Contents lists available atScienceDirect
Materials Science & Engineering C
journal homepage:www.elsevier.com/locate/msecPreparation of pillar[5]arene immobilized trypsin and its application in
microwave-assisted digestion of Cytochrome c
Keziban Atacan
a,⁎, Ahmed Nuri Kursunlu
b, Mustafa Ozmen
baBiomedical, Magnetic and Semiconductor Materials Application and Research Center (BIMAS-RC), Sakarya University, 54187 Sakarya, Turkey bDepartment of Chemistry, Faculty of Science, Selçuk University, 42250 Konya, Turkey
A R T I C L E I N F O Keywords: Pillar[5]arene Trypsin Immobilization Cytochrome c Microwave digestion A B S T R A C T
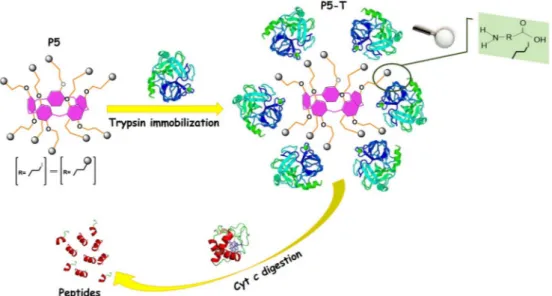
This work presents the immobilization of trypsin on the synthesized pillar[5]arene derivative (P5) containing ten iodo-terminals. The characterization has been carried out by Thermogravimetric Analysis (TGA), Fourier Transform Infrared (FTIR) and Raman spectra, Scanning Electron Microscopy (SEM) and Zeta Potential. Furthermore, Cytochrome c (Cyt c) was chosen as a model protein for evaluation of the performance of the pillar [5]arene-immobilized trypsin (P5-T), and its microwave-assisted digestion conditions were investigated by using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) for 15 second. The peptide numbers of 10 and sequence coverage of 93% for 15 second microwave-assisted digestion were obtained forP5-T. The results can be further applied in future proteomics studies due to high efficiency, good reproducibility and stability of the pillar[5]arene-immobilized trypsin.
1. Introduction
Macromolecular compounds have a remarkable potential for med-icine, biochemistry and chemistry. The novel derivatives of macrocyclic molecules can contribute the development of supramolecular science. Among the macromolecular compounds, pillararenes have a perfect inclusion complex character owing to the function with several organic groups on the aromatic units. Pillar[n]arenes have beenfirstly prepared in 2008 and they have a great potential to be used as sensitive binders for a range of organic/inorganic compounds [1]. Pillar[n]arenes are unique tubular-shaped macromolecules after other host supramolecules (crown ether, calixarene, porphyrin, cucurbituril, cyclodextrin etc.) including large cavity and exhibit unique inclusion properties [2–4]. Pillar[n]arenes can easily encapsulate to smaller molecules due to their tubular structures. They are generally synthesized by a Lewis acid (BF3.OEt2) from a 1,4-dialkoxybenzene group and paraformaldehyde [1]. Pillararenes which possess novel host–guest binding properties and a type of macromolecules, are consist of organic group bound by eCH2e units at 1, 4 positions [5]. Pillar[5]arenes, which containfive repeating units, can be obtained in high yields so they have most widely used in numerous applications. Ogoshi et al. obtained a new pillar[5] arene derivatives in 2011 and published a lot of papers, and the studies have speeded up pillararene science [4]. Their excellent symmetrical architecture differs from the known calixarene compounds that these
macromolecules have been detected to link both neutral and ionic groups [6,7].
The properties of a useful host compound can be listed by the fol-lowing points, (i) ergonomic shape, (ii) cheap synthesis, (iii) highly solubility, (iv) easy functionality, (v) homologues and host−guest ability-cavity size. Before pillar[n]arenes, the most well-known host molecules are cyclodextrins, crown ethers, calix[n]arenes, cucurbit[n] urils. Pillar[n]arenes combine many of the interesting features of the all well-known macrocyclic compounds. Cyclodextrins homologues can be provided from starch using an enzymatic process at a moderate price. In contrast, other host-molecules can only be produced by expensive synthetic methods in low yields [8,9]. On the other hand, excellent shape of pillar[5]arenes having decameric branches and appropriate cavity enables to more complexometric interactions in enzymatic ap-plications when other macromolecules compared [10].
The pillar[n]arenes can be practically derived and functionalized for many applications. The pillar[n]arenes with 5 or 6-member rings can be generally synthesized by the condensation of 1,4-dialkoxybenzene and paraformaldehyde using BF3.(OC2H5) or FeCl3as catalyst in different solvents. Other than these catalysts, although various catalysts have been tried for the synthesis of pillar[n]arenes, sufficient yield has not been achieved [11]. So, they facilitate many amazing molecular structure such as controllable release, bioimaging, stabilization of na-noparticles, catalysis and drug delivery [12,13]. In spite of the crucial
https://doi.org/10.1016/j.msec.2018.10.043
Received 6 December 2017; Received in revised form 6 September 2018; Accepted 9 October 2018 ⁎Corresponding author.
E-mail address:kezibanatacan@sakarya.edu.tr(K. Atacan).
Available online 11 October 2018
0928-4931/ © 2018 Elsevier B.V. All rights reserved.
attention that pillararenes have received from the researchers, their biological functions have been rarely investigated [6].
