Diosmin ameliorates intestinal injury induced by hepatic ischemia reperfusion in rats
Tam metin
(2) Bratisl Lek Listy 2011; 112 (10) 545 551. on ischemia-reperfusion injury were attributed to its antioxidant and antiinflammatory effects (14, 15). In the present study, we investigated the effects of diosmin on intestinal injury, and the possible mechanisms of its effects in the experimental liver ischemia-reperfusion model. Materials and methods Animals Fourty Wistar-Albino female rats, weighing 230±30 g, were housed under constant temperature (21±2 °C) individually in wire cages with 12 h light-dark cycle, fed with water and rodent chow ad libitum. Twelve hours before anesthesia, the animals were deprived of food but had free access to water 2 h before anesthesia. No enteral or parenteral antibiotics were administered at any time. The procedures in this experimental study were performed in accordance with the National Guidelines for The Use and Care of Laboratory Animals and approved by Animal Ethics Committee of Ankara Research and Training Hospital. Study groups and operative procedure Rats were randomly divided into four groups, each including 10 animals: sham group (Group 1), control group (Group 2), perop diosmin treatment group (Group 3) and preop diosmin treatment group (Group 4). Animals were anesthetized by intramuscular injection of 80 mg/kg ketamine hydrochloride (Ketalar®; Parke- Davis, Istanbul, Turkey) and 20 mg/kg xylasine (Rompun®, Bayer, Istanbul, Turkey). After shaving and disinfecting the abdomen, a midline incision was made and rats underwent either sham surgery or ischemia-reperfusion. Ischemia was carried out by clamping the hepatic pedicle for 60 min with a vascular clamp (Vascu-Statt® II Plus, U.S.A). After the ischemic period, the liver was reperfused by opening the clamp, and reperfusion was achieved for 90 min. In the perop treatment group (Group 3), diosmin was given to the rats just after ischemia induction at a dose of 50 mg/kg in gavage form with orogastric tube; therefore the peak plasma levels of diosmin had been achieved before the reperfusion was initiated. In the preop treatment group (Group 4), 50 mg/kg/day diosmin (Vendios®; Bilim, Istanbul, Turkey) was given to the rats one dose daily for ten days before the operation, in gavage form with orogastric tube (7 Gauge feeding tube) that was inserted daily and taken off after drug administration. At the end of procedures, blood and ileum tissue samples were obtained for biochemical and histopathological assessments. Biochemical analyses A portion of ileum was stored at 80 °C for oxidative stress analyses. Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) activities were measured for evaluating the liver functions by using Olympus Au 640 autoanalyzer. To assess the oxidative injury, malondialdehyde (MDA) levels, glutathione peroxidase (GSHPx) and xanthine oxidase (XO) enzyme activities were determined in blood and ileum samples. 546. Evaluation of oxidative stress After scarifying the animals, ileum samples were removed and kept in ice bath until homogenization. The sample of ileum was first washed with distilled water, the tissues were homogenized in physiological saline (20 % w/v, approximately 1 g in 5 mL for each), then they were centrifuged 4000xg for 15 min and upper clear supernatants were used in the assays. All procedures were performed at +4 °C throughout the experiments. The protein level of clear supernatants was studied by Lowrys method (16), and then adjusted to