Contents lists available atScienceDirect
Journal of Photochemistry & Photobiology A: Chemistry
journal homepage:www.elsevier.com/locate/jphotochemSynthesis of novel tetrazine based D-
π-A organic dyes for
photoelectrochemical and photocatalytic hydrogen evolution
Emre Aslan
a,b, Merve Karaman
c, Gizem Yanalak
b, Hakan Bilgili
d, Mustafa Can
c, Faruk Ozel
e,f,
Imren Hatay Patir
g,*
aSelcuk University, Faculty of Science, Chemistry Department, Campus, Konya, Turkey bSelcuk University, Faculty of Science, Biochemistry Department, Konya, Turkey cIzmir Katip Celebi University, Engineering Sciences Department, Cigli, Izmir, Turkey dIzmir Katip Celebi University, The Central Research Laboratory, Cigli, Izmir, Turkey
eKaramanoglu Mehmetbey University, Metallurgical and Materials Engineering Department, Karaman, Turkey
fKaramanoglu Mehmetbey University, Scientific and Technological Research and Application Center, 70200, Karaman, Turkey gSelcuk University, Faculty of Science, Biotechnology Department, Konya, Turkey
A R T I C L E I N F O Keywords: Donor-π-acceptor dyes Hydrogen evolution Dye sensitization A B S T R A C T
Two novel donor-π-acceptor (D-π-A) dyes, called as MK-2 and MK-8, are synthesized. Their structural, optical and electrochemical properties are investigated by NMR, absorption/photoluminescence spectroscopies and cyclic voltammetry techniques, respectively. Photocatalytic and photoelectrochemical hydrogen evolution properties of these D-π-A dyes are explored by using triethanolamine (TEOA) as a sacrificial electron donor under anaerobic conditions and visible light irradiation with or without co-catalysts (Cu2WS4and Pt) for thefirst time. Photoelectrochemical and photocatalytic hydrogen evolution reaction (HER) activities of these dyes are studied by using TiO2 coated FTO electrodes and powdered TiO2 (Degussa P25), respectively. Photoelectrochemical response of MK-2/TiO2and MK-8/TiO2arefigured out in the order of 180 μA cm−1and 80μA cm−1. The photocatalytic hydrogen evolution amounts of MK-2/TiO2, MK-2/TiO2/Cu2WS4, MK-2/TiO2/ Pt, MK-8/TiO2, MK-8/TiO2/Cu2WS4and MK-8/TiO2/Pt are turned out to be 565, 920, 1828, 374, 522 and 1260 μmolg−1h−1, respectively. Dye/TiO
2photocatalysts are displayed good stability in the both photochemical HER experiments. The alteration in the HER activities of MK-2 and MK-8 is explained by molecule structures of dyes. The proposed mechanism of photocatalytic hydrogen evolution is clarified by using electrochemical band levels of each constituent.
1. Introduction
Light-driven water splitting into H2and O2gases has been attracted great interest by using visible light-driven photocatalyst in the photo-chemical hydrogen evolution systems. Fujishima and Honda are reported that well-known photocatalyst TiO2 was used for the first time to cleavage the water by illumination with UV light [1]. TiO2has two main disadvantages, which is not absorbed visible light because of its wide band gap and shows high recombination rates. The recombination rate decrement and the visible light absorption of TiO2can be provided by the addition of co-catalyst and the dye sensitization on the photo-catalyst, respectively [2]. Dye sensitization is generally carried out by using xanthene-based dyes [3–6]. Recently, organic semiconductor dyes with the donor-acceptor (D-A), donor-π-acceptor (D-π-A), donor-ac-ceptor-π-acceptor (D-A-π-A) and donor-π-acceptor-π-acceptor
(D-π-A-π-A) structures have been arouse interest for the sensitization of visible or solar light owing to tunable absorption characteristics, electro-chemical energy levels and intramolecular charge transfer properties in dye-sensitized photoelectrochemical and photocatalytic hydrogen evo-lution reaction (HER), phoelectrochemical cells (DS-PEC) and solar cells (DSSC) [7–12]. Triphenylamine, carbazole, merocyanine, phe-nothiazine, coumarin and their derivatives have been usually played a part of donor groups in the organic semiconductor D-A, D-π-A and D-A-π-A dyes [7]. The HER in the neutral medium have been explored by D-A type dye sensitized TiO2photocatalyst under solar illumination and the difference of photoactivities of dyes have been related to different substituents by changing methine chain length [13,14]. The photo-catalytic hydrogen evolution by TiO2/Pt photocatalyst have been in-vestigated by the amount of loading dye, steric and hydrophilic effect, the length of spacer groups and number of anchoring groups of D-π-A
https://doi.org/10.1016/j.jphotochem.2019.112301
Received 23 August 2019; Received in revised form 10 December 2019; Accepted 11 December 2019 ⁎Corresponding Author.