Cytochrome c (Cyt c) is a highly water-soluble protein and has a molecular weight of 12 kDa consisting of a single 104 amino acid. A tryptic digest of a small (and relatively inexpensive) protein such as Cyt c can be used for performance of analytical instrumental [14]. The tryptic enzymes have gained a great deal of attention to carry out highly efficient and low-level protein digestion, which could be performed in a few minutes or even seconds [15]. The protein immobilization is very important for responsiveness and reversibility in supramolecular chemistry [16,17]. The host–guest couples in supramolecular chemistry are specially a hopeful candidate for the surface-immobilized protein due to the cavity diameter of macromolecules [18,19]. Proteins' quantitation is vital for the monitoring of disease progression, early diagnosis [20]. Mass spectrometry (MS) is a popular technique for protein identification [21]. Mass spectrometry-based proteomics is currently the most valuable analytical tool for identification of proteins present in complex biological samples such as cell lysates, bodyfluids, or tissues. Proteins can be enzymatically or chemically digested into peptide fragments, before a MS analysis. Peptide mass mapping and MS/MS sequence analysis are the key methods currently used in protein identification [22]. The immobilized proteolytic enzymes define a number of benefits including increased stability of activity, reduced extent of autodigestion, and ease of reusability [23].
Microwave technology is an alternative approach which speeds up the enzymatic cleavage of proteins for mass spectrometry analysis by reducing the time of digestion from several hours to a few minutes in many biological applications [24]. Microwaves have been applied to digest standard protein solutions, such as Cyt c, myoglobin, lysozyme, bovine serum albumin and proteins captured on the affinity surfaces of protein chips as well as ultrafast microwave-assisted in-tip digestion of standard proteins and milk extract. Moreover, the low cost, enhanced speed and digestion efficiency are big advantages of microwave irra-diation over other tools accelerating proteolysis for high-throughput analysis [25,26].
Although some papers on pillararene derivatives have been pub-lished, there has been no investigation on the interaction between pil-lararenes and an enzyme for the digestion of a protein. The goal of this research is to prepareP5 containing trypsin and their application for digestion of Cyt c. The present study demonstrates a great potential for usingP5-T in rapid and effective digestion of a small protein.
2. Experimental details 2.1. Materials and apparatus
The synthesis method ofP5 and other synthesized products were given inScheme 1that all syntheses were carried out in argon medium. Trypsin from bovine pancreas, Nα-Benzoyl-DL-arginine 4-nitroanilide
hydrochloride (BAPNA, 98%), albumin from bovine serum (BSA, 98%), Bradford Reagent, 4-nitroaniline (p-nitroaniline), Cyt c from bovine heart, carbon tetraiodide, 1,4-Bis(2-hydroxyethoxy)benzene, paraf-ormaldehyde, triphenylphosphine, boron trifluoride etherate,
chloroform, dimethyl sulfoxide (DMSO), petroleum ether, potassium carbonate and dichloromethane were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. All aqueous solutions were prepared with deionized water that had been passed through a Millipore Milli-Q Plus water purification system.
1H NMR and 13C NMR spectra were carried out by a Varian (400 MHz, ppm). TGA was performed on SII SEIKO thermogravimetric analyzer (Japan) under N2. Bruker FT-IR/FT-NIR Fourier spectrometer was used for FTIR measurements in r.t. Similar results were obtained from Raman spectra (Raman microscope, Renishaw) just as FTIR. SEM images were obtained using a Zeiss EVO-LS10field emission SEM in-strument equipped with an Inca Energy 350 X-Max (Oxford Instruments, UK) spectrometer. The enzymatic activities of free and immobilized trypsin were monitored at 410 nm by a Shimadzu UV-2401PC spectrophotometer. The zeta potential measurements were conducted with NanoPlus zeta/nano particle analyzer (Particulate Systems) at 30 °C. MALDI-MS spectra were acquired in linear modes with average of 50 shots on a Bruker Daltonics Microflex mass spec-trometer (Bremen, Germany) equipped with a nitrogen UV-Laser op-erating at 337 nm.
2.2. The synthesis of 1,4-bis(2-iodoethoxy)benzene
Carbon tetraiodide (6.12 g, 12 mmol) was poured slowly to a solu-tion of 1,4-Bis(2-hydroxyethoxy)benzene (1.19 g, 6 mmol) and triphe-nylphosphine (3.15 g, 6 mmol) in 50 mL of dry acetonitrile at 0 °C and the solution was stirred for 3 h at room temperature under Ar atmo-sphere. Ice particles were poured to the reactionflask, where 1,4-bis(2-iodoethoxy)benzene quickly precipitated. The raw product wasfiltrated by vacuum and the precipitation was washed with water/methanol (70:30, v/v) to give a white solid product (2.17 g, 86%). M.P.: 92 °C.1H NMR (400 MHz, CDCl3, r.t.)δ (ppm): 6.91 (s, 4H), 4.32 (t, J = 5.2 Hz, 4H), 3.51 (t, J = 5.7 Hz, 4H).13C NMR (100 MHz) δ (ppm): 150.35, 116.15, 75.20, 4.29.
2.3. The synthesis ofP5
P5 was synthesized by a known literature procedure [13]. BF3.OEt2 (0.4 g, 3.38 mmol) was added to a solution of 1,4-bis(2-iodoethoxy) benzene (1.41 g, 3.38 mmol) and paraformaldehyde (0.25 g, 9 mmol) in 50 mL of 1,2-dichloroethane at r.t.. The reaction solution was stirred for 3 h under Ar. 0.5 g ofP5 (37%) have been obtained in column (P. ether/ DCM, 1:1). M.P.: 275 °C.1H NMR (400 MHz, CDCl3, r.t.)δ (ppm): 6.91 (s, 10H), 4.32 (t, J = 5.4 Hz, 20H), 3.86 (s, 10H), 3.51 (t, J = 5.4 Hz, 20H). 13C NMR (100 MHz)δ (ppm): 149.90, 125.90, 116.33, 67.41, 29.69, and 3.95. Elemental Analysis calcd.: C55H60I10O10: C, 30.71; H, 2.82; found: C, 30.56; H, 3.01.