equal concentrations before other analyses. All results were expressed as unit/mg protein for those of ileum tissues. Malondialdehyde (MDA) level (nmol/mg), glutathione peroxidase (GSH-Px) enzyme activity (mlU/mg) and xanthine oxidase (XO) enzyme activity (µIU/mg) were measured in the supernatants. The MDA level was measured by the thiobarbituric acid reactive substances method. The GSH-Px activity was measured by following the changes in NADPH absorbance at 340 nm. The xanthine oxidase activity was determined by measuring the uric acid formation at 293 nm by using a spectrophotometer. The extinction coefficient of uric acid was used in the activity (international unit, IU) calculation of the XO enzyme. Plasma MDA levels, GSH-Px and XO activities were determined by the same methods and expressed as unit/ml plasma (1719). Histological evaluation For light microscope analyses, tissue samples from the ileum were obtained from all animals. In order to avoid mucosal damage, the intestinal lumen was carefully cannulated and gently washed with normal saline solution before sampling. The ileal samples were fixed in 10% neutral buffered formalin solution for 2 days. Tissues were washed in flowing water and dehydrated with rising concentrations of ethanol (50 %, 75 %, 96 %, and 100 %). After dehydration, the specimens were put into xylene to obtain transparency and then infiltrated and embedded in paraffin. Embedded tissues were cut into sections of 5-µm thickness by Leica RM 2125 RT. Randomly selected sections were systematically stained with hematoxylin and eosin (H&E) and Periodic Acid-Schiff reaction. Histopathological examinations were performed by two histologists blinded to the study design, and photographed with a Nikon eclipse E 600, panelled and marked with scale bars. The specimens were evaluated by Chiu scale consisting values from 0 to 5, where 0 is normal mucosa; 1 is development of subepithelial (Gruenhagens) spaces; 2 is extension of the subepithelial space with moderate epithelial lifting from the lamina propria; 3 is extensive epithelial lifting with occasional denuded villi tips; 4 is denuded villi to the level of lamina propria and dilated capillaries; and 5 is disintegration of the lamina propria, hemorrhage, and ulceration (20). Statistical analysis The data analysis was performed using SPSS 15.0 package program. The data were presented as mean ± standard deviation. The fact whether there were any differences among the groups.
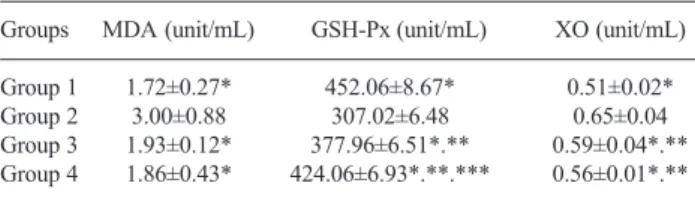
(3) Tanrikulu Y et al. Diosmin ameliorates intestinal injury induced by hepatic ischemia…. was evaluated by One-Way ANOVA or Kruskal Wallis variance analysis, where appropriate. When the p-value from the variance analysis was statistically significant, Mann-Whitney U test for multiple comparisons was used to know which group differs from which others. All results were accepted as statistically significant when p<0.05. Results All rats were sacrificed on postoperative day 10. No rat died during the experimental period. Biochemical analyses The results of liver function tests of the groups are summarized in Table 1. There was a significant difference between the control group (Group 2) and the other groups (p<0.001 for all). The difference between the sham group (Group 1), perop group (Group 3) and preop group (Group 4) were also significant (p<0.001 for all). There was no significant difference between the perop and preop groups according to the levels of AST and LDH (p>0.05). ALT levels were