E-mail address:imrenhatay@gmail.com(I.H. Patir).
Available online 12 December 2019
1010-6030/ © 2019 Elsevier B.V. All rights reserved.
nm) [30]. The photovoltaic performance and photocatalytic HER ac-tivities of two D‐A‐π‐A dyes have been researched covered by limited wavelength frame (780 nm >λ > 420 nm), which are showed both high photoelectric conversion efficiency and photocatalytic HER ac-tivity because of high molar extinction coefficient of the dyes [31]. The photocatalytic HER by dye sensitized TiO2 photocatalyst have been usually performed in the presence of co-catalyst to host active sites, enhance photogenerated charge transport yield and stability of reac-tions, reduce photocorrosion and charge recombination rates. Platinum group metals (PGMs) like Pt, Pd, etc. are usually operated as a co-cat-alyst for photocatalytic HER [9]. Hydrogenase-like metal sulfides such as molybdenum/tungsten sulfides co-catalysts are promising candidate as an alternative to PGMs [32,33]. The catalytic activities on HER of metal sulfide co-catalysts could be increased by alloyed/doped struc-tures on HER studies [22,34,35].
In this study, we have synthesized two novel photosensitizer D-π-A organic dyes, which are named MK-2 and MK-8, to sensitize of TiO2for the absorption of visible light. The chemical structures of synthesized D-π-A organic dyes have been explored by FT-IR, NMR and HRMS tech-niques; optical and electrochemical properties have been clarified by absorption/photoluminescence spectroscopies, and cyclic voltammetry methods, respectively. The MK-2/MK-8 sensitized TiO2have been used first time in the literature as the visible light-driven photocatalysts in the photochemical hydrogen evolution reaction (HER). Photoelectrochemical features of the dye sensitized electrodes have been investigated in the aqueous TEOA/Na2SO4 solution by linear sweep voltammetry (LSV) and chronoamperometry (CA) techniques. There are no changing transient photocurrent densities by dye/TiO2 electrodes during the light on/off cycles in the photoelectrochemical experiments, which is indicated the stabilities of MK-2/MK-8 sensitized TiO2electrodes. Photocatalytic hydrogen evolution experiments have beenfigured out in the aqueous sacrificial electron donor TEOA solu-tion. In addition, ternary metal sulfide Cu2WS4has been used as the co-catalyst in the photocatalytic HER and its catalytic activity has been compared to Pt. The photocatalytic HER by MK-2/MK-8 sensitized TiO2 shows good stabilities due to linearly increasing hydrogen evolution during the photocatalytic reactions. Rates of HER have been explained by molecular variation of D-π-A organic dyes. Finally, the hydrogen evolution mechanism has been stated by energy levels of each con-stituent, which determined electrochemically.
2. Experimental Section 2.1. Dye sensitization process
In a dye-sensitized system, to prepare the photocatalytic sample, TiO2(Degussa P25) powder was annealed in a furnace at 450 °C for 45 min in order to eliminate of some organic impurities and adsorbed water. Followed by annealing step, calcined TiO2was taken out; pre-pared donor-π-acceptor dye species (10−5M in THF) was added to the TiO2and magnetically stirred under dark conditions. After the TiO2 coating with dye, thefiltered-out solution was typically washed on the filter with THF and ethanol, respectively, for three times. TiO2coated FTO electrodes were sintered (450 °C for 1 h). Then, TiO2 coated
trodes, respectively. Two types electrochemical techniques were ap-plied in the photoelectrochemical HER experiments, which is chronoamperometry (CA) and linear sweep voltammetry (LSV) tech-niques. The LSV was used to investigate the stability of dyes with a potential range between -0.6 V and 0.6 V that has shown good stability and the CA was used for determination of dye stability with a potential of 0 V for 350 s light on/off periods of 50 s each. Photocatalytic activity experiments were performed in n a Pyrexflask (135 ml) under the visible light (Solar Light XPS-300™, λ ≥ 420 nm). (MK-2 or MK-8) / TiO2hybrid photocatalysts (10 mg), with and without Cu2WS4or Pt co-catalysts, and TEOA (0.33 M) sacrificial agent were added into the flask under N2 atmosphere. After that the flask was sealed with silicon septum prior to the photocatalytic HER. Ultrasonic treatment was ap-plied to the solution to supply the uniform distribution of photo/cata-lysts. Eventually, hydrogen production is evaluated by Shimadzu GC-2010 Plus GC.
