food products by removing water in the solid to a level at which mi‐ crobial spoilage and deterioration resulting from chemical reactions is significantly reduced (Sablani, 2006). Several drying techniques such as air, oven, and freeze‐drying are using for various products in food industry. Generally, air‐drying and oven drying are favored due to processing cost and efficiency (Vega‐Gàlvez et al., 2009). The main objective of current study was to determine the effect of dry‐ ing time on oil content, total phenol, antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents of arils and aril oils of Hicaznar and 33 N 16Keben pomegranate varieties growing Mediterranean region in Turkey.
2 | MATERIAL AND METHODS
2.1 | Materials
Pomegranate fruits were purchased from a local market in Mersin, Turkey. All of them were provided when fully ripe. All samples were kept in refrigerator till analysis. Arils (pulp and seeds) were manually separated. All reagents and solvents were analytical grade and pur‐ chased from Sigma‐Aldrich Co. (St. Louis, MO, USA).
2.2 | Methods
2.2.1 | Drying process
Pomegranate arils were dried in an oven (Nüve FN055 Ankara, Turkey, 55 L volume) at 70°C for 20 hr and taken samples at inter‐ vals of 5 hr fresh and dried (for 5, 10, 15, and 20 hr) samples were analyzed. Initial moisture contents of pomegranate samples were measured at at 105°C.
2.2.2 | Sample extraction
Pomegranate aril samples were extracted according to Madrigal‐ Carballo, Rodriguez, Krueger, Dreher, and Reed (2009) with some
modifications. 0.5 g of ground samples were added to 20 ml of methanol (50% v/v). The mixture was sonicated for 15 min, followed by centrifugation at 6,000 rpm for 10 min. The supernatant was col‐ lected. Prior to injection, the extract was filtered with 0.45 µm nylon filter.
2.2.3 | Total phenolic content
Total phenol contents of pomegranate aril extracts were determined with the Folin–Ciocalteu (FC) reagent according to method of Yoo, Lee, Park, Lee, and Hwang (2004). After 1 ml of Folin–Ciacueltau was added, sample was mixed strongly for 5 min. A 10‐ml Na2CO3 solution was added to the sample, followed by stirring, and the final volume was completed with 25 ml of distilled water. Total phenolic contents of samples were measured at 750 nm in spectrophotom‐ eter at the end of 1 hr, and results were given as mg GAE/100 g. Gallic acid (0–200 mg/ml) was used as the standard for calibration.
2.2.4 | Antioxidant activity
The antioxidant activity of two pomegranate aril samples was deter‐ mined according to DPPH (1,1‐diphenyl‐2‐picrylhydrazyl) reported in study of Lee et al., (1998). The aril extract was mixed with 2 ml methanolic DPPH solution, and the mixture was shaken strongly. After sample was kept at room temperature for 30 min, the absorb‐ ance of sample was measured at 517 nm in a spectrophotometer.
2.2.5 | Determination of phenolic compounds
Phenolic compounds of two pomegranate aril extracts were deter‐ mined by Shimadzu‐HPLC equipped with PDA detector and Inertsil ODS‐3 (5 µm; 4.6 × 250 mm) column. The flow rate of the mobile phase and the injection volume were 1 ml/min at 30°C and 20 µl, respectively. At 280 and 330 nm, the peak records were carried out. The total running time was 60 min for each sample.2.2.6 | Oil extraction
The oil contents of ground aril samples were obtained by petroleum benzine in Soxhlet apparatus for 5 hr, and the solvent was separated with a rotary evaporator at 50°C (AOAC, 1990).
2.2.7 | Fatty acid composition
The fatty acid methyl esters of aril oils were identified by comparing the retention time of the samples and appropriate fatty acids methyl esters standards. The fatty acid methyl ester was injected in a Varian 5,890 gas chromotograph with a capillary column, CP‐Sil 88 (100 m long, 0.25 mm ID, film thickness 0.2 μm) (Matthaus & Özcan, 2016).
2.2.8 | Tocopherol contents
Twenty microliter of a solution of 250 mg of oil in 25 ml of n‐hep‐ tane was directly injected to a Diol phase HPLC column 25 cm × 4.6
mmID (Merck, Darmstadt, Germany) used with a flow rate of 1.3 ml/ min (Balz, Schulte, & Their, 1992; Matthäus & Özcan, 2006).
2.3 | Statistical analysis
The results are mean ± standard deviation (MSTAT C) of pomegran‐ ate aril and oils. All analyses were carried out 3 times (Püskülcü & İkiz, 1989).