higher in perop group than preop group while the difference was significant (p<0.001). Oxidative stress The plasma levels of MDA, GSH-Px and XO are summarized in Table 2. There was a significant difference between the control (Group 2) and other groups (p<0.05 for all). The difference between the sham group (Group 1), perop group (Group 3) and preop group (Group 4) were also significant for GSH-Px and XO (p<0.001 for all) but there was no significant difference between these groups according to MDA levels (p>0.05). There was no significant difference between the perop and preop groups according to MDA, while XO (p>0.05) as well as GSH-Px levels were significantly higher in preop group than perop group (p<0.001). The ileum tissue levels of MDA, GSH-Px and XO are summarized in Table 3. There was a significant difference between the control (Group 2) and other groups (p<0.05 for all). The difference between sham group (Group 1), perop group (Group 3), and preop group (Group 4) were also significant for MDA, GSHPx and XO (p?0.001 for all). There was no significant difference between the perop and preop groups according to the levels of MDA and GSH-Px (p>0.05). XO levels were significantly higher in preop group than perop group (p=0.041).. Tab. 1. Liver function tests. Groups. AST (U/L). ALT (U/L). LDH (U/L). Group 1 197.60±18.15* 36.60±3.13* 992.10±92.45* Group 2 465.60±43.49 201.20±49.49 4649.00±360.32 Group 3 318.60±108.32*.** 159.40±83.59*.**.*** 1829.50±365.60*.** Group 4 282.60±38.02*.** 78.40±16.04*.** 1606.00±254.62*.** Abbreviations: ALT alanine aminotransferase, AST aspartate aminotransferase, LDH lactate dehydrogenase, *p<0.001 vs. control, **p<0.001 vs. sham, ***p<0,001 vs. group 4. Tab. 2. Plasma oxidative stress activities of the groups. Groups. MDA (unit/mL). Group 1 Group 2 Group 3 Group 4. 1.72±0.27* 3.00±0.88 1.93±0.12* 1.86±0.43*. GSH-Px (unit/mL). XO (unit/mL). 452.06±8.67* 307.02±6.48 377.96±6.51*.** 424.06±6.93*.**.***. 0.51±0.02* 0.65±0.04 0.59±0.04*.** 0.56±0.01*.**. Abbreviations: MDA malondialdehyde, GSH-Px glutathione peroxidase enzyme activity, XO xanthine oxidase enzyme activity, *p<0.05 vs. control, **p<0.001 vs. sham, ***p<0,001 vs. group 3. Tab. 3. Tissue oxidative stress activities of the groups. Groups. MDA (nmol/mg). Group 1 Group 2 Group 3 Group 4. 0.33±0.03* 0.53±0.07 0.41±0.02*.** 0.41±0.02*.**. GSH-Px (mlU/mg) 19.50±3.18* 11.27±1.92 12.42±0.91*.** 12.90±1.16*.**. XO (µIU/mg) 112.56±10.38* 163.39±21.96 141.40±18.84*.** 142.27±4.05*.**.***. Abbreviations: MDA malondialdehyde, GSH-Px glutathione peroxidase enzyme activity, XO xanthine oxidase enzyme activity, *p<0.05 vs. control, **p≤0.001 vs. sham, ***p=0.041 vs. group 3. Histopathological results We evaluated the specimens in a systematic fashion including the assessment of villous architecture, surface and crypt epithelia, lamina propria constituents and submucosal structures. In all specimens from the sham group (Group 1), the histologic features showed a regular appearance of ileal tissue (Figs 1 A1, 2, 3, 4). The specimens of the ischemia-reperfusion group (Group 2) showed specific morphological abnormalities. The epithelial lining of the apical surface of villi was degenerated and desqua-. Tab. 4. Histopathological scores of the groups*. Number of rats. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Group 1 Group 2 Group 3 Group 4. 0 4 0 0. 1 4 0 0. 0 4 0 0. 0 4 1 0. 0 4 0 0. 0 5 2 0. 0 4 0 0. 0 4 0 1. 0 4 0 0. 0 5 0 0. * The specimens were evaluated by Chiu scale.. 547.