3. Results and discussion
3,6-bis [4-methylthien-2-yl]-stetrazine(1) molecule was prepared according to reported methods [36,37]. 4-[5-{6-[5-Bromo-4-(2-ethyl-hexyll)-2-thienyl]-1,2,4,5-tetrazin-3-yl}-3-(2-ethylhexyl)-2-thienyl -N,N-bis[4-(hexyloxy)phenyl]aniline (3), methyl-4[5-{6-[5-(4-{bis[4- (hexyloxy)phenyl]amino}phenyl)-4-(2-ethylhexyll)-2-thienyl]-1,2,4,5-tetrazin-3-yl)-3-(2-ethylhexyl-2-thienyl]benzoate(4) and 4-[5-{6-[5-(4- {bis[4-(hexyloxy)phenyl]amino}phenyl)-4-(2-ethylhexyl)-2-thienyl]-1,2,4,5-tetrazin-3-yl}-3-(2-ethylhexyl)-2-thienyl] benzaldehyde (5) were synthesized by Suzuki-Miyaura reaction. Molecule(5) was con-verted by Knoevenagel condensation reaction intoMK-8 dye. The de-tailed synthesis steps of MK-2 and MK-8 are given in the SI. Structural characterizations of MK-2 and MK-8 are carried out by using NMR, FT-IR and HRMS techniques (see SI). The synthetic pathways of dyes are shown inScheme 1.
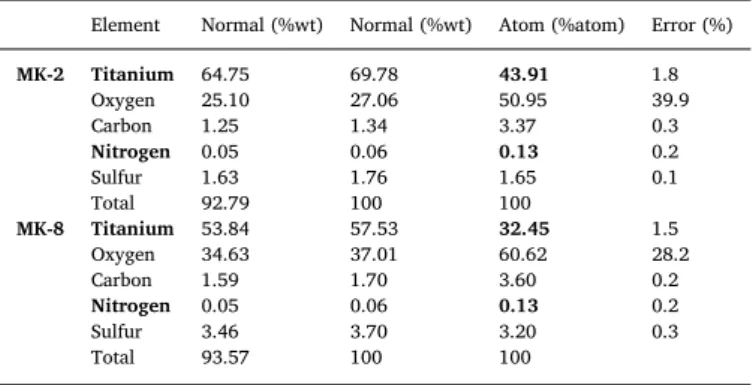
Binding ratios between dyes (MK-2 or MK-8) and TiO2have been investigated by using energy dispersive x-ray spectroscopy (EDX). According to elemental analysis data obtained from EDX spectra (see SI, Figure S1), N/Ti ratios have been calculated for the MK-2/TiO2, and MK-8/TiO2due to the common elements in the MK-2/MK-8 dyes sen-sitized TiO2. N/Ti ratios were found out 2.96 × 10−3and 4.01 × 10−3 for the MK-2/TiO2and MK-8/TiO2, respectively (Table 1). These results show that aggregate formation isfigured out in the MK-8 on the surface of TiO2[38–41]. The effect of aggregate formation has been discussed in the results of photoelectrochemical and photocatalytic HER.
UV-Vis absorption spectra have been carried out by using 10−5M dye solution in THF as shown inFig. 1a. The sharp absorption peaks of dyes are seen in 349 nm and 296 nm for MK-2; 343 nm and 298 nm for MK-8. Thefirst peaks about 300 nm and second peaks about 350 nm are assigned to the localizedπ-π* transition and delocalized π-π* transi-tion, respectively [12]. In comparison with MK-8, the maximum ab-sorption wavelength of MK-2 shows a red-shift. This can be explained by the addition of a cyano group, which increases the electron with-drawing ability and decrease the electron density in MK-8 dye [42]. Molar extinction coefficients have been estimated MK-2 and MK-8 as 915 M-1cm-1(349 nm) and 2938 M-1cm-1(343) by Beer–Lambert Law
[43]. Fluorescence spectra of dyes have been demonstrated in the Fig. 1b. Emission peaks of the MK-2 and MK-8 have been 556 nm and 571 nm, respectively. The original emission spectra of MK-2 and MK-8 dyes depicted infigure S2. It is obvious that MK-2 was higher emissive, which suggesting more energy loss was occur upon photoexcitation [44]. The emission band intensity of MK-8 were lower when compared to MK-2. The cyanocarboxyl group is known as cyanoacrylic acid group which is containing strong electron-withdrawing cyano and carboxylic acid groups. The presence of electronegative groups near the carboxyl group, act to increase the acidity and it also has a unique charge-transport property. It reduces electron density on the MK-8 molecule in the presence of cyanoacyrilic acid group. Therefore, compared with MK-2, the maximum wavelength offluorescence red-shifted (15 nm) in normalized PL spectra given inFig. 1b [45–48]. Fluorescent quantum yield is the ratio of the number of photons emitted to the number of photons absorbed and gives efficiency of fluorescence process [49]. The fluorescence emission quantum efficiency yield of MK-2 and MK-8 dyes were found out as the 19 % and 34 %, referenced to Rhodamine. HRMS analysis were performed by Thermo Fisher Scientific TSQ Fortis (LC-MS/MS). Vaporizer Temperature was 325 °C because ionization was not successful due to the size of the masses at room temperature (Figure S3).