3 | RESULTS AND DISCUSSION
The antioxidant activities, moisture and total phenolic contents of fresh and dried pomegranate arils are reported in Table 1. The mois‐ ture contents of Hicaznar pomegranate arils were decreased from 84.322% to 21.232% depending on drying times, while the mois‐ ture contents of 33 N 16Keben pomegranate arils decreased from 81.633% to 23.813%. The DPPH method was used to investigate the effect of free radical scavenging activity of the extracts. The antioxidant activity of Hicaznar and 33 N 16Keben varieties ranged from 32.213% to 68.492% and from 47.885% to 85.195%, respec‐ tively. The total phenolic contents changed between 508.174 and 875.642 for Hicaznar; between 535.960 and 1,221.137 mgGAE/ kg for 33 N 16Keben. Additionally, 33 N 16Keben variety demon‐ strated higher both antioxidant activity and total phenolic content in comparison with variety of Hicaznar. Results showed that both pomegranate varieties were significantly affected from drying times. Similarly, Tontul and Topuz (2017) applied different drying methods of pomegranate pestil and they observed that the antioxidant activ‐ ity and total phenolic of pomegranate pestil are affected by the dry‐ ing conditions. On the other hand, the highest antioxidant activity and total phenolic content of both varieties were observed in sam‐ ples dried for 10 hr. A significant increase in the scavenging effects of the methanol extract of pomegranate varieties was determined at different times of drying compared to control samples. However, the increase appeared to be concentrated the antioxidants during drying. Similarly, Gan, Lui, Chan, and Corke (2017) observed that hot
air‐drying incresaed the phenolic content and antioxidant capacity of mung bean sprouts. In general, there was an important reduction in total phenolic content of both varieties, except the samples dried for 10 hr, when fruits were dried. Indeed, the amount of total phe‐ nolics decreased from 797.297 to 508.174 mg GAE/100 g (DW) for Hicaznar variety; from 1,037.469 to 535.960 mg GAE/100 g (DW) for 33 N 16Keben variety at the last stage of drying process (20 hr). It was observed stastistically significant differences among mois‐ ture contents, antioxidant activiy values, and total phenolic con‐ tents of Hicaznar and 13 N 16Keben pomegranate arils compared to control group (p < 0.05). According to the study of Calin‐Sanchez et al. (2013), total phenolic contents were found 7.57 mg GAE/g (DW) in fresh pomegranate arils (P. granatum L. cv. Mollar de Elche) and 2.01 mg GAE/g (DW) in samples dried at 70°C. Elbandy and Ashoush (2012) demonstrated 14.76 mg/g total phenol and 98.2 an‐ tioxidant activity in pomegranate seed oil. Dried pomegranate peel contains phenolic acid (p‐coumaric), flavan‐3‐ols (+catechin,‐epicat‐ echin), flavanone (hesperidin), flavonol (rutin), ellagitannin (punicalin) (Mphahlele, Fawole, Makunga, & Opara, 2016). Punicalin values of pomegranate peel changed between 559.60 and 888.40 mg/kg dw (Mphahlele et al., 2016), and punicalin is hydrolyzable tannin which is known to account for high antioxidant activity in pomegranate peel (Lin, Hsu, Lin, & Hsu, 2001; Sun, Tao, Men, Xu, & Wang, 2017; Tzulker et al., 2007). In another study, pomegranate juice contained 22.8 ± 0.69 μg/100 g vitamin A, 57.8 ± 0.59 mg/100 g vitamin C, and 0.07 ± 0.01 mg/100 g vitamin E (Anahita, Asmah, & Fauziah, 2015). In addition, total phenol contents of pomegranate juice, pomegranate seed, and pomegranate seed‐juice were determined as 2,502 ± 54, 165 ± 49, and 2,696 ± 49 mg GAE/L, respectively (Anahita et al., 2015). Also, antioxidant activity values of pomegran‐ ate juice, pomegranate seed, and pomegranate seed‐juice were es‐ tablished as 32 ± 5.1, 20 ± 2.8, and 47 ± 5.5 mmol/L, respectively (Anahita et al., 2015). Pomegranate juice is obtained from arils, which are rich sources of bioactive compound like phenolic and flavonoids (Kalaycıoglu & Erim, 2017; Li et al., 2006). Phenolic con‐ tent is the main compound attribute for the most of the functional properties of many fruits such as pomegranate and grapes (Viuda,
Sample Drying time (hr) Moisture (%) Antioxidant activity (%) Total phenolic content (mgGAE/100 g DW)
Hicaznar Control 84.3 ± 0.1*a 32.2 ± 0.02e 797.3 ± 0.01b
5 71.8 ± 0.4b** 44.3 ± 0.01d 732.1 ± 0.01c
10 58.6 ± 0.1c 68.5 ± 0.01a 875.6 ± 0.02a
15 24.9 ± 0.2d 64.4 ± 0.01b 555.5 ± 0.02d
20 21.2 ± 0.3e 59.9 ± 0.01c 508.2 ± 0.01e
33 N 16Keben Control 81.6 ± 0.2a 47.9 ± 0.02e 1,037.5 ± 0.02b
5 73.6 ± 0.32b 65.9 ± 0.01d 969.6 ± 0.01c
10 43.2 ± 0.6c 85.2 ± 0.01a 1,221.1 ± 0.02a
15 25.0 ± 0.6d 76.5 ± 0.01b 574.4 ± 0.01d
20 23.8 ± 0.7e 74.5 ± 0.02c 536.0 ± 0.02e
*Mean ± standard deviation. **Values within each column followed by different letters are signifi‐ cantly different (p < 0.05).
TA B L E 1 Antioxidant activity and total
4 of 9
|
ÖZCAN etAl.Ruiz, Fernandez, & Perez, 2011). Total phenolic content, vitamin C, and antioxidant activity of the fruit (aril) juice obtained from fruits harvested from three locations in the southern dry zone (DL 1a) were 22.2 ± 6.5, 8.7 ± 2.3 g/L, and 17–33 mmol/L, respectively (Amararatne, Weerakkody, & Jayakody, 2012). The total phenolic contents of hot water, ethanol, and acetone extracts of pome‐ granate peels were determined as 87.32 mg GAE/100 g, 72.84 mg GAE/100 g, and 64.60 mg GAE/100 g using the Folin–Ciocalteu method, respectively (Nuamsetti et al., 2012).