(4) Bratisl Lek Listy 2011; 112 (10) 545 551. Fig. 1. This panel of rat ileum is stained with hematoxylin and eosin (1st and 2nd micrographs of each group on the left two columns of panel) and Periodic Acid-Schiff reaction (3rd and 4th micrographs of each group on the right two columns of panel). A) The first line is the micrographs of Group I, B) The second line is the micrographs of Group II, C) The third line is the micrographs of Group III and D) The fourth line is the micrographs of Group IV. A The normal appearance of ileum with muscularis layer (M) and villi (V) surrounding the lumen (L). The enterocytes and goblet cells (G) lining the surface of villi and the lamina propria (Lp) under the epithelium are in regular aspect. B The lumen filled with debris (D), inflammation (I) and congestion (*) in the lamina propria and desquamation of the epithelial tissue (arrow) on the tips of villi (Tv) are seen in the ischaemia-reperfusion group. C, D Diosmin groups showing morphology similar to the sham group.. mated and thus the lumen was filled with cellular debris and deposits (Fig. B1). Capillary dilatation and congestion was widespread in the lamina propria under the epithelium. The tips of villi became blunt; lamina propria was disintegrated, while the covering epithelium was absent. The congestion and mononuclear leukocyte infiltration were present in the lamina propria of villi. The disintegration of lamina propria was prevalent (Figs B2). The PAS-stained sections of ileum yielded a significant depletion in the number of goblet cells. The disintegration of lamina propria and the debris of desqumated epithelial cells were easily viewed in these PAS-stained sections (Figs B3,4). The peroperative diosmin-administered group (Group 3), showed an ileal histomorphology similar to that of the sham group. The villus integrity was well preserved in the whole tissue (Figs C1, 2, 3, 4). The covering epithelial tissue was intact. Although the congestion and increased cellularity were current in the lamina propria of villi, only a small number of villi presented villous blunting due to congestion (Fig. C2). The number of goblet cells was slightly reduced in contrast to sham group (Figs C3, 4). Ten days preoperatively, the diosmin-administered group (Group 4) represented a regular architecture of the ileum (Fig. D1). The 548. congestion and increased cellularity in lamina propria was minimal (Fig. D2). The reduction in the number of goblet cells was not observed in this group (Figs D3, 4). The pathological scores of the groups were given in Table 4. According to the pathological scores, there was a significant difference between Group 2 and other groups (p<0.001 for all groups). There was no significant difference between the sham group (Group 1) and treatment groups (Groups 3 and 4) (p>0.05). Discussion Ischemia reperfusion injury is a phenomenon whereby the cellular damage in a hypoxic organ is accentuated following the restoration of oxygen delivery. An interruption of organ's blood flow with its subsequent lack of oxygen and nutrient supply is an inherent phenomenon during diverse surgical procedures. In liver surgery, there are clinical situations in which the ischemic periods can be particularly long, such as during the resection of large hepatic tumors, management of hepatic trauma of diverse origins, vascular reconstructions, and liver procurement for transplantation. Once the blood flow and oxygen supply are reestab-.
(5) Tanrikulu Y et al. Diosmin ameliorates intestinal injury induced by hepatic ischemia…. lished, the reperfusion enhances the injury caused by the ischemic period, thus aggravating the damage caused at the cellular level (3, 21). During the ischemic period, several functional changes occuring at the cellular level promote cell injury. The initial phase (<2 h after reperfusion) is characterized by oxidant stress, where the production and release of reactive oxygen species appear to directly result in hepatocellular injury. The complement activation is also critical in the initial phase of ischemia-reperfusion injury since the depletion of complement reduces the Kupffer cell-induced oxidant stress. The late phase of liver injury from 6 to 48 h after hepatic reperfusion is an inflammatory disorder mediated by recruited neutrophils; these damage the hepatocytes, at least partly through releasing the reactive oxygen species. A significant portion of the injury is a result of leukocyte-dependent damage. It appears that resident lymphocytes modulate the inflammation by producing the cytokines. The expression of these mediators will then result in the recruitment of more lymphocytes and neutrophils (3, 21, 22). All cells generate reactive oxygen species and the most general source is the mitochondrion. Other known sources of reactive oxygen species are phagocytic cells, neutrophils and monocytes. These produce large quantities of extracellular reactive oxygen species. Oxidative stress results from an imbalance between prooxidants and antioxidants, in which the former predominate. Cells can adapt to increased oxidative stress up to a threshold, particularly