The cyclic voltammetry measurements of MK-2 and MK-8 dyes were performed by CH Instruments 760D potentiostat in three-electrode cell using glassy carbon working electrode, platinum wire counter electrode and Ag/AgCl reference electrode in acetonitrile in the presence of 0.1 M tetrabutylammonium hexafluorophosphate as supporting electrolyte solution. The HOMO and LUMO energy levels of MK-2 and MK-8 dyes have been calculated from their oxidation and reduction potentials, respectively [50]. The oxidation potentials of MK-2 and MK-8 have been found at 0.81 V and 0.83 V, respectively. The reduction potentials of MK-2 and MK-8 have beenfigured out at -0.77 V / -1.56 V and -0.90 V / -1.71 V, respectively, by using cyclic voltammetry (Fig. 2).
visible light illumination on/off. The LSV studies have been figured out from 0.6 V to -0.6 V, which are displayed well durability over a wide range of potentials (Fig. 3a). The CA studies were performed at the 0 V potential with 50 s light on and 50 s light off during 350 s. Only TiO2 electrode shows very low photocurrent density under visible light ir-radiation. However, dye sensitized electrodes have been displayed en-hancing photocurrent density under illuminated by visible light. Pho-togenerated electron-hole separation efficiency is also understood by photocurrent density [51,52]. As shown inFig. 3b, the changing of photocurrent densities by illuminated condition have been raised by using MK-2 and MK-8 sensitized TiO2electrodes in comparison non-sensitized TiO2electrode. Photocurrent densities have been attained 180 and 80 μA cm−1by MK-2 and MK-8 sensitized TiO
2electrodes, respectively. There are no changing transient photocurrent densities by dye/TiO2 electrodes during the light on/off cycles in the photoelec-trochemical experiments, which is indicated the stabilities of MK-2/ MK-8 sensitized TiO2electrodes. D-π-A dyes have been developed the intramolecular electron-hole separation efficiencies due to the in-creased dipole moment in the dye structure [7,17,53,54].
The photocatalytic HER have been carried out by using 5 % aqueous TEOA as the sacrificial agent with solar irradiation (λ > 420 nm). Firstly, optimal pH has been determined by using different pH of electron donor solution, which pH values changes from 7 to 10, and the best pH value has found out as the 9 (Fig. 4).
The amount of evolved hydrogen gas against time has beenfigured out at pH 9 aqueous TEOA solution with dispersed photocatalyst/cat-alyst under solar illumination [17,22,23,55–58]. The produced hy-drogen gas is normalized to the 1 g of photocatalyst. Ternary metal sulfide Cu2WS4, which is synthesized by our previous published pro-cedures [22,59], have been used as the noble metal-free co-catalyst. The hydrogen evolution rate of TiO2/ MK-2, TiO2/ MK-2 / Cu2WS4, TiO2/ MK-8 and TiO2/ MK-8 / Cu2WS4have been found 565, 920, 374 and 522μmolg−1h−1, respectively. After 8 h photocatalytic reaction, the produced hydrogen amount of TiO2/ MK-2, TiO2/ MK-2 / Cu2WS4, TiO2/ MK-8 and TiO2/ MK-8 / Cu2WS4have been found 2588, 3620, 2140 and 2650μmolg−1, respectively. No hydrogen gas observed in the absence of dye (using only Cu2WS4, Pt, TiO2or TiO2/Cu2WS4, TiO2/Pt). Moreover, activity of PGM-free co-catalyst Cu2WS4is compared to Pt,
Nitrogen 0.05 0.06 0.13 0.2 Sulfur 3.46 3.70 3.20 0.3 Total 93.57 100 100
which is achieved by photoreducing chloroplatinic acid hexahydrate, in the equal conditions. The hydrogen production rates for TiO2/ MK-2 / Pt and TiO2 / MK-8 / Pt have been figured out as 1828 and 1260 μmolg−1 h−1, respectively. During 8 h of photocatalytic reactions 18590 and 4370μmol g−1hydrogen gas have produced by TiO
2/ MK-2 / Pt and TiO2/ MK-8 / Pt, respectively. Adding co-catalysts (Cu2WS4 and Pt) give rise to increase the amount of evolved hydrogen when compared in the absence of co-catalyst (Fig. 5a-b). There is also no decreasing the photocatalytic activities after 8 h of reaction as shown in Fig. 5by linearly increasing. According to the experimental results, the photocatalytic HER performance differences between MK-2 and MK-8 have been in harmony with the photoelectrochemical studies men-tioned above. This photoactivity difference in the HER can be arised from molecular structures of dyes, which changing the strong acceptor groups of the MK-2 (carboxylic acid) and MK-8 (cyanocarboxylic acid) dyes. It is expected that MK-8 would exhibit higher HER activity due to the more electronegativity of acceptor group in the MK-8, and also more molecular absorption coefficient, which means that more ab-sorption of light than that of MK-2. However, MK-2 have been displayed more photochemical activity for the both photoelectrochemical and photocatalytic HER because of the displaying aggregate formation of MK-8 on the surface of TiO2[38–41]. Aggregate formation of MK-8 may be explained by cis–trans photoisomerization of the C]C bonds. MK-8
molecule has cyanoacrylic acid group and this group contains addi-tional double bond, which led to decrease in the photoefficiency be-cause of the nonrigid bridging moiety. It could be be-caused energy losses by photoisomerization [46]. Moreover, the photocatalytic HER activ-ities of MK-2/MK-8 sensitized TiO2 are compared to xanthane dye eosin-y (EY) in the equal conditions (Fig. 5c). Although the photo-catalytic activity of MK-2/TiO2is higher comparing to that of EY/TiO2. In addition, MK-8/TiO2is lower than EY/TiO2, which can be explained by aggregation of MK-8 dye on TiO2. The differences of photocatalytic HER activities of MK-2 and MK-8 dyes are more effective than EY dye owing to intramolecular electron-hole separation efficiencies of the dyes [7–10]. Eventually, we have speculated that D-π-A organic dyes are more efficient than xanthane dyes due to the having more dipole moment in the dye structure. This situation supplies enhanced in-tramolecular electron-hole separation efficiencies.