2.4. Trypsin immobilization onP5 and protein assay
0.2 g ofP5 was dissolved and added to a solution of trypsin (10 mg/ 10 mL) prepared in equivalent amount of sodium phosphate buffer so-lution (PBS) (0.1 M, pH 7.5) and DMSO. The mixture was stirred for 3 h
Scheme 1. The synthesis procedure of P5.
at 4 °C. Then, the resulting solution was centrifuged at 1500 ×g for 5 min. The P5-T was collected and then washed with PBS (0.1 M, pH 7.5) for three times. TheP5-T are stored at 4 °C until further use (Scheme 2).
The amount of protein in the trypsin solution and the elution so-lutions was determined by the Bradford's method using BSA as a stan-dard [27]. The amount of the trypsin bound toP5 was determined from the difference between the initial and the residual protein content in the solution [28].
2.5. Trypsin activity assay
The enzymatic activity of free trypsin and P5-T was assayed by using BAPNA. The enzymatic activity of free trypsin was measured by hydrolysis of 0.1% BAPNA solution (10 mg BAPNA, 0.2 mL DMSO, 9.8 mL deionized water) for 10 min at room temperature. The enzy-matic activity ofP5-T depends on the hydrolysis of 0.1% BAPNA. P5-T was incubated in an orbital shaker for 10 min at room temperature. After the incubation, P5-T was centrifuged and measured absorbance value. One unit (U) of trypsin activity was expressed as the amount of enzyme that formed 1μmol of p-nitroaniline per minute under optimum reaction conditions [28].
2.6. Microwave-assisted digestion of Cyt c, yeast and BSA
Standard stock Cyt c solution (2 mg/mL) was prepared by the dis-solving of 20 mg of Cyt c in 10 mL NH4HCO3solution (100 mM, pH 8.0). Before the digestion, Cyt c was denatured in a 95 °C water bath for 15 min to increase efficiently digestion [23]. Then, 250μL stock solu-tion of Cyt c and 750μL NH4HCO3 solution (100 mM, pH 8.0) were mixed and obtained to Cyt c in 0.5 mg/mL concentration.
10 mg ofP5-T was transferred into an Eppendorf tube (1.5 mL) and then, 100μL of the denaturated and diluted Cyt c solution (100 μg/μL) was added to the same tube. The mixture solution was conducted by a microwave irradiation (CEM Mars model) with a controlled power of 700 W for 15 s [29]. For comparison, 50μL solution of denaturated Cyt c was transferred into an Eppendorf tube, and 10μL of trypsin in NH4HCO3solution (0.1 M, pH 8.0) with a concentration of 5 mg/10 mL was added to the same tube. After the mixture solution was conducted by the microwave irradiation with a controlled power of 700 W for 15 s, the digestion was quenched by the addition of 11μL of acetic acid. Finally, two different digestive products were examined by MALDI-MS [30,31].
For the microwave-assisted BSA digestion, the similar method was
conducted. 10 mg of P5-T was transferred into an Eppendorf tube (1.5 mL) and then, 100μL of the denaturated and diluted BSA solution (100μg/μL) was added to the same tube. The mixture solution was conducted by a microwave irradiation (CEM Mars model) with a con-trolled power of 700 W for 15 s [30].
In order to investigate the digestion of yeast (Saccharomyces cere-visiae) obtained from Dr. Oetker, free trypsin andP5-T was carried out according to above method and MALDI-MS were recorded [32–34]. 3. Results and discussion
3.1. Characterization of compounds
In1H NMR of 1,4-bis(2-iodoethoxy)benzene, the signals observed at 3.52 ppm (triplet) and 4.33 ppm (triplet) and 6.92 ppm (singlet) for aliphatic and aromatic protons, respectively. Except of the signals in 1,4-bis(2-iodoethoxy)benzene, new peak was observed at 3.85 ppm in 1
H NMR ofP5 that it assigned to the methylene bridge protons. The –CH2protons ofP5 are a singlet signal owing to-the circular rotation. In 13C NMR spectrum of 1,4-bis(2-iodoethoxy)benzene, four peaks are observed for two aromatic and two aliphatic carbons whereasP5 have six signals. After the cyclization of 1,4-bis(2-iodoethoxy)benzene, the equal signals of methylene bridge carbons raised at 29.65 ppm (Suppl. Material, Fig. S1).
Fig. 1shows a representative weight losses curve with the heating rate of 10 °C/min, and temperatures ranged from 25 to 1000 °C forP5 andP5-T. The weight losses up to 150 °C are attributed to physisorbed water (Fig. 1a and b) [35]. Thermo-gravimetric analysis ofP5 (Fig. 1a) showed a 6–8% weight loss of some organic groups between 150 and 290 °C. Then, the percentage loss of weight quickly increased toward 60% until 320 °C that assigned the decomposition of P5 [36]. The weight losses continued toward 1000 °C as hillocks, which assign to several organic fragments, and total loss carried out by 65%. In contrast toP5, thermo-gravimetric graph of P5-T (Fig. 1b) gave a drastic weight loss between 150 and 305 °C. This loss can be attributed to the de-composition of trypsin unit as a macromolecule. Finally, total weight loss was observed as 76%, which may be due to theeCOOH and eNH2 functional groups as a whole are decomposed. All TGA results shown that the interaction betweenP5 and trypsin enzyme carried out e ffec-tively [37].
The FTIR spectra ofP5 and P5-T were recorded by FTIR spectro-metry as given in Fig. 2. The band around 2950 cm−1 in the FTIR spectrum ofP5 was attributed to the vibrations of aliphatic and aro-matic CeH stretching (eCH and eCH2). The multiple bands between Scheme 2. The trypsin immobilization on P5 and digestion of Cyt c using P5-T.