if it is imposed slowly. However, when oxidative stress becomes overwhelming, the impairment of cellular function becomes irreversible and cell death occurs (21). Since the endogenous antioxidant levels decrease significantly during reperfusion, the administration of exogenous antioxidants, particularly in the early stages of reperfusion, could significantly decrease the severity of ischemia-reperfusion damage (3). Although the results are still not entirely clear, there is accumulative evidence, mainly from in vitro and in vivo experimental studies, that the administration of antioxidant substances can reduce the postreperfusion injury (21-23). In conclusion, hepatic ischemia-reperfusion injury involves an interaction between different cell types and a variety of cellular and molecular mechanisms including Kupffer cell activation, formation of reactive oxygen species, release of cytokines and chemokines, and neutrophil recruitment. Ischemia reperfusion leads also to microcirculatory failure. The loss of delicate equilibrium between nitric oxide and endothelin induces vasoconstriction, narrows the sinusoidal lumen, and compromises the leukocyte flow, thus bringíng the latter corpuscles in close contact with the capillary wall. The increased contact between leukocytes and endothelial cells promotes leukotaxis, and although not occluding the capillary lumen completely, the trapped leukocytes interfere with the flow of blood through the sinusoidal capillaries. The platelet aggregation within the hepatic sinusoids further aggravates the turbulent flow rate through the partially occluded capillaries (3). The gastrointestinal tract performs a variety of functions in digestion, namely selective absorption, and secretion. However,. its barrier function preventing the spread of intraluminal bacteria and endotoxins to organs and tissues plays the key role. Gut barrier failure may result from one or more of the three basic pathophysiologic conditions, namely disruption of the normal ecologic balance of the indigenous gut microflora, impaired host immune defenses, and physical disruption of the gut mucosal barrier (24-27). Intestinal ischemia-reperfusion produces a systemic inflammatory response with multiple organ failures (28). Hepatic ischemia-reperfusion injury induced by total occlusion of the portal vein and hepatic artery causes more serious damage to liver, compared with the partial liver ischemia. The more severe damage to liver in turn produces and releases greater quantities of destructive proinflammatory cytokines and oxygenderived radicals into circulation, thus causing subsequent damage to other organs including the kidney, lung, and intestine (4) Moreover, the portal venous congestion is an important event in hepatic ischemia-reperfusion that slows down the blood flow in the intestinal wall thus causing intestinal bacterial overgrowth. Portal venous congestion may result in extensive mesenteric venous congestion, which greatly slows down the blood flow within the intestinal wall thus causing stagnant anoxia in tissues, intestinal mucosal lesions, and abnormalities in the coordinated motor function of small bowel (29). Xing et al (5) found an alteration in bacterial population of the intestine in liver ischemia-reperfusion injury model. This alteration was associated with the damage of microvilli in the epithelial apical surface, as well as with the disruption of epithelial tight junctions in ileum. They concluded that these results strongly suggested that ischemia-reperfusion liver injury could alter the intestinal microflora and damage the intestinal mucosal barrier function, and thus possibly lead to the elevation of plasma endotoxin and BT to multiple organs. Considering the complex and multiple mechanisms that are involved in the pathogenesis of ischemic cell injury, it is not surprising that different substances and methods have been tried to abolish or reduce the concequences of ischemia-reperfusion. Diosmin is a naturally occurring flavonoid glycoside that can be isolated from various plant sources or derived from flavonoid hesperidin. Diosmin is considered a vascular-protecting agent used to treat chronic venous insufficiency, hemorrhoids, lymphedema, and varicose veins. As a flavonoid, diosmin also exhibits anti-inflammatory, free-radical scavenging, and antimutagenic properties. Diosmin acts by improving the venous tone, increasing the lymphatic drainage, protecting the capillary bed microcirculation, inhibiting the inflammatory reactions, and reducing the capillary permeability (30). The micronised purified flavonoid fraction inhibits the activation, migration and adhesion of leukocytes at the capillary level. This leads to a reduction in the release of inflammatory mediators such as oxygen free radicals, prostoglandins and thromboxane, resulting in a decrease in capillary hyperpermeability (6). Diosmin-hesperidin was reported to have radical scavenging properties in previous reports (31-33). Bouskela et al (33) has found that diosmin-hesperidin prevents reperfusion injury in hamster cheek pouch ischemia/reperfusion injury model. It was 549.