The photocatalytic HER performance of some D-π-A dyes on TiO2 with or without co-catalysts have been also compared to this paper [23]. These results show that MK-2 and MK-8 dyes have competitive amount of produced hydrogen from photocatalytic reactions with dif-ferent D-π-A dye sensitized TiO2.
Fig. 2. Cyclic voltammograms of MK-2 (a) and MK-8 (b) dyes (Scan rate: 100 mV s−1).
Table 2
Photophysical and energy parameters of dyes.
Dyes MK-2 MK-8
Absorption Wavelength (λ) / nm 349 343 Molar Absorption Coefficients (ε) / M−1
cm−1
915 2938
Emission Wavelength (λ) / nm 556 571 Fluorescence Quantum Yield / % 19 34 Oxidation Potentials / V 0.81 0.83 Reduction Potentials / V −0.77 / -1.56 −0.90 / -1.71 HOMO/LUMO energy levels / eV −3.63 / -5.21 −3.50 / -5.23
Fig. 3. Transient photoelectrochemical current changing of TiO2, TiO2/ MK-2 and TiO2/ MK-8 by using LSV(a) and CA (b) methods. Fig. 4. pH dependency of photocatalytic HER for dyes/TiO2.
As illustrated in theFig. 6, the proposed mechanism of HER by MK-2/MK-8 dyes and Cu2WS4is presented by energy band levels of each constituent. Light is absorbed by dyes on TiO2 and electrons in the HOMO levels are excited to LUMO levels. The photoexcited electron is injected into TiO2because of the more positive conduction band (CB) energy level of titania than dyes’ LUMO levels [22,60]. H2O could be reduced by excited electrons in the CB of TiO2to produce hydrogen without co-catalyst (Pt or Cu2WS4) because of more positive redox level of H2O/H2 (0 V) than CB energy levels of TiO2 [2]. Photoexcited electrons in the CB of TiO2could be transferred to the co-catalyst in the presence of Pt or Cu2WS4and hydrogen could be taken place on the surfaces of Pt or Cu2WS4. The HER is thermodynamically favorable in the presence of Cu2WS4because the CB energy level of Cu2WS4is po-sitioned between redox level of H2O/H2and CB energy level of TiO2 [22]. Eventually, electron donor TEOA (TEOA+/TEOA: 0.82 V) gives an electron to HOMO levels of dye molecules for the regeneration of the photocatalytic HER in our system.
4. Conclusions
Two different novel tetrazine based D-π-A dyes, entitled as MK-2 and MK-8, were synthesized for the first time. Optical and
electrochemical properties of dyes have been investigated by using absorption/photoluminescence spectroscopies and cyclic voltammetry techniques, respectively. These dyes have been used as the sensitizer for visible light the TiO2and obtained dye/TiO2applied as a photocatalyst in the photochemical HER. The HER activities of dyes have been found out unexpected results due to the aggregate formation of MK-8 on the surface of TiO2, which is proofed from EDX spectra data. Photocatalytic activities of these photocatalysts have been increased by adding co-catalyst because of having new active sites for evolving hydrogen. Dye/ TiO2photocatalysts have been displayed the good stability in the both photoelectrochemical and photocatalytic HER studies. These results show that D-π-A type MK-2 and MK-8 dyes could be used as the sen-sitizer for different solar energy conversion applications.
Author statement
Emre Aslan and Gizem Yanalak performed hydrogen evolution re-actions. Merve Karaman and Mustafa Can synthesized the organic dyes. Hakan Bilgili calculated quantum yields of dyes. Faruk Ozel synthesized the co-catalyst. Emre Aslan, Mustafa Can and Imren Hatay Patir ana-lyzed data and wrote paper.