1600 and 1400 cm−1show the C]C stretching of benzene rings on P5. The sharp broad band at 1210 cm−1corresponds to the etheric CeOeC stretching. By the interaction betweenP5 and trypsin, three new peaks appeared at 1732 cm−1, 1655 cm−1and 1611 cm−1that it attributed to the C]O vibrations of amides and ketones in trypsin unit, respectively [38]. After immobilization, the peaks between 1500 cm−1 and 4000 cm−1have changed. Moreover, the broad band at 3391 cm−1can be attributed to OeH vibration of water. The broad stretching of AreOeC bond at 1232 cm−1shifted toward 1196 cm−1in the spectrum of P5. The sharp vibration of the etheric C–O–C bond prominently shifted to 1210 cm−1from 1245 cm−1[39].
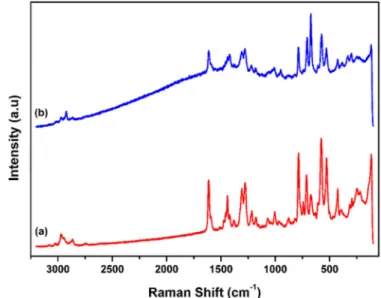
The Raman spectra ofP5 and P5-T (Fig. 3) gave multi vibrations as the aromatic ring stretching (C=C) mainly appeared between 1450 and 1610 cm−1, signals associated with weak asymmetriceCH bending of the alkyl chain around 1400 cm−1, the weak signal at 1125 cm−1 is CeOeC stretching, CeC bonding of the aliphatic chains are around 250 cm−1and CeH stretching between 2850 and 3000 cm−1. CeI vi-bration (medium signal) at 480 cm−1that it disappeared in the Raman spectrum ofP5-T (Fig. 3b) depending on the interaction of enzyme and macromolecule [40].
The morphologies ofP5 and P5-T were shown inFig. 4. The surface morphology shows that the P5 is continuous and compact (Fig. 4a) [41]. As could be seen, the trypsin immobilization significantly changed
the morphology of the surface (Fig. 4b). Comparison of the surface morphology of theP5-T with the P5 indicated that the size of particles are complicated due to the trypsin immobilization.
Since zeta-potential can contribute to the understanding of elec-trostatic interactions between the enzyme and its support, it has been used as a useful technique.Fig. 5depicts the zeta potential curves ofP5 andP5-T. The zeta potential value of P5 and P5-T gives negative po-tential value at varying pH ranges from 4 to 11. From zeta popo-tential measurement, P5 contained negative charges because of functionali-zation with 1,4-bis(2-iodoethoxy)benzene ofP5. Because of the ethoxy groups on the rims are electron-donating moieties, the inner surface of P5 cavity is negative. Therefore, P5 is an excellent host which may guest trypsin containing the positively charged in its cavity at pH 7.5. As you seen inFig. 5(a) and (b), the immobilization has carried out according to electrostatic interactions, sinceP5 and trypsin have op-posite charges at the pH 7.5. So, the immobilization was actualized easily [42]. There are several literatures that reported the binding of protein and enzyme through electrostatic interaction with different support materials [42–44]. It was demonstrated that zeta potential of P5 increased ranges pH between 7 and 8, also decreased after this pH value compared with that of P5-T (≈−13 mV). After trypsin im-mobilization, the zeta potential value of P5-T increased (above −13 mV) due to the positively charged trypsin added to the anionic P5. As result,P5-T has been negatively charged due to the negative char-acteristic structure of P5 [37]. After the isoelectric point of trypsin (about pI = 10.5),P5-T has the zeta potential of negative value (below −13 mV).
3.2. Immobilization of trypsin on P5 and stabilities of free and immobilized trypsin
The method of immobilization can affect to the activity of enzyme when the coupling point is situated near the enzyme active site. Electrostatic interactions can have a strong influence on the enzyme immobilization by promoting of the binding among the charged sur-faces [31]. So, the binding mode between trypsin andP5 can be con-sidered as a strong electrostatic interaction. However, iodine atoms of P5-T are possessing a large effective spin–orbit property because of its heavy atom. Although many types of molecular interactions are pos-sible, it can be mentioned that iodine atom can hold negative charges owing to its large diameter. Thus, iodine atoms having partial negative charge can easily interact with the hydroxyl groups of trypsin. There-fore, the affinity between trypsin and P5 was remarkably strong in-termolecular interaction, but non-covalent interactions can ingenerate Fig. 1. TGA curves of P5 (a) and P5-T (b).
Fig. 2. FTIR spectra of P5 (a) and P5-T (b).
Fig. 3. Raman spectra of P5 (a) and P5-T (b).
between nitrogen/oxygen atoms and iodine atoms of P5. The strong electrostatic interaction between trypsin andP5 might be the reason for reusability of P5-T. The determination of unbound trypsin was per-formed by using Bradford method for the content of immobilized trypsin on P5 [27]. The amount of binding is 80% that it shows an electrostatic interaction betweenP5 and trypsin. The immobilized en-zymes show usually a better thermal and pH stability and can be reused for practical applications. All applications of free trypsin and the im-mobilized trypsin (P5-T) on the enzymatic activity were given in
Supplementary Material (Figs. S2–S7).