Tables 2 and 3 show the phenolic compounds of Hicaznar and 33 N 16Keben varieties, respectively. Gallic acid (111.232 mg/100 g in Hicaznar; 62.953 mg/100 g in 33 N 16Keben), 3,4‐dihydroxybenzoic acid (76.857 mg/100 g in Hicaznar; 42.147 mg/100 g in 33 N 16Keben), (+)‐cat‐ echin (42.309 mg/100 g in Hicaznar; 17.645 mg/100 g in 33 N 16Keben), 1,2‐dihydroxybenzene (86.417 mg/100 g in Hicaznar; 55.335 mg/100 g in 33 N 16Keben), and isorhamne‐ tin (37.291 mg/100 g in Hicaznar; 44.629 mg/100 g in 33 N 16Keben) were determined as the main phenolic compounds of fresh pomegranate arils. The concentrations of catechin and 1,2‐dihydroxybenzene in mg/100 g increased from 17.645 to 372.447 and from 55.335 to 321.003 in 33 N 16Keben variety; from 42.309 to 255.284 and from 86.417 to 220.197 in Hicaznar variety, respectively, when the samples dried for 10 hr. Similarly, Tontul and Topuz (2017) reported that the phenolic profile of pomegranate pestil are affected by drying conditions (method, temperature, and time). On the other hand, drying process caused a decrease in gallic acid content of both Hicaznar (ranging from 111.232 mg/100 g to 45.313 mg/100 g) and 33 N 16Keben (var‐ ied from 66.036 mg/100 g to 52.737 mg/100 g) in comparison with control samples. The reduction of quantities of phenolic
compounds during drying processes has also been observed in different studies (Mphahlele et al., 2016; Tontul & Topuz, 2017). It was observed statistically significant differences among phe‐ nolic compounds of Hicaznar pomegranate arils compared to control group (p < 0.05). But, it was not observed statistically significant differences among 1,2‐dihydroxybenzene contents of Hicaznar pomegranate arils compared to control group during 15 and 20 hr drying times. Also, It was not observed statistically significant differences among trans‐cinnamic acid and naringenin contents of 33 N 16Keben pomegranate arils compared to con‐ trol group during 15 and 20 hr drying times. In the experiments reported by Elfalleh et al., (2011), phenolic compounds of pome‐ granate pulp were observed as gallic acid (17.50 mg/100 g), el‐ lagic acid (23.10 mg/100 g), caffeic acid (13.52 mg/100 g), and p‐coumaric acid (11.45 mg/100 g) and the contents of these phenolics showed differences between genotypes. According to Poyrazoğlu, Gökmen, and Artık (2002), gallic acid was estimated in highest level with 45.57 mg/100 ml. The amounts of p‐couma‐ ric acid and caffeic acid in pomegranate juice were determined as 0.67 and 7.87 mg/100 ml, respectively. Total polyphenol con‐ tents of pomegranate fruit peels were 85.60 ± 4.87 mg GAE/g, dw (Elfalleh et al., 2012). Aqueous and methanolic extracts of pomegranate seed, leave, flower, and peel exhibited good antiox‐ idant activities (Elfalleh et al., 2012). Dried arils contained 1.6 mg eq GA/g total polyphenols and they have high antioxidant capac‐ ity (0.6 mg eq Trolox/g) (Calin‐Sanchez et al., 2013). Antioxidant capacity of pomegranate arils changed between 0.57 and 1.20 mg eq Trolox/g (dw), while total phenol vary between 1.57 and 7.57 mg eq GA/g (dw) (Calin‐Sanchez et al., 2013). The highest pu‐ nicalin (888.04 ± 141.03 mg CE/kg dw) and catechin (674.51 mg/ kg dw) were found in oven drying at 60°C and freeze‐dried peels,
TA B L E 2 Phenolic compounds of pomegranate “Hicaznar” (mg/100 g)
Phenolic compounds
Drying time
Control 5 hr 10 hr 15 hr 20 hr
Gallic acid 111.2 ± 7.9*a 50.8 ± 2.4d 53.9 ± 2.7c 69.8 ± 3.9b 45.3 ± 3.8e
3,4‐Dihydroxybenzoic Aci 76.9 ± 3.4a** 63.9 ± 5.8b 51.5 ± 3.5c 63.6 ± 2.3b 26.8 ± 2.4d
(+)‐Catechin 42.3 ± 2.5e 4,670 ± 13.0d 255.3 ± 11.2a 196.6 ± 13.1b 184.9 ± 15.4c
1,2‐Dihydroxybenzene 86.4 ± 5.7b 71.4 ± 3.7c 220.2 ± 13.2a 55.3 ± 3.7d 55.3 ± 4.5d
Syringic acid 2.4 ± 0.5a 2.5 ± 0.9a 2.6 ± 1.1a 1.9 ± 0.8b 2.2 ± 0.8a
Caffeic acid 2.1 ± 0.3c 1.0 ± 0.3e 1.1 ± 0.9d 2.5 ± 0.9b 3.6 ± 0.7a
Rutin trihydrate 2.0 ± 0.5c 2.7 ± 0.5a 2.0 ± 0.7b 1.0 ± 0.3e 1.9 ± 0.5d
p‐Coumaric acid 0.4 ± 0.2e 0.5 ± 0.3d 0.8 ± 0.3b 1.0 ± 0.5a 0.5 ± 0.3c
trans‐Ferulic acid 1.4 ± 0.1c 1.5 ± 0.3c 1.0 ± 0.3d 2.8 ± 0.7a 2.0 ± 0.9b
Apigenin 7 glucoside 2.3 ± 09c 3.0 ± 0.7b 2.2 ± 0.7d 3.2 ± 0.9a 1.3 ± 0.5e
Resveratrol 1.2 ± 0.7c 2.2 ± 0.9a 1.6 ± 0.5b 1.1 ± 0.3d 0.8 ± 0.3e
Quercetin 8.3 ± 0.9d 9.9 ± 1.1a 8.7 ± 10.1c 9.4 ± 0.7b 4.6 ± 0.9e
trans‐Cinnamic Acid 4.8 ± 0.8d 3.9 ± 0.7e 7.6 ± 1.1a 6.1 ± 0.3c 7.0 ± 1.1b
Naringenin 20.1 ± 1.7a 15.8 ± 012c 12.5 ± 1.9e 16.8 ± 1.6b 12.8 ± 1.3d
Isorhamnetin 37.3 ± 2.2e 60.0 ± 3.5a 48.2 ± 1.1b 44.7 ± 3.1c 43.4 ± 4.2d
respectively. In addition, high level of vitamin C (31.19 μg AAE/g dw) was observed in the oven‐dried (40°C) pomegranate peel (Mphahlele et al., 2016). The curent results are nealy in accor‐ dance with those obtained by Calin‐Sanchez et al. (2013), Başlar, Karasu, Kiliçli, Us, and Sağdiç (2014) and Poyrazoğlu et al., (2002). This study provided valuable information for developing value‐ added utilization of pomegranate fruits as nutraceuticals.