Fig. 5. Photocatalytic HER activities against time of (a) TiO2/ MK-2, TiO2/ MK-2 / Cu2WS4, TiO2/ MK-2 / Pt,(b) TiO2/ MK-8, TiO2/ MK-8 / Cu2WS4, TiO2/ MK-8 / Pt and(c) TiO2/ EY, TiO2/ EY / Cu2WS4, TiO2/ EY / Pt.
Declaration of Competing Interest
The authors declare no competingfinancial interest. Acknowledgment
This article has been funded by Turkish Academy of Sciences via a TUBA-GEBIP fellowship, UNESCO-Loreal and The Scientific and Technological Research Council of Turkey (TUBITAK) (215M309). This study is prepared by part of Emre Aslan’s Ph.D thesis.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jphotochem.2019. 112301.
References
[1] A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature 238 (1972) 37–38.
[2] Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han, C. Li, Titanium dioxide-based nanoma-terials for photocatalytic fuel generations, Chem. Rev. 114 (2014) 9987–10043. [3] K.B. Dhanalakshmi, S. Latha, S. Anandan, P. Maruthamuthu, Dye sensitized
hy-drogen evolution from water, Int. J. Hyhy-drogen Energy 26 (2001) 669–674. [4] L. Sang, L. Lei, J. Lin, H. Ge, Co-sensitization of TiO2 electrode with Eosin Y dye and
carbon dots for photoelectrochemical water splitting: the enhanced dye adsorption and the charge transfer route, Int. J. Hydrogen Energy 42 (2017) 29686–29693. [5] J. Xu, Y. Li, S. Peng, Photocatalytic hydrogen evolution over Erythrosin B-sensitized
graphitic carbon nitride with in situ grown molybdenum sulfide cocatalyst, Int. J. Hydrogen Energy 40 (2015) 353–362.
[6] W. Zhang, R. Xu, Hybrid photocatalytic H2 evolution systems containing xanthene dyes and inorganic nickel based catalysts, Int. J. Hydrogen Energy 37 (2012) 17899–17909.
[7] Y. Wu, W. Zhu, Organic sensitizers from D-π-A to D-A-π-A: effect of the internal electron-withdrawing units on molecular absorption, energy levels and photo-voltaic performances, Chem. Soc. Rev. 42 (2013) 2039–2058.
[8] Z. Yu, F. Li, L. Sun, Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components, Energy Environ. Sci. 8 (2015) 760–775.
[9] X. Zhang, T. Peng, S. Song, Recent advances in dye-sensitized semiconductor sys-tems for photocatalytic hydrogen production, J. Mater. Chem. A 4 (2016) 2365–2402.
[10] Y. Park, W. Kim, D. Monllor-Satoca, T. Tachikawa, T. Majima, W. Choi, Role of interparticle charge transfers in agglomerated photocatalyst nanoparticles: de-monstration in aqueous suspension of dye-sensitized TiO2, J. Phys. Chem. Lett. 4 (2013) 189–194.
[11] B.E. Hardin, H.J. Snaith, M.D. McGehee, The renaissance of dye-sensitized solar cells, Nat. Photonics 6 (2012) 162.
[12] G.-G. Luo, H. Lu, Y.-H. Wang, J. Dong, Y. Zhao, R.-B. Wu, A D-π-A-π-A metal-free organic dye with improved efficiency for the application of solar energy conversion, Dye. Pigment. 134 (2016) 498–505.
[13] A. Tiwari, I. Mondal, U. Pal, Visible light induced hydrogen production over thio-phenothiazine-based dye sensitized TiO2 photocatalyst in neutral water, RSC Adv. 5 (2015) 31415–31421.
[14] A. Tiwari, U. Pal, Effect of donor-donor-π-acceptor architecture of triphenylamine-based organic sensitizers over TiO2 photocatalysts for visible-light-driven hydrogen production, Int. J. Hydrogen Energy 40 (2015) 9069–9079.
[15] W.-S. Han, K.-R. Wee, H.-Y. Kim, C. Pac, Y. Nabetani, D. Yamamoto, T. Shimada, H. Inoue, H. Choi, K. Cho, S.O. Kang, Hydrophilicity control of visible-light drogen evolution and dynamics of the charge-separated state in Dye/TiO2/Pt hy-brid systems, Chem. Eur. J. 18 (2012) 15368–15381.
[16] S.-H. Lee, Y. Park, K.-R. Wee, H.-J. Son, D.W. Cho, C. Pac, W. Choi, S.O. Kang, Significance of hydrophilic characters of organic dyes in visible-light hydrogen generation based on TiO2, Org. Lett. 12 (2010) 460–463.
[17] S.K. Choi, H.S. Yang, J.H. Kim, H. Park, Organic dye-sensitized TiO2 as a versatile photocatalyst for solar hydrogen and environmental remediation, Appl. Catal. B 121–122 (2012) 206–213.