(6) Bratisl Lek Listy 2011; 112 (10) 545 551. reported that ischemia/reperfusion induced an increase in microvascular permeability while leukosyte adhesion was inhibited by diosmin-hesperidin, which is consistent with the improvement in blood flow through the microvascular network. This protective effect might include a decrease in endothelial cell swelling with a consequent decrease in flow resistance. Another possibility for the observed effects of diosmin-hesperidin is an antioxidant effect (13, 34-36). Pehlivan et al (11) found that diosminhesperidin was effective in preventing the intestinal reperfusion injury after oral administration. Prophylaxis of intestinal reperfusion injury via orally administrated diosmin-hesperidin may be preferential for clinical purposes especially in high-risk patients for mesenteric ischemia. In the present study; we investigated the effect of diosmin on intestine in the hepatic ischemia-reperfusion injury. There was a significant difference between the control and diosmin groups according to the results of liver function tests. We evaluated the specimens of ileum in a systematic fashion including the assessment of villous architecture, surface and crypt epithelia, lamina propria constituents and submucosal structures. The specimens of the ischemia-reperfusion group showed specific morphological abnormalities. The epithelial lining of the apical surface of villi was degenerated and desquamated, and thus the lumen was filled with cellular debris and deposits. Capillary dilatation and congestion was widespread in the lamina propria under the epithelium. The tips of villi became blunt, lamina propria was disintegrated and the covering epithelium was absent. The preoperative and peroperative diosmin-administered groups showed an ileal histomorphology similar to the sham group. The villus integrity was well preserved in the whole tissue. The covering epithelial tissue was intact. Although the number of goblet cells was slightly reduced in the peroperative group, the reduction in the number of goblet cells was not observed in the preoperatively treated group. We also evaluated the possible mechanisms of beneficial effects of diosmin on intestine in hepatic ischemia reperfusion injury. Although the mechanism is not clear, the protective activity of diosmin seems to rely on antioxidant and antiinflammatory effects. Flavonoids can prevent injury caused by free radicals in various ways. One of them is based on direct scavenging of free radicals. Flavonoids stabilize the reactive oxygen species by reacting with the reactive compound of the radical. Since the reactivity of the hydroxyl group of flavonoids is high, the radicals become inactive. The xanthine oxidase pathway has been implicated as an important route in the oxidative injury to tissues, especially after ischemia-reperfusion. In the reperfusion phase, xanthine oxidase reacts with molecular oxygen, thereby releasing the superoxide free radicals. Flavonoids inhibit xanthine oxidase activity, thereby resulting in decreased oxidative injury (15, 37, 38). Glutathione is a tripeptide present in millimolar concentrations in virtually all cells. It is an important component of the endogenous antioxidant system. The main function of glutathione is to act as a cosubstrate of glutathione peroxidase to reduce intracellularly generated peroxides. Glutathione also scavenges 550. directly reactive oxygen species and reactive nitrogene species (23). Malondialdehyde (MDA) is the principal and most studied product of polyunsaturated fatty acid peroxidation. This molecule is able to interact with nucleic acid bases to form several different adducts. All of these potentially genotoxic activities of MDA may lead to mutations and subsequently to cancer. MDA is able to impair several physiological mechanisms of the human body through its ability to react with molecules such as DNA and proteins. It is therefore useful to consider this molecule as something more than a lipid peroxidation product (39). In the present study, we