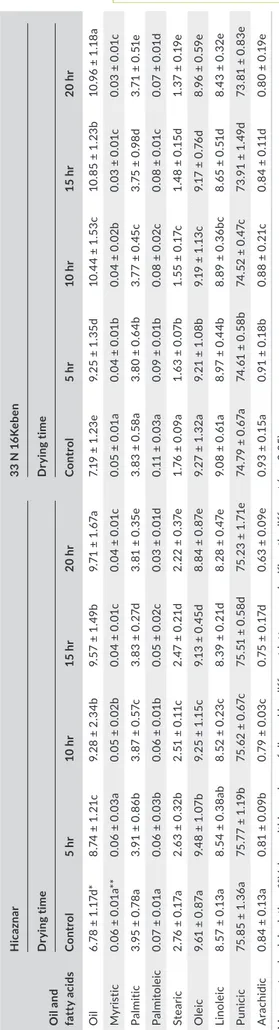
Oil content and fatty acid compositions of dried pomegranate arils of Hicaznar and 33 N 16Keben pomegranate fruits are given in Table 4. Oil contents of Hicaznar and 33 N 16Keben arils increased depending on drying times compared to control group. While oil con‐ tents of Hicaznar aril oil change between 6.78% (control) and 9.71% (20 hr), oil contents of 33 N 16Keben aril varied between 7.19% (con‐ trol) and 10.96% (control). This range was in agreement with results (4.44 and 13.70% for Valencia and Katırbaşı cultivars, respectively) of Fernandes et al. (2015), and results (11.4% and 14.8% for the Suanshiliu and Sanbaitian varieties) of Jing et al. (2012). Generally, fatty acids of Hicaznar aril oil were found higher than those of fatty acid values of 33 N 16Keben aril oil. Fatty acid compositions of both varieties were affected from drying depending on drying times. So, fatty acids of both varieties compared to control group decreased depending on drying times. However, Cano‐Lamadrid et al. (2018) reported that fatty acids of pomegranate pomace were not affected by drying temperature and microwave power. The dif‐ ference between these studies could be attributed to the variation in the pomegranate genotypes, drying conditions, and parts used (arils and pomace). Generally, unsaturated fatty acids were affected more compared to saturated fatty acids of aril oils. Oleic acid con‐ tents of Hicaznar aril oil are determined between 9.61% (20 hr) and 8.84% (control), oleic acid contents of 33 N 16Keben aril oil changed
between 8.96% (20 hr) and 9.27% (control). Also, while punicic acid contents of Hicaznar aril oil vary between 75.23% (20 hr) and 75.85% (control), punicic acid contents of 33 N 16Keben oil ranged from 73.81% (20 hr) to 74.79% (control). In addition, while linoleic acid contents of Hicaznar aril oil change between 8.28% (20 hr) and 8.57% (control), linoleic acid contents of 33 N 16Keben aril oil var‐ ied between 8.43% (20 hr) and 9.08% (control). Punicic, oleic, and linoleic acids were the predominant fatty acid of two pomegran‐ ate cultivar oils. It was observed statistically significant differences among phenolic compounds of Hicaznar and 33 N 16Keben pome‐ granate aril oils compared to control group (p < 0.05). But, it was not observed statistically significant differences among myristic acid contents of Hicaznar and 33 N 16Keben pomegranate aril oils com‐ pared to control group during 15 and 20 hr drying times. In previous study, Habinnia, Ghavami, Ansaripour, and Vosough (2012) deter‐ mined 2.95%–3.57% palmitic, 1.99%–2.54% stearic, 5.71%–7.48% oleic, 5.22%–7.08% linoleic, and 78.25%–82.40% punicic acids in pomegranate seed oil samples. In other study, Melo, Carvalho, and Mancini‐Filho (2014) studied on fatty acid of pomegranate seed oil, and they determined 2.45%–5.70% palmitic, 1.52%–4.20% stearic, 4.19%–9.00% oleic, 4.08%–10.80% linoleic, and 36.98%–81.22% pu‐ nicic acids in pomegranate seed oils. Fernandes et al. (2015) stated that pomegranate seed oil contained 77.3% and 83.6% punicic acid, and followed by linoleic, oleic, and palmitic acids. Pomegranate seeds are a rich origins of polyunsaturated mostly linolenic (n‐3), and linoleic (n‐2) (Li et al., 2006). According to results, the pomegranate are rich source of phytochemicals. Our fatty acid composition was partly similar to previous results (Fernandes et al., 2015; Habinnia et al., 2012; Hernandez, Melgarejo, Martnez, Martnez, & Legua, 2011; Melo et al., 2014).