Fig. S2 shows thatP5-T is highly active at pH 9 while the optimum pH value of free trypsin is pH 7.5. These results reveal to the number of positively charged groups of enzyme included to the iodo groups de-creases on upon immobilization [45]. The maximum activity was ob-served at 45 °C for both forms of trypsin (Fig. S3). The functionalized trypsin is more stable at higher temperatures than free form.P5-T is thermally more stable than free enzyme at 55 °C (Fig. S4).P5-T retained 55% of enzymatic activity at 55 °C while free trypsin withheld 16% of activity after 120 min. These results showed that the thermo-stability of P5-T improved considerably depending on the immobilization [46]. Due to the stability is an important parameter in storage, it should be taken into account in systems, and use in the functionalized form of enzymes. Both free trypsin andP5-T were stored at 4 °C under same conditions and the activity measurements were carried out for 120-day period (Fig. S5). P5-T lost only 10% of its initial activity over same period of time. The kineticfitting for BAPNA (as a substrate) hydrolysis reaction was calculated according to the Michaelis–Menten equation. Michaelis constant (Km) and the maximum reaction velocity (Vmax) of free trypsin and immobilized forms were obtained from Lineweaver-Burk plots (1/V versus 1/S) as shown in Fig. S6. Furthermore, the re-lative activity ofP5-T decreased toward 59% of its initial activity after 8 cycles of reuse (Fig. S7). All these results demonstrate that theP5 are good support for trypsin immobilization and the process canfind more biological applications.
3.3. Digestion of Cyt c
A lot of MALDI matrices were tried tofind an intense molecular ion peak and low fragmentation under the MALDI-MS conditions for free trypsin andP5-T [32,33]. They yielded a good spectra by using 3,5-Fig. 4. SEM images of P5 (a) and P5-T (b).
Fig. 5. Zeta potential curves for P5 (a) and P5-T (b) at various pH (error bars represent ± standard deviations, n = 3).
hydroxypbenzoic acid MALDI matrix [47]. In this section, Cyt c, which contain 100 amino acids, is cleaved by free trypsin andP5-T. We used Cyt c as a model protein which molecular weight is about 12 kDa. Cyt c is a heme protein associated with the inner membrane of the mi-tochondrion and it has used wide applications in biological and bio-medical research owing to its small size (about 100 amino acids) and solubility in water. So, Cyt c is an electron-transfer protein having one or several heme c groups bound to the surrounding protein structure by one or, more generally, two thioether bonds involving sulfhydryl groups of cysteine residues [14].Fig. 6shows the MALDI-MS spectra of Cyt c digested by the free trypsin andP5-T. MALDI matrix cluster ions obscure low m/z species (< 600). So, the important peptide fragments have values of m/z≥ 500.
As seen inFig. 6a,5peptides were determined for 15 s in the mass spectra with a sequence coverage of 48%, which is apparent that only a few peaks with low intensity were detected. For the comparison, Cyt c was well digested and detected with a high intensity and 10 peptides can be matched and identified with a sequence coverage of 93% for P5-T (Fig. 6b). Apparently, the results obtained Cyt c digestion usingP5-T for 15 s is quite comparable to and in general better than the results obtained using the 15 s in solution method.Table 1andTable 2give to the detailed information for the identified peptides for free trypsin and P5-T, respectively. Microwave irradiation expedited and improved the protein digestion by generating the results of 93% sequence coverage
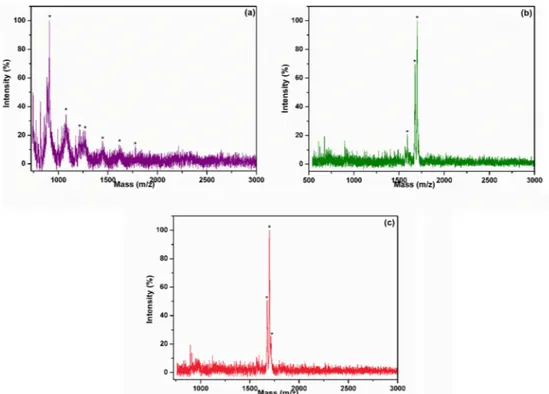
within 15 s of Cyt c digestion. BSA was hydrolyzed efficiently by P5-T to peptide fragments. Also, the P5-T is capable of hydrolyzing yeast, suggesting it has the potential for the utilization in various protein degradation processes in food, pharmaceuticals, diagnostics, and waste treatments. Both BSA and yeast digestion were carried out microwave digestion in as little as 15 s [30]. Even for 15 s digestion, the protein molecule can be confidently identified from the corresponding peaks, and according to the data, 15-second digestion outperformed the other digestions, which is also a sufficiently short digestion period for the high molecular weight proteins in proteomics applications. At least three peptides for yeast and BSA protein were observed and these peptides could thought to be sufficient to identify the proteins using MALDI-MS inFig. 7(a), (b) and (c).
According to theTable 3, Cheng et al., studied tryptic digestion for 30 min using magnetic Fe3O4@PDA-Trypsin nanosystem and they found the identified protein with sequence coverage of 92% [48]. Li et al., found the average sequence coverage of 77% for Cyt c in 5 min using trypsin immobilized on-chip microreactor [15]. Cao et al., ob-served peptide sequence coverage of 90% for Cyt c in 15 min [23]. Shi et al., reported that the sequence coverage obtained from MG@PDA-trypsin nanocomposites was 62% for Cyt c in 10 min [49]. Bao et al., studied IR-assisted digestion for 5 min and they found the identified Cyt c with sequence coverage of 67% [50]. Jiang et al., prepared magnetic Fe3O4 nanoparticles modified graphene oxide nanocomposites (GOeCOeNHeFe3O4) and they immobilized trypsin on GOe-COeNHeFe3O4by covalent bonding. Also, they observed peptide se-quence coverage of 64% for Cyt c in 15 s by using microwave assisted digestion [51]. The long digestion times can cause the formation of autocatalysis products of trypsin in high amounts [52]. The reduction of protein digestion time from min to sec can significantly improve the throughput and turnaround time of proteomic analysis. This finding confirms that the microwave-assisted digestion increased the protein digestion with a shorter time interval.