[18] K. Jae-Hong, A. Kwang-Soon, Tri-branched tri-anchoring organic dye for visible light-responsive dye-sensitized photoelectrochemical water-splitting cells, J. Appl. Phys. 49 (2010) 060219.
[19] F.T. Yu, S.C. Cui, X. Li, Y.Y. Peng, Y. Yu, K. Yun, S.C. Zhang, J. Li, J.G. Liu, J.L. Hua, Effect of anchoring groups on N-annulated perylene-based sensitizers for dye-sen-sitized solar cells and photocatalytic H-2 evolution, Dye. Pigment. 139 (2017) 7–18. [20] M. Watanabe, H. Hagiwara, A. Iribe, Y. Ogata, K. Shiomi, A. Staykov, S. Ida,
K. Tanaka, T. Ishihara, Spacer effects in metal-free organic dyes for visible-light-driven dye-sensitized photocatalytic hydrogen production, J. Mater. Chem. A 2 (2014) 12952–12961.
[21] D.D. Konieczna, H. Biller, M. Witte, W.G. Schmidt, A. Neuba, R. Wilhelm, New pyridinium based ionic dyes for the hydrogen evolution reaction, Tetrahedron 74
(2018) 142–149.
[22] E. Aslan, M.K. Gonce, M.Z. Yigit, A. Sarilmaz, E. Stathatos, F. Ozel, M. Can, I.H. Patir, Photocatalytic H2 evolution with a Cu2WS4 catalyst on a metal free D-π-A organic dye-sensitized TiO2, D-π-Appl. Catal. B 210 (2017) 320–327.
[23] I.H. Patir, E. Aslan, G. Yanalak, M. Karaman, A. Sarilmaz, M. Can, M. Can, F. Ozel, Donor-π-acceptor dye-sensitized photoelectrochemical and photocatalytic hy-drogen evolution by using Cu2WS4 co-catalyst, Int. J. Hyhy-drogen Energy 44 (2019) 1441–1450.
[24] E. Aslan, M. Karaman, G. Yanalak, M. Can, F. Ozel, I.H. Patir, The investigation of novel D-π-A type dyes (MK-3 and MK-4) for visible light driven photochemical hydrogen evolution, Dye. Pigment. 171 (2019) 107710.
[25] K.A. Click, D.R. Beauchamp, Z. Huang, W. Chen, Y. Wu, Membrane-inspired acidically stable dye-sensitized photocathode for solar fuel production, J. Am. Chem. Soc. 138 (2016) 1174–1179.
[26] L. Li, L. Duan, F. Wen, C. Li, M. Wang, A. Hagfeldt, L. Sun, Visible light driven hydrogen production from a photo-active cathode based on a molecular catalyst and organic dye-sensitized p-type nanostructured NiO, Chem. Commun. 48 (2012) 988–990.
[27] E.A. Gibson, Dye-sensitized photocathodes for H2 evolution, Chem. Soc. Rev. 46 (2017) 6194–6209.
[28] Z. Li, Y. Chen, Y. Du, X. Wang, P. Yang, J. Zheng, Triphenylamine-functionalized graphene decorated with Pt nanoparticles and its application in photocatalytic hydrogen production, Int. J. Hydrogen Energy 37 (2012) 4880–4888. [29] Y. Lu, D. Wang, Z. Mou, J. Huang, Y. Du, P. Yang, Triphenylamine-based dye
functionalized platinum colloid for photocatalytic hydrogen evolution from water: synthesis, characterization, electron transfer, and photocatalysis, Colloids Surf. A Physicochem. Eng. Asp. 457 (2014) 282–287.
[30] Y. Xu, N. Mao, C. Zhang, X. Wang, J. Zeng, Y. Chen, F. Wang, J.-X. Jiang, Rational design of donor-π-acceptor conjugated microporous polymers for photocatalytic hydrogen production, Appl. Catal. B 228 (2018) 1–9.
[31] X. Li, S. Cui, D. Wang, Y. Zhou, H. Zhou, Y. Hu, J.-g. Liu, Y. Long, W. Wu, J. Hua, H. Tian, New organic donor–Acceptor–π–Acceptor sensitizers for efficient dye-sensitized solar cells and photocatalytic hydrogen evolution under visible-light ir-radiation, ChemSusChem 7 (2014) 2879–2888.
[32] E. Reisner, D.J. Powell, C. Cavazza, J.C. Fontecilla-Camps, F.A. Armstrong, Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 na-noparticles, J. Am. Chem. Soc. 131 (2009) 18457–18466.