TA B L E 3 Phenolic compounds of pomegranate “33 N 16Keben”
Phenolic compounds (mg/100 g)
Drying time
Control 5 hr 10 hr 15 hr 20 hr
Gallic acid 66.0 ± 3.5*a 63.0 ± 4.2b 53.9 ± 3.9d 59.0 ± 7.6c 52.7 ± 3.2e
3,4‐Dihydroxybenzoic acid 42.1 ± 2.7c** 36.0 ± 3.1d 34.8 ± 5.3e 55.4 ± 4.3a 50.8 ± 5.6b
(+)‐Catechin 17.6 ± 1.3d 200.6 ± 12.4b 372.4 ± 13.7a 100.2 ± 5.8c 62.6 ± 7.6e
1,2‐Dihydroxybenzene 55.3 ± 6.2c 98.6 ± 8.1b 321.0 ± 15.3a 38.8 ± 2.7d 23.3 ± 3.2e
Syringic acid 2.5 ± 1.1e 6.3 ± 1.7d 8.4 ± 1.1c 12.5 ± 3.1b 15.9 ± 1.4a
Caffeic acid 2.2 ± 0.9d 1.5 ± 0.9e 4.2 ± 1.3c 21.8 ± 2.3a 11.6 ± 1.3b
Rutin trihydrate 1.5 ± 0.7e 4.3 ± 1.3c 2.5 ± 1.0d 9.1 ± 1.3a 6.0 ± 1.1b
p‐Coumaric acid 0.4 ± 0.2d 0.7 ± 0.3c 0.2 ± 0.1e 1.4 ± 0.9a 1.0 ± 0.9b
trans‐Ferulic acid 1.4 ± 0.3c 1.2 ± 0.7d 1.0 ± 0.3e 5.9 ± 1.1a 4.4 ± 1.1b
Apigenin 7 glucoside 1.4 ± 0.5e 2.4 ± 0.5c 2.0 ± 0.5d 5.6 ± 1.7a 4.1 ± 0.9b
Resveratrol 1.1 ± 0.3d 1.4 ± 0.3c 1.0 ± 0.3e 1.6 ± 0.7b 1.8 ± 0.7a
Quercetin 6.8 ± 1.1b 5.6 ± 1.4d 4.5 ± 1.6e 6.7 ± 1.3c 8.3 ± 1.9a
trans‐Cinnamic acid 8.0 ± 1.3a 1.8 ± 0.9b 0.8 ± 0.3d 0.8 ± 0.3d 1.1 ± 0.5 cd
Naringenin 9.5 ± 1.2c 21.2 ± 1.7a 12.7 ± 3.1b 3.7 ± 1.2d 3.1 ± 1.4d
Isorhamnetin 44.6 ± 6.1c 47.7 ± 5.2b 48.7 ± 3.2a 11.2 ± 1.1e 12.0 ± 3.1d
6 of 9
|
ÖZCAN etAl. T A B LE 4 O il c on te nt s a nd f at ty a ci d c om po si tio n o f “ H ic az na r” a nd “ 33 N 1 6K eb en ” a ril s ( % ) O il an d fa tt y a ci ds H ic azn ar 33 N 1 6K eb en D ry in g tim e D ry in g tim e C on tr ol 5 h r 10 h r 15 h r 20 h r C on tr ol 5 h r 10 h r 15 h r 20 h r O il 6. 78 ± 1 .1 7d* 8. 74 ± 1 .2 1c 9. 28 ± 2 .3 4b 9. 57 ± 1 .4 9b 9. 71 ± 1 .6 7a 7. 19 ± 1 .2 3e 9. 25 ± 1 .3 5d 10 .4 4 ± 1. 53 c 10 .8 5 ± 1. 23 b 10 .9 6 ± 1. 18 a M yr is tic 0.0 6 ± 0.0 1a ** 0.0 6 ± 0.0 3a 0.0 5 ± 0.0 2b 0.0 4 ± 0.0 1c 0.0 4 ± 0.0 1c 0.0 5 ± 0.0 1a 0.0 4 ± 0.0 1b 0.0 4 ± 0.0 2b 0.0 3 ± 0.0 1c 0.0 3 ± 0.0 1c Pa lmi tic 3. 95 ± 0 .78 a 3. 91 ± 0 .8 6b 3. 87 ± 0 .5 7c 3. 83 ± 0 .2 7d 3. 81 ± 0 .3 5e 3. 83 ± 0 .5 8a 3. 80 ± 0 .6 4b 3. 77 ± 0 .4 5c 3. 75 ± 0 .9 8d 3. 71 ± 0 .5 1e Pa lmi to leic 0.0 7 ± 0.0 1a 0.0 6 ± 0.0 3b 0.0 6 ± 0.0 1b 0.0 5 ± 0.0 2c 0.0 3 ± 0.0 1d 0. 11 ± 0.0 3a 0.0 9 ± 0.0 1b 0.0 8 ± 0.0 2c 0.0 8 ± 0.0 1c 0.0 7 ± 0.0 1d St ea ric 2. 76 ± 0 .1 7a 2. 63 ± 0 .3 2b 2. 51 ± 0 .1 1c 2. 47 ± 0 .2 1d 2. 22 ± 0 .3 7e 1. 76 ± 0 .0 9a 1. 63 ± 0 .0 7b 1. 55 ± 0 .1 7c 1. 48 ± 0 .1 5d 1. 37 ± 0 .1 9e O leic 9. 61 ± 0 .8 7a 9. 48 ± 1 .0 7b 9. 25 ± 1 .1 5c 9. 13 ± 0 .4 5d 8.8 4 ± 0.8 7e 9. 27 ± 1 .3 2a 9. 21 ± 1 .0 8b 9.1 9 ± 1.1 3c 9. 17 ± 0 .7 6d 8. 96 ± 0 .5 9e Lin ol eic 8. 57 ± 0 .1 3a 8. 54 ± 0 .3 8a b 8. 52 ± 0 .2 3c 8. 39 ± 0 .2 1d 8. 28 ± 0 .4 7e 9. 08 ± 0 .6 1a 8. 97 ± 0 .4 4b 8. 89 ± 0 .3 6b c 8. 65 ± 0 .5 1d 8. 43 ± 0 .3 2e Pu nic ic 75 .8 5 ± 1. 36 a 75 .7 7 ± 1. 19b 75 .6 2 ± 0. 67 c 75 .5 1 ± 0. 58 d 75 .2 3 ± 1. 71e 74 .7 9 ± 0. 67 a 74 .6 1 ± 0. 58 b 74 .5 2 ± 0. 47 c 73 .9 1 ± 1. 49 d 73 .8 1 ± 0. 83 e A ra ch idic 0. 84 ± 0 .1 3a 0. 81 ± 0 .0 9b 0. 79 ± 0.0 3c 0. 75 ± 0 .1 7d 0. 63 ± 0.0 9e 0. 93 ± 0 .1 5a 0. 91 ± 0 .1 8b 0. 88 ± 0 .2 1c 0. 84 ± 0 .1 1d 0. 80 ± 0 .1 9e *M ea n ± s ta nd ar d d ev ia tio n. * *V al ue s w ith in e ac h r ow f ol lo w ed b y d iff er en t l et te rs a re s ig ni fic an tly d iff er en t ( p < 0.0 5) . T A B LE 5 To co ph er ol c on te nt s o f “ H ic az na r” a nd “ 33 N 1 6K eb en ” a ril o ils ( m g/ 10 0 g) Toc oph er ol s H ic azn ar 33 N 1 6K eb en D ry in g tim e D ry in g tim e C on tr ol 5 h r 10 h r 15 h r 20 h r C on tr ol 5 h r 10 h r 15 h r 20 h r α‐ to co phe ro l 98 .6 3 ± 1. 17 *a 98 .5 5 ± 2. 67 a 97 .8 8 ± 3. 65 b 97 .2 8 ± 2. 83 c 96 .7 4 ± 1. 52d 87 .8 6 ± 3. 49 a 87 .7 5 ± 2. 61 ab 87 .1 8 ± 3. 84 b 86. 75 ± 1 .38c 85 .4 7 ± 1. 38 d ɣ‐ to co phe ro l 22 7. 84 ± 7. 65 a* * 22 6. 48 ± 9 .6 5b 22 5. 64 ± 7 .4 9c 22 5. 07 ± 6 .9 2c 22 4. 86 ± 5 .9 8d 28 9. 44 ± 9 .6 4a 28 6. 47 ± 8 .5 1b 28 6. 21 ± 7 .5 6c 28 5. 16 ± 1 1. 89 d 28 4. 36 ± 1 4. 87 e δ‐ to co phe ro l 6. 