4. Conclusions
In conclusion, we have synthesized a macromolecule based on pillar [5]arene and it was interacted with trypsin without compromising the enzyme activity. The interaction was supported by thermogravimetric method, infrared and Raman spectroscopies. Finally, the microwave-assisted digestion conditions of Cyt c are illuminated by using MALDI-MS for the efficiency of P5-T that MALDI-MS is a vital tool in mass analysis of biomolecules. Trypsin, which divides peptidic bonds into the C-terminal group of lysine or arginine, is a pancreatic serine en-doprotease used for protein digestion. Thus, we demonstrated a high efficient and fast microwave-assisted digestion of Cyt c using MALDI-MS. The experimental results showed that Cyt c protein was identified with 15 s immobilized tryptic digestion, which was slightly more than those obtained by 15 s in solution digestion. Trypsin interacted withP5, fabricated via simple attachment without any crosslinker, and was Fig. 6. Mass spectra of Cyt c microwave-digested by 15 s for free trypsin (a) and P5-T (b).
Table 1
The identified peptides of Cyt c digests for free trypsin.
Peak MWa Start–end Sequence
1 2 559.03 671.89 101–105 1–6 K.KATNE.--.MGDVEK.G 3 780.61 81–87 K.MIFAGIK·K 4 5 869.84 1218.76 1–8 29–40 -.MGDVEKGK·K K.TGPNLHGLFGRK.T a Molecular weight. Table 2
The identified peptides of Cyt c digests for P5-T.
Peak MWa Start-End Sequence
1 683.12 1–6 -.MGDVEK.G 2 768.80 9–14 K.KIFVQK·C 3 4 5 955.52 1017.56 1226.63 93–100 15–23 29–40 R.EDLIAYLK·K K.CAQCHTVEK.G K.TGPNLHGLFGRK.T 6 7 8 1454.37 1593.09 1616.72 41–54 40–54 10–23 K.TGQAPGFSYTDANK·N R.KTGQAPGFSYTDANK·N K.IFVQKCAQCHTVEK.G 9 10 1701.52 1953.88 41–56 57–73 K.TGQAPGFSYTDANKNK.G K.GITWGEETLMEYLENPK·K a Molecular weight.
highly stable. The study demonstrated in this paper can be further de-veloped to be fully automated thus leading to a high-throughput pro-teome lab.
Acknowledgments
The authors thank Prof. J.-F. Nierengarten and Dr. I. Nierengarten for laboratory studies and scientific comments. The authors would like to thank Prof. Dr. Mahmut Ozacar for his support. The authors also would like to appreciate the kind help to Assoc. Prof. Dr. Bunyemin Cosut for MALDI-MS analyses. This work isfinanced by the Scientific Research Projects Commission of Sakarya University (Project number: 2016-02-04-047) and is gratefully acknowledged.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.msec.2018.10.043.
References
[1] N.L. Strutt, H. Zhang, S.T. Schneebeli, J.F. Stoddart, Amino-functionalized pillar[5] arene, Chem. Eur. J. 20 (2014) 10996–11004.
[2] X. Ji, S. Dong, P. Wei, D. Xia, F. Huang, A novel diblock copolymer with a supra-molecular polymer block and a traditional polymer block: preparation, controllable self-assembly in water, and application in controlled release, Adv. Mater. 25 (2013)
5725–5729.
[3] Q. Zhang, D.H. Qu, J. Wu, X. Ma, Q. Wang, H. Tian, A dual-modality photo-switchable supramolecular polymer, Langmuir 29 (2013) 5345–5350. [4] T. Ogoshi, T. Yamagishi, Pillar[5]- and pillar[6]arene-based supramolecular
as-semblies built by using their cavity-size-dependent host-guest interactions, Chem. Commun. 50 (2014) 4776–4787.
[5] J.-F. Chen, X. Liu, J.-F. Ma, B.-B. Han, J.-D. Ding, Q. Lin, H. Yao, Y.-M. Zhang, T.-B. Wei, A pillar[5]arene-based multiple-stimuli responsive metal–organic gel was constructed for facile removal of mercury ions, Soft Matter 1 (2017) 5214–5218. [6] Y. Guan, M. Ni, X. Hu, T. Xiao, S. Xiong, C. Lin, L. Wang, Pillar[5]arene-based
polymeric architectures constructed by orthogonal supramolecular interactions, Chem. Commun. 48 (2012) 8529.
[7] Y. Jia, Y. Fang, Y. Li, L. He, W. Fan, W. Feng, Y. Yang, J. Liao, N. Liu, L. Yuan, Pillar [5]arenes bearing phosphine oxide pendents as Hg2+selective receptors, Talanta 125 (2014) 322–328.
[8] W.A. Freeman, W.L. Mock, N.Y. Shih, Cucurbituril, J. Am. Chem. Soc. 103 (1981) 7367–7368.
[9] J. Kim, I.S. Jung, S.Y. Kim, E. Lee, J.K. Kang, S. Sakamoto, K. Yamaguchi, K. Kim, New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8), J. Am. Chem. Soc. 122 (2000) 540–541.
[10] M.V. Rekharsky, T. Mori, C. Yang, Y.H. Ko, N. Selvapalam, H. Kim, D. Sobransingh, A.E. Kaifer, S. Liu, L. Isaacs, W. Chen, S. Moghaddam, M.K. Gilson, K. Kim, Y. Inoue, A synthetic host-guest system achieves avidin-biotin affinity by overcoming en-thalpy-entropy compensation, Proc. Natl. Acad. Sci. 104 (2007) 20737–20742. [11] H. Tao, D. Cao, L. Liu, Y. Kou, L. Wang, H. Meier, Synthesis and host-guest
prop-erties of pillar[6]arenes, SCIENCE CHINA Chem. 55 (2012) 223–228. [12] C.-C. Zhang, S.-H. Li, C.-F. Zhang, Y. Liu, Size switchable supramolecular
nano-particle based on Azobenzene derivative within anionic pillar[5]arene, Sci. Rep. 6 (2016) 37014.