[33] S. Ye, C. Ding, R. Chen, F. Fan, P. Fu, H. Yin, X. Wang, Z. Wang, P. Du, C. Li, Mimicking the key functions of photosystem II in artificial photosynthesis for photoelectrocatalytic water splitting, J. Am. Chem. Soc. 140 (2018) 3250–3256. [34] D. Jing, M. Liu, Q. Chen, L. Guo, Efficient photocatalytic hydrogen production
under visible light over a novel W-based ternary chalcogenide photocatalyst pre-pared by a hydrothermal process, Int. J. Hydrogen Energy 35 (2010) 8521–8527. [35] N. Li, M. Liu, Z. Zhou, J. Zhou, Y. Sun, L. Guo, Charge separation in
facet-en-gineered chalcogenide photocatalyst: a selective photocorrosion approach, Nanoscale 6 (2014) 9695–9702.
[36] S. Wen, Q. Dong, W. Cheng, P. Li, B. Xu, W. Tian, A benzo[1,2-b:4,5-b′]dithiophene-based copolymer with deep HOMO level for efficient polymer solar cells, Sol. Energy Mater. Sol. Cells 100 (2012) 239–245.
[37] Z. Li, J. Ding, N. Song, X. Du, J. Zhou, J. Lu, Y. Tao, Alternating copolymers of Dithienyl-s-Tetrazine and cyclopentadithiophene for organic photovoltaic applica-tions, Chem. Mater. 23 (2011) 1977–1984.
[38] J. Cheng, F. Zhang, K. Li, J. Li, X. Lu, J. Zheng, K. Guo, S. Yang, Q. Dong, A planar dithiafulvene based sensitizer forming J-aggregates on TiO2 photoanode to enhance the performance of dye-sensitized solar cells, Dye. Pigment. 136 (2017) 97–103. [39] M. Más‐Montoya, A.J. Janssen René, The effect of H‐ and J‐Aggregation on the
photophysical and photovoltaic properties of small thiophene–Pyridine–DPP mo-lecules for bulk‐heterojunction solar cells, Adv. Funct. Mater. 27 (2017) 1605779. [40] M. Pastore, F. De Angelis, Aggregation of organic dyes on TiO2 in dye-sensitized
solar cells models: an ab initio investigation, ACS Nano 4 (2009) 556–562. [41] L. Zhang, J.M. Cole, Dye aggregation in dye-sensitized solar cells, J. Mater. Chem. A
5 (2017) 19541–19559.
[42] Z.-S. Huang, T. Hua, J. Tian, L. Wang, H. Meier, D. Cao,
Dithienopyrrolobenzotriazole-based organic dyes with high molar extinction coef-ficient for efficient dye-sensitized solar cells, Dye. Pigment. 125 (2016) 229–240. [43] D.F. Swinehart, The beer-lambert law, J. Chem. Educ. 39 (1962) 333.
[44] S. Cai, X. Hu, Z. Zhang, J. Su, X. Li, A. Islam, L. Han, H. Tian, Rigid triarylamine-based efficient DSSC sensitizers with high molar extinction coefficients, J. Mater. Chem. A 1 (2013) 4763–4772.
[45] Y. Zhao, D. Xue, H. Qi, C. Zhang, Twisted configuration pyrene derivative: ex-hibiting pure blue monomer photoluminescence and electrogenerated chemilumi-nescence emissions in non-aqueous media, RSC Adv. 7 (2017) 22882–22891. [46] A. Mishra, M.K.R. Fischer, P. Bäuerle, Metal-free organic dyes for dye-sensitized
solar cells: from structure: property relationships to design rules, Angew. Chem. Int. Ed. 48 (2009) 2474–2499.
[47] C.-C. Chiu, Y.-C. Sheng, W.-J. Lin, R. Juwita, C.-J. Tan, H.-H.G. Tsai, Effects of internal electron-withdrawing moieties in d–a−π–a organic sensitizers on photo-physical properties for DSSCs: a computational study, ACS Omega 3 (2018) 433–445.
[48] A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson, Dye-sensitized solar cells, Chem. Rev. 110 (2010) 6595–6663.
[49] J. Wu, H.-Y. Li, Q.-L. Xu, Y.-C. Zhu, Y.-M. Tao, H.-R. Li, Y.-X. Zheng, J.-L. Zuo, X.-Z. You, Synthesis and photoluminescent properties of series ternary lanthanide (Eu (III), Sm(III), Nd(III), Er(III), Yb(III)) complexes containing 4,4,4-trifluoro-1-(2-naphthyl)-1,3-butanedionate and carbazole-functionalized ligand, Inorganica Chim. Acta 363 (2010) 2394–2400.
their implications in photovoltaic materials design, J. Am. Chem. Soc. 134 (2012) 4142–4152.
[55] M.K. Gonce, E. Aslan, F. Ozel, I. Hatay Patir, Dye-sensitized Cu2 XSnS4 (X=Zn, Ni,
with varied anchoring groups as photosensitizers in TiO2‐Based dye‐sensitized solar cells (DSSCs), Chem. € “Asian J. 6 (2011) 1525–1532.