17 ± 1 .3 8a 6. 07 ± 1 .5 4a b 5. 97 ± 1 .0 9b 5. 81 ± 1 .7 3c 5. 38 ± 1 .7 3 5. 61 ± 2 .6 8a 5. 50 ± 1 .4 6b 5. 43 ± 1 .9 5c 5. 09 ± 1 .2 7d 4. 93 ± 1 .2 9e *M ea n ± s ta nd ar d d ev ia tio n. * *V al ue s w ith in e ac h r ow f ol lo w ed b y d iff er en t l et te rs a re s ig ni fic an tly d iff er en t ( p < 0.0 5) .Tocopherol contents of dried pomegranate aril oils belong to Hicaznar and 33 N 16Keben pomegranate varieties are shown in Table 5. Tocopherol contents of Hicaznar and 33 N 16Keben decreased compared to control group and depending on drying times. While α‐tocopherol contents of Hicaznar aril oil change between 96.7 mg/100 g (20 hr) and 98.63 mg/100 g (control), α‐tocopherol contents of 33 N 16Keben aril oil varied between 85.47 mg/100 g (20 hr) and 87.86 mg/100 g (control). In addi‐ tion, ɣ‐tocopherol contents of Hicaznar aril oil are determined between 224.86 mg/100 g (20 hr) and 227.84 mg/100 g (con‐ trol), ɣ‐tocopherol contents of 33 N 16Keben aril oil changed be‐ tween 284.36 mg/100 g (20 hr) and 289.44 mg/100 g (control). Generally, α‐and ɣ‐tocopherol contents of Hicaznar aril oil were found higher compared to α‐and ɣ‐tocopherol contents of 33 N 16Keben aril oil (except δ‐tocopherol) It was observed ststisti‐ cally partially differences among tocopherol contents of Hicaznar and 33 N 16Keben pomegranate aril oils compared to control group (p < 0.05). Fernandes et al. (2015) determined 159.7 and 586.2 mg/100 g ɣ‐tocopherol, 8.9 and 26.1 mg/100 g α‐tocoph‐ erol, and 6.0 and 15.2 mg/100 g δ‐tocopherol in pomegranate seed oil. Our ɣ‐tocopherol contents (224–227.84 mg/100 g for Hicaznar and 284.36–289.44 mg/100 g for Keben) were similar to results (120.62 and 672.56 mg/100 g oil) of Liu et al. (2012). On the other hand, our α‐tocopherol values 96.74 to 98.63 mg/100 g for Hicaznar and 85.47 and 87.86 for 33 N 16Keben) were found lower than results (between 161.2 and 173.7 mg/100 g) of Pande and Akoh (2009). In other study, 543.6–1,134.6 ppm α‐tocopherol and 1856.6–7,106.1 ppm ɣ‐tocopherol were determined in pome‐ granate seed oils (Habinnia et al., 2012). In the current study, the following order was found: ɣ‐tocopherol > α‐tocopherol > δ‐to‐ copherol. These differences can be probably due to climatic factor, cultivation, and varieties.
4 | CONCLUSION
Drying significantly affected the chemical composition, phenolic com‐ pounds, and bioactive properties attributes of pomegranate and arils. In general, when drying process, significant reductions in the con‐ tents of total polyphenols and phenolic compounds were observed. The traditional drying method has disadvantages for nutritional com‐ pounds and degradation of foods in the high temperature. The ob‐ servations made in this study reveal that the pomegranate arils are rich in phenolic compounds with potentially high antioxidant activities. Pomegranate aril oil is rich in punicic acid and ɣ‐tocopherol, although contain other tocopherols in minor amounts.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through re‐ search group no. RG‐1439‐80. Technical support of RSSU at King Saud University is also well appreciated.
CONFLIC T OF INTEREST
The authors have declared no conflicts of interest for this article.
ORCID
Mehmet Musa Özcan https://orcid.org/0000‐0002‐5024‐9512
Isam A. Mohamed Ahmed https://orcid.org/0000‐0002‐6578‐0795
REFERENCES
Amararatne, D. I. M., Weerakkody, W. A. P., & Jayakody, J. A. L. P. (2012). Bioactive properties of fruit juice of pomegranate (Punica granatum) grown in dry regions of Sri Lanka. Tropical Agricultural Research, 23(4), 370–375. doi:10.4038/tar.v23i4.4873
Anahita, A., Asmah, R., & Fauziah, O. (2015). Evaluation of total phenolic content, total antioxidant activity, and antioxidant vitamin compo‐ sition of pomegranate seed and juice. International Food Research
Journal, 22(3), 1212–1217.
AOAC (1990). Official Methods of Analysis, 15th ed.. Washington, DC: Author.