[13] A.N. Kursunlu, Y. Acikbas, M. Ozmen, M. Erdogan, R. Capan, Preparation of pillar [5]arene-quinoline Langmuir–Blodgett thin films for detection of volatile organic compounds with host–guest principles, Analyst 142 (2017) 3689–3698.
Fig. 7. Mass spectra of yeast microwave-digested by 15 s for free trypsin (a), P5-T (b) and Mass spectra of BSA microwave-digested by 15 s for P5-T (c).
Table 3
Comparison of Cyt c digestion for this study and other studies.
Support materials Digestion method Digestion time Sequence coverage (%) References
Trypsin immobilized on-chip microreactor Incubated at 50 °C 5 min 77 [15]
AuNP@Fe3O4enzymatic nanosystem
Fe3O4@PDA-Trypsin nanosystem Incubated at 37 °C Incubated at 37 °C 15 min 30 min 90 92 [23] [48]
MG@PDA-trypsin nanocomposites Incubated at 37 °C 10 min 62 [49]
Trypsin solution
Trypsin immobilized on GOeCOeNHeFe3O4
IR-assisted Microwave-assisted 5 min 15 s 67 64 [50] [51]
[14] G.W. Pettigrew, G.R. Moore, Cytochromes c, The Enzymes 3 (1987) 397–547. [15] Y. Li, B. Yan, C. Deng, W. Yu, X. Xu, P. Yang, X. Zhang, Efficient on-chip proteolysis
system based on functionalized magnetic silica microspheres, Proteomics 7 (2007) 2330–2339.
[16] B. Rybtchinski, Adaptive supramolecular nanomaterials based on strong non-covalent interactions, ACS Nano 5 (2011) 6791–6818.
[17] D.A. Uhlenheuer, K. Petkau, L. Brunsveld, Combining supramolecular chemistry with biology, Chem. Soc. Rev. 39 (2010) 2817.
[18] H.D. Nguyen, D.T. Dang, J.L.J. Van Dongen, L. Brunsveld, Protein dimerization induced by supramolecular interactions with cucurbit[8]uril, Angew. Chem. Int. Ed. 49 (2010) 895–898.
[19] L.M. Heitmann, A.B. Taylor, P.J. Hart, A.R. Urbach, Sequence-specific recognition and cooperative dimerization of N-terminal aromatic peptides in aqueous solution by a synthetic host, J. Am. Chem. Soc. 128 (2006) 12574–12581.
[20] P. Picotti, R. Abersold, Ruth Hüttenhain, Johan Malmström, Perspectives of tar-geted mass spectrometry for protein biomarker verification, Curr. Opin. Chem. Biol. 13 (2010) (2009) 518–525.
[21] J. Qiao, J.Y. Kim, Y.Y. Wang, L. Qi, F.Y. Wang, M.H. Moon, Trypsin immobilization in ordered porous polymer membranes for effective protein digestion, Anal. Chim. Acta 906 (2016) 156–164.
[22] S. Longobardi, A.M. Gravagnuolo, R. Funari, B. Della Ventura, F. Pane, E. Galano, A. Amoresano, G. Marino, P. Giardina, A simple MALDI plate functionalization by Vmh2 hydrophobin for serial multi-enzymatic protein digestions, Anal. Bioanal. Chem. 407 (2015) 487–496.
[23] Y. Cao, L. Wen, F. Svec, T. Tan, Y. Lv, Magnetic AuNP@Fe3O4nanoparticles as reusable carriers for reversible enzyme immobilization, Chem. Eng. J. 286 (2016) 272–281.
[24] M. Montowska, E. Pospiech, Processed meat protein and heat-stable peptide marker identification using microwave-assisted tryptic digestion, Food Technol. Biotechnol. 54 (2016) 482–488.
[25] N.Y. Ha, S.H. Kim, T.G. Lee, S.Y. Han, Rapid characterization of protein chips using microwave-assisted protein tryptic digestion and MALDI mass spectrometry, Langmuir 27 (2011) 10098–10105.
[26] H.W. Hahn, M. Rainer, T. Ringer, C.W. Huck, K. Bonn, Ultrafast microwave-assisted in-tip digestion of proteins, Research Articles, J. Proteome Res. (2009) 4225–4230. [27] M.M. Bradford, A rapid and sensitive method for the quantitation of microgram
quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248–254.
[28] K. Atacan, M. Özacar, Characterization and immobilization of trypsin on tannic acid modified Fe3O4nanoparticles, Colloids Surf. B: Biointerfaces 128 (2015) 227–236. [29] G. Cheng, P. Chen, Z.G. Wang, X.J. Sui, J.L. Zhang, J.Z. Ni, Immobilization of
trypsin onto multifunctional meso-/macroporous core-shell microspheres: a new platform for rapid enzymatic digestion, Anal. Chim. Acta 812 (2014) 65–73. [30] K. Atacan, B. Çakıroğlu, M. Özacar, Efficient protein digestion using immobilized
trypsin onto tannin modified Fe3O4magnetic nanoparticles, Colloids Surf. B: Biointerfaces 156 (2017) 9–18.
[31] C. Xia, H. Wang, F. Jiao, F. Gao, Q. Wu, Y. Shen, Y. Zhang, X. Qian, Rational synthesis of MoS 2-based immobilized trypsin for rapid and effective protein di-gestion, Talanta 179 (2018) 393–400.