evaluated the oxidative stress by measuring the plasma and ileum tissue levels of MDA, GSH-Px and XO. Diosmin treatment significantly decreased the oxidative stress both in preoperative and peroperatively treated groups (Tabs 2 and 3). Histopathologically, we determined the inflammatory status of the ileum specimens from control and diosmin-treated groups. Although a significant congestion and mononuclear leukocyte infiltration were present in the lamina propria of villi in the control group, these morphological findings of inflamation were minimal in diosmin-treated groups. Conclusion The present study was the first study dealing with the effect of diosmin on intestine subjected to hepatic ischemia-reperfusion injury. When we compared the preoperatively and peroperatively treated groups, better results were achieved in the preopratively treated group but the difference was not statistically significant. According to these results, we concluded that diosmin could be administered for protection from destructive effects of hepatic ischemia-reperfusion injury on intestine in both emergent and elective hepatic surgical operations in which the possible ischemic periods are expected. References 1. Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury-a fresh look. Exp Mol Pathol 2003; 74: 8693. 2. Suzuki S, Inaba K, Konno H. Ischemic preconditioning in hepatic ischemia and reperfusion. Curr Opin Organ Transplant 2008; 13: 1427. 3. Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA et al. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res 2008; 147: 153159. 4. Jiang H, Meng F, Li W et al. Splenectomy ameliorates acute multiple organ damage induced by liver warm ischemia reperfusion in rats. Surgery 2007; 141: 3240. 5. Xing HC, Li LJ, Xu KJ et al. Intestinal microflora in rats with ischemia/reperfusion liver injury. J Zhejiang Univ Sci B 2005; 6: 1421. 6. Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003; 63: 71100. 7. Cotelle N, Bernier JL, Catteau JP et al. Antioxidant properties of hydroxy-flavones. Free Radic Biol Med 1996; 20: 3543..
(7) Tanrikulu Y et al. Diosmin ameliorates intestinal injury induced by hepatic ischemia… 8. Bors W, Heller W, Michel C et al. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol 1990; 186: 343355. 9. Unlu A, Sucu N, Tamer L et al. Effects of Daflon on oxidative stress induced by hindlimb ischemia/reperfusion. Pharmacol Res 2003; 48: 1115. 10. Bouskela E, Cyrino FZ, Lerond L. Leukocyte adhesion after oxidant challenge inthe hamster cheek pouch microcirculation. J Vasc Res 1999; 36: 1114. 11. Pehlivan M, Hazinedaroglu SM, Kayaoglu HA et al. The effect of diosmin hesperidin on intestinal ischaemiareperfusion injury. Acta Chir Belg 2004; 104: 715718. 12. Pickelmann S, Nolte D, Schütze E, Messmer K. Effect of Daflon 500 mg onreperfusion damage following ischemia and reperfusion of striated muscle. Langenbecks Arch Chir Suppl Kongressbd 1998; 115 (Suppl I): 353356. 13. Bouskela E, Donyo KA. Effects of oral administration of purified micronized flavonoid fraction on increased microvascular permeability induced by various agents and on ischemia/reperfusion in the hamster cheek pouch. Angiology 1997; 48 (5): 391399. 14. Korthui RJ, Gute DC. Anti-inflammatory actions of a micronized, purified flavonoid fraction in ischemia/reperfusion. Adv Exp Med Biol 2002; 505: 181190. 15. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001; 74 (4): 418425. 16. Lowry OH, Rosebrough NJ, Farr AL et al. Protein measurement with folin phenol reagent. J Biol Chem 1951; 182: 265275. 17. Dahle LK, Hill EG, Holman RT. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys 1962; 98: 253261. 18. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70: 158169. 19. Hashimoto S. A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal Biochem 1974; 62: 426435. 20. Chiu CJ, McArdle AH, Brown R et al. Intestinal mucosal lesion in low-flow states. I. A. morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970; 101: 478483. 21. Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 2003; 18: 891902. 22. Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol 2007; 82: 457464. 23. Glantzounis GK, Salacinski HJ, Yang W et al. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl 2005; 11: 10311047.. 24. Assimakopoulos SF, Vagianos CE, Charoni SA et al. Intestinal failure in obstructive jaundice. World J Gastroenterol 2005; 11: 38063807. 25. Kayama S, Mitsuyama M, Sato N et al. Overgrowth and translocation of Escherichia coli from intestine during prolonged enteral feeding in rats. J Gastroenterol 2000; 35: 1519. 26. De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med 2005; 33: 11251135. 27. Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil 2005; 26: 383391. 28. Stefanutti G, Pierro A, Parkinson EJ et al. Moderate hypothermia as a rescue therapy against intestinal ischemia and reperfusion injury in the rat. Crit Care Med 2008; 36: 15641572. 29. Kirimlioglu V, Kirimlioglu H, Yilmaz S, et al. Effect of steroid on mitochondrial oxidative stress enzymes, intestinal microflora, and bacterial translocation in rats subjected to temporary liver inflow occlusion. Transplant Proc 2006; 38: 378381. 30. Monograph. Diosmin. Altern Med Rev 2004; 9: 308311. 31. Pickelmann S, Nolte D, Leiderer R, et al. Effects of the phlebotropic drug Daflon 500 mg on postischemic reperfusion injury in striated skin muscle: a histomorphologic study in the hamster. J Lab Clin Med 1999; 134: 536545. 32. Korthui RJ, Gute DC. Adhesion molecule expression in postischemic microvascular dysfunction: activity of a micronized purified flavonoid fraction. J Vasc Res 1999; 36: 1523. 33. Bouskela E, Cyrino FZ, Lerond L. Effects of oral administration of different doses of purified micronised flavonoid fraction on microvascular reactivity after ischaemia/reperfusion in the hamster cheek pouch. Br J Pharmacol 1997; 122: 16111616. 34. Walker PM, Lindsay TF, Labbe R et al. Salvage of skeletal muscle with free radical scavengers. J Vasc Surg 1987; 5: 6875. 35. Potter RF, Dietrich HH, Tyml K et al. Ischemia-reperfusion induced microvascular dysfunction in skeletal muscle: application of intravital video microscopy. Int J Microcirc Clin Exp 1993; 13: 173186. 36. Wolin MS. Reactive oxygen species and vascular signal transduction mechanisms. Microcirculation 1996; 3: 117. 37. Korkina LG, Afanasev IB. Antioxidant and chelating properties of flavonoids. Adv Pharmacol 1997; 38: 151163. 38. Sanhueza J, Valdes J, Campos R, Garrido A, Valenzuela A. Changes in the xanthine dehydrogenase/xanthine oxidase ratio in the rat kidney subjected to ischemia-reperfusion stress: preventive effect of some flavonoids. Res Commun Chem Pathol Pharmacol 1992; 78 (2): 211218. 39. Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 2005; 15 (4): 316328. Received April 1, 2010. Accepted May 25, 2011.. 551.
(8)
Şekil
Benzer Belgeler
These cations precipitate as sulfides and hydroxides when reacted with hydrogen sulfide (H 2 S) or thioacetamide solution in basic medium buffered with NH 4 OH – NH 4 Cl..
This group contains Ag + , ve Pb 2+ ions, and the main prominent feature of this group is that they precipitate as insoluble chloride salts in water in the presence of diluted
Cation group 1- All students complete the procedure given in the flowchart below for KNOWN sample analysis.. Cation group 1- Each student complete the procedure for
When the species leaves the flame, the excited electron returns to the lower energy level and the energy of the transition is emitted as light, with a
In all analysis schemes, precipitates are enclosed in boxes with solid lines, solutions are contained in boxes with dashed lines... Qualitative analysis flowchart for
• Students are asked to search for suitable herbal remedies for each case.. • Presentation of each group:
Normal control (NC) group: 餵食 餵食 chow chow 飲食 飲食 Control group:. Control group: 餵食 餵食 semi purified semi purified 飲食 飲食
● Her orta gerilim hücresi için bir veya daha fazla pano ısıtıcı ve ayrı bir termostat (var ise) kullanılmalıdır.. ● Termostat kullanılmamalı veya çok geniş