Attanasio, G., Cianquanta, L., & Matteo, M. D. (2004). Effect of drying temperature on physic‐chemical properties of dried and rehydrated chestnuts (Castanea sativa). Food Chemistry, 88, 583–590. https://doi. org/10.1016/j.foodchem.2004.01.071
Balz, M., Schulte, E., & Their, H. P. (1992). Trennung von tocophero‐ len und tocotrienolen durch HPLC. Fat Science and Technology, 94, 209–213. https://doi.org/10.1002/lipi.19920940604
Başlar, M., Karasu, S., Kiliçli, M., Us, A. A., & Sağdiç, O. (2014). Degradation kinetics of bioactive compounds and antioxidant ac‐ tivityof pomegranate arils during the drying process. International
Journal of Food Engineering, 10(4), 839–848. https://doi.org/10.1515/
ijfe‐2014‐0080
Bhat, M. M., Thakur, N. S., & Jindal, N. (2014). Studies on the effect of drying methods and packaging on quality and shelf life of dried wild pomegranate arils. Asian Journal of Dairying and Foods Research, 33(1), 18–24. https://doi.org/10.5958/j.0976‐0563.33.1.005
Calin‐Sanchez, A., Figiel, A., Hernandez, F., Melgarejo, P., Lech, K., & Carbonell‐Barrachina, A. A. (2013). Chemical composition, an‐ tioxidant capacity, and sensory quality of pomegranate (Punica
granatum L.) arils and rind as affected by drying method. Food and Bioprocess Technology, 6, 1644–1654. https://doi.org/10.1007/
s11947‐012‐0790‐0
Cano‐Lamadrid, M., Lech, K., Calın‐Sanchez, A., Rosas‐Burgos, E. C., Figiel, A., Wojdyło, A., … Carbonell‐Barrachina, A. A. (2018). Quality of pomegranate pomace as affected by drying method. Journal
of Food Science and Technology, 55(3), 1074–1082. https://doi.
org/10.1007/s13197‐017‐3022‐9
Elbandy, M. A., & Ashoush, I. S. (2012). Phytochemicals in pomegranate seeds and their effect as hypolipidemic agent in hypercholesterol‐ emic rats. World Journal of Dairy & Food Sciences, 7, 85–92. https:// doi.org/10.5829/idosi.wjdfs.2012.7.1.1107
Elfalleh, W., Hannachi, H., Tlili, N., Yahia, Y., Nasri, N., & Ferchichi, A. (2012). Total phenolic contents and antioxidant activities of pome‐ granate peel, seed, leaf and flower. Journal of Medicinal Plants
Research, 6, 4724–4730. https://doi.org/10.5897/JMPR11.995
Elfalleh, W., Tlili, N., Nasri, N., Yahia, Y., Hannachi, H., Chaira, N., … Ferchichi, A. (2011). Antioxidant capacities of phenolic com‐ pounds and tocopherols from Tunisian pomegranate (Punica gra‐
natum) fruits. Journal of Food Science, 76, 707–771. https://doi.
org/10.1111/j.1750‐3841.2011.02179.x
Fernandes, L., Pereira, J. A., Lopez‐Cortes, I., Salazar, D. M., Ramalhosa, E., & Casal, S. (2015). Lipid composition of seed oils of different
8 of 9
|
ÖZCAN etAl.pomegranate (Punica granatum L.) cultivars from Spain. International
Journal of Food Studies, 4, 95–103.
Gan, R.‐Y., Lui, W.‐Y., Chan, C.‐L., & Corke, H. (2017). Hot air drying in‐ duces drowning and enhances phenolic content and antioxidant capacity in mung bean (Vigna radiata L.) sprouts. Journal of Food
Processing and Preservation, 41(1), e12846. https://doi.org/10.1111/
jfpp.12846
Goula, A. M., & Adamopoulos, K. G. (2012). A method for pomegran‐ ate seed application in food industries: Seed oil encapsulation. Food
and Bioproducts Processing, 90(4), 639–652. https://doi.org/10.1016/
j.fbp.2012.06.001
Habinnia, M., Ghavami, M., Ansaripour, M., & Vosough, S. (2012). Chemical evaluation of oils extracted from five different variet‐ iesof Iranian pomegranate seeds. Journal of Food Biosciences and
Technology, 2, 35–40.
Hernandez, F., Melgarejo, P., Martnez, J. J., Martnez, R., & Legua, P. (2011). Fatty acid composition of seed oils from important Spanish pomegranate cultivars. Italian Journal of Food Science, 23(2), 188–193. Jing, P., Ye, T., Shi, H., Sheng, Y., Slavin, M., Gao, B., … Yu, L. (2012).
Antioxidant properties and phytochemical composition of China‐ grown pomegranate seeds. Food Chemistry, 132, 1457–1464. https:// doi.org/10.1016/j.foodchem.2011.12.002
Kalaycıoglu, Z., & Erim, F. B. (2017). Total phenolic contents, antioxi‐ dant activities, and bioactive ingredients of juices from pomegran‐ ate cultivars worldwide. Food Chemistry, 221, 496–507. https://doi. org/10.1016/j.foodchem.2016.10.084
Kulkarni, A. P., & Aradhya, S. M. (2005). Chemical changes and an‐ tioxidant activity in pomegranate arils during fruit develop‐ ment. Food Chemistry, 93, 319–324. https://doi.org/10.1016/j. foodchem.2004.09.029
Lee, S. K., Mbwambo, Z. H., Chung, H. S., Luyengi, L., Games, E. J. C., & Mehta, R. G. (1998). Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen, 1, 35–46.
Li, Y., Guo, C., Yang, J., Wei, J., Xu, J., & Cheng, S. (2006). Evaluation of an‐ tioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Journal of Food Chemistry, 96(2), 254–260. https://doi.org/10.1016/j.foodchem.2005.02.033
Lin, C. C., Hsu, Y. F., Lin, T. C., & Hsu, H. Y. (2001). Antioxidant and hepa‐ toprotective effects of punicalagin and punicalin on acetaminophen‐ induced liver damage in rats. Phytotherapy Research, 15, 206–212. https://doi.org/10.1002/ptr.816
Liu, G., Xu, X., Gong, Y., He, L., & Gao, Y. (2012). Effects of supercrit‐ ical CO2 extraction parameters on chemical composition and free radical‐scavenging activity of pomegranate (Punica granatum L.) seed oil. Food and Bioproducts Processing, 90(3), 573–578. https://doi. org/10.1016/j.fbp.2011.11.004
Madrigal‐Carballo, S., Rodriguez, G., Krueger, C. G., Dreher, M., & Reed, J. D. (2009). Pomegranate (Punica granatum) supplements: Authenticity, antioxidant and polyphenol composition. Journal of Functional Foods,
1(3), 324–329. https://doi.org/10.1016/j.jff.2009.02.005
Matthäus, B., & Özcan, M. M. (2006). Quantitation of fatty acids, ste‐ rols, and tocopherols in turpentine (Pistacia terebinthus Chia) grow‐ ing wild in Turkey. Journal of Agriculture and Food Chemistry, 54(20), 7667–7671. https://doi.org/10.1021/jf060990t
Matthaus, B., & Özcan, M. M. (2016). Pomegranate plant (Punica grana‐
tum L.) composition, antioxidant activity, therapeutic effect, antimi‐
crobial activity – A review. Zeitschrift Fur Arznei‐& Gewurzpflanzen,
21, 160–167.