[32] T. Piper, U. Mareck, H. Geyer, U. Flenker, M. Thevis, P. Platen, W. Schanzer, Cysteine-capped ZnSe quantum dots as affinity and accelerating probes for micro-wave enzymatic digestion of proteins via direct matrix-assisted laser desorption/ ionization time-of-flight mass spectrometric analysis, Rapid Commun. Mass Spectrom. 22 (2008) 2161–2175.
[33] S.K. Kailasa, H.F. Wu, Functionalized quantum dots with dopamine dithiocarba-mate as the matrix for the quantification of efavirenz in human plasma and as
affinity probes for rapid identification of microwave tryptic digested proteins in MALDI-TOF-MS, J. Proteome 75 (2012) 2924–2933.
[34] S.C. Repair-and-go, G.V. Kolmakov, R. Revanur, R. Tangirala, T. Emrick, T.P. Russell, A.J. Crosby, A.C. Balazs, Using Nanoparticle-filled Microcapsules, 4 (2010), pp. 1115–1123.
[35] B. Sahoo, S.K. Sahu, D. Bhattacharya, D. Dhara, P. Pramanik, A novel approach for efficient immobilization and stabilization of papain on magnetic gold nano-composites, Colloids Surf. B: Biointerfaces 101 (2013) 280–289.
[36] T. Zhou, N. Song, H. Yu, Y.W. Yang, Pillar[5,6]arene-functionalized silicon dioxide: synthesis, characterization, and adsorption of herbicide, Langmuir 31 (2015) 1454–1461.
[37] A.L. Barrán-Berdón, M. Martínez-Negro, L. García-Río, Ò. Domènech, C. Tros De Ilarduya, E. Aicart, E. Junquera, A biophysical study of gene nanocarriers formed by anionic/zwitterionic mixed lipids and pillar[5]arene polycationic macrocycles, J. Mater. Chem. B 5 (2017) 3122–3131.
[38] K. Atacan, B. Cakiroglu, M. Ozacar, Covalent immobilization of trypsin onto mod-ified magnetite nanoparticles and its application for casein digestion, Int. J. Biol. Macromol. 97 (2017) 148–155.
[39] F. Ye, R. Wei, L. Wang, H. Meier, D. Cao, A pillar[5]arene-containing cross-linked polymer: synthesis, characterization and adsorption of dihaloalkanes and n-alky-lene dinitriles, RSC Adv. 6 (2016) 89810–89814.
[40] R.T. Forbes, B.W. Barry, A.A. Elkordy, Preparation and characterisation of spray-dried and crystallised trypsin: FT-Raman study to detect protein denaturation after thermal stress, Eur. J. Pharm. Sci. 30 (2007) 315–323.
[41] V.B. Stepanova, D.N. Shurpik, V.G. Evtugyn, I.I. Stoikov, G.A. Evtugyn, Y.N. Osin, T. Hianik, Label-free electrochemical aptasensor for cytochrome c detection using pillar[5]arene bearing neutral red, Sensors Actuators B Chem. 225 (2016) 57–65. [42] N. Schultz, G. Metreveli, M. Franzreb, F.H. Frimmel, C. Syldatk, Zeta potential
measurement as a diagnostic tool in enzyme immobilisation, Colloids Surf. B: Biointerfaces 66 (2008) 39–44.
[43] C. Lei, Y. Shin, J. Liu, E.J. Ackerman, Entrapping enzyme in a functionalized na-noporous support, J. Am. Chem. Soc. 124 (2002) 11242–11243.
[44] F. Turci, E. Ghibaudi, M. Colonna, B. Boscolo, I. Fenoglio, B. Fubini, An integrated approach to the study of the interaction between proteins and nanoparticles, Langmuir 26 (2010) 8336–8346.
[45] K. Kubiak-Ossowska, P.A. Mulheran, What governs protein adsorption and im-mobilization at a charged solid surface? Langmuir 26 (2010) 7690–7694. [46] C. Daglioglu, F. Zihnioglu, Covalent immobilization of trypsin on
glutaraldehyde-activated silica for protein fragmentation, Artif. Cells Blood Substit. Immobil. Biotechnol. 40 (2012) 378–384.
[47] S.O. Tümay, E. Okutan, I.F. Sengul, E. Özcan, H. Kandemir, T. Doruk, M. Çetin, B. Çoşut, Naked-eye fluorescent sensor for Cu(II) based on indole conjugate BODIPY dye, Polyhedron 117 (2016) 161–171.
[48] G. Cheng, S.-Y. Zheng, Construction of a high-performance magnetic enzyme na-nosystem for rapid tryptic digestion, Sci. Rep. 4 (2014) 6947.
[49] C. Shi, C. Deng, Y. Li, X. Zhang, P. Yang, Hydrophilic polydopamine-coated mag-netic graphene nanocomposites for highly efficient tryptic immobilization, Proteomics 14 (2014) 1457–1463.
[50] H. Bao, T. Liu, X. Chen, G. Chen, Efficient in-gel proteolysis accelerated by infrared radiation for protein identification, J. Proteome Res. 7 (2008) 5339–5344. [51] B. Jiang, K. Yang, Q. Zhao, Q. Wu, Z. Liang, L. Zhang, X. Peng, Y. Zhang,
Hydrophilic immobilized trypsin reactor with magnetic graphene oxide as support for high efficient proteome digestion, J. Chromatogr. A 1254 (2012) 8–13. [52] G. Bayramoglu, V.C. Ozalp, M.Y. Arica, Magnetic polymeric beads functionalized
with different mixed-mode ligands for reversible immobilization of trypsin, Ind. Eng. Chem. Res. 53 (2014) 132–140.