Melo, I. L. P., Carvalho, E. B. T., & Mancini‐Filho, J. (2014) Pomegranate seed oil (Punica granatum L.): A source of punicic acid (conjugated α‐linolenic acid). Journal of Human Nutrition & Food Science 2(1), 1024–1035.
Mousavinejad, G., Emam‐Djomeh, Z., & Rezaei, K. (2009). Identification and quantification of henolic compouds and their effects on
anioxidant activity in pomegranate juices of eight Iranian culti‐ vars. Food Chemistry, 115, 1274–1278. https://doi.org/10.1016/ j.foodchem.2009.01.044
Mphahlele, R. R., Fawole, O. L., Makunga, N. P., & Opara, U. L. (2016). Effect of drying on the bioactive compounds, antioxidant, anti‐ bacterial and antityrosinase activities of pomegranate peel. BMC
Complementary and Alternative Medicine, 16, 143. https://doi.
org/10.1186/s12906‐016‐1132‐y
Negi, P. S., & Jayaprakasha, G. K. (2003). Antioxidant and antibacterial activities of Punica granatum peel extracts. Journal of Food Science,
68(4), 1473–1477. https://doi.org/10.1111/j.1365‐2621.2003.
tb09669.x
Nuamsetti, T., Dechayuenyong, P., & Tantipaibulvut, S. (2012). Antibacterial activity of pomegranate fruit peels and arils.
Science Asia, 38, 319–322. https://doi.org/10.2306/scienceasia1
513‐1874.2012.38.319
Pande, G., & Akoh, C. C. (2009). Antioxidant capacity and lipid charac‐ terization of six Georgia‐grown pomegranate cultivars. Journal of
Agriculture and Food Chemistry, 57(20), 9427–9436.
Poyrazoğlu, E., Gökmen, V., & Artık, N. (2002). Organic acids and pheno‐ lic compounds in pomegranates (Punica granatum L.) grown in Turkey.
Journal of Food Composition and Analysis, 15, 567–575. https://doi.
org/10.1016/S0889‐1575(02)91071‐9
Prakash, C. V. S., & Prakash, I. (2011). Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel – A review.
International Journal of Research in Chemistry and Environment, 1,
1–18.
Püskülcü, H., & İkiz, F. (1989). Introduction to statistic (p. 333). İzmir, Turkey: Bilgehan Press. (in Turkish).
Rowayshed, G., Salama, A., Abul‐Fadl, M., Akila‐Hamza, S., & Mohamed, E. A. (2013). Nutritional and chemical evaluation for pomegran‐ ate (Punica granatum L.) fruit peel and seeds powders by products.
Middle East Journal of Applied Sciences, 3, 169–179.
Sablani, S. S. (2006). Drying of fruits and vegetables: Retention of nutri‐ tional/functional quality. Drying Technology, 24, 123–135. https://doi. org/10.1080/07373930600558904
Singh, R. P., Murthy, K. N., & Jayaprakasha, G. K. (2002). Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. Journal of Agriculture and Food
Chemistry, 50, 81–86.
Soetjipto, H., Pradipta, M., & Timotius, K. H. (2010). Fatty acids compo‐ sition of red and purple pomegranate (Punica granatum L.) seed oil.
Indonesian Journal of Cancer Chemoprevention, 1, 74–77. https://doi.
org/10.14499/indonesianjcanchemoprev1iss2pp74‐77
Sun, Y.‐Q., Tao, X., Men, X.‐M., Xu, Z.‐W., & Wang, T. (2017). In vitro and
in vivo antioxidant activities of three major polyphenolic compounds
in pomegranate peel: Ellagic acid, punicalin, and punicalagin. Journal
of Integrative Agriculture, 16(8), 1808–1818. https://doi.org/10.1016/
S2095‐3119(16)61560‐5
Tontul, I., & Topuz, A. (2017). Effects of different drying methods on the physicochemical properties of pomegranate leather (pestil). LWT –
Food Science and Technology, 80, 294–303. https://doi.org/10.1016/
j.lwt.2017.02.035
Tzulker, R., Glazer, I., Ilan, I. B., Holland, D., Aviram, M., & Amir, R. (2007). Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. Journal of Agriculture and Food Chemistry,
55, 9559–9570. https://doi.org/10.1021/jf071413n
Vega‐Gàlvez, A., Di Scala, K., Rodriguez, K., Lemus‐Mondaca, R., Miranda, M., & Lopez, J. (2009). Effect of air‐drying temperature on physico‐chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum L. var. hun‐
garian). Food Chemistry, 117, 647–653. https://doi.org/10.1016/
Viuda, M. M., Ruiz, N. Y., Fernandez, L. J., & Perez, J. A. (2011). Spices as functional foods: A review. Journal of Critical Reviews in Food Science
and Nutrition, 51(1), 13–28.
Yoo, K. M., Lee, K. W., Park, J. B., Lee, H. J., & Hwang, I. K. (2004). Variation in major antioxidants and total antioxidant activity of Yuzu (Citrusjunos SiebexTanaka) during maturation and between cultivars.
Journal of Agriculture and Food Chemistry, 52, 5907–5913. https://doi.
org/10.1021/jf0498158
How to cite this article: Özcan MM, Aljuhaimi F, Uslu N, et al.
Effect of oven drying on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents of pomegranate aril and oils. J Food Process Preserv.