Abstract.
Background/Aim: The aim of the study was to
evaluate the effect of rutin, which is a vitamin P1 flavonoid
with anti-inflammatory and anti-edema effects, on traumatic
brain injury (TBI) and edema in rats. Materials and Methods:
Rats were divided into 3 groups as sham group without brain
trauma (SG), brain trauma without medication (BT) group
and Rutin treated brain trauma (RBT) group. Fifty mg/kg rutin
was administered to the RBT group once a day for three days.
On the fourth day, rats were sacrificed. Extracted brain tissues
were examined biochemically and histopathologically. Results:
We found that the levels of malondialdehyde, nuclear
factor-kappa B and tumor necrosis factor-alpha decreased, and those
of total glutathione increased significantly. Furthermore, rutin
administration reduced pyramidal neuron degeneration and
poly-morpho-nuclear-leucocyte accumulation due to trauma
in brain tissue, while eliminating edema. Conclusion: Rutin
might be effective in the treatment of TBI and TBI-related
brain edema.
Traumatic brain injury (TBI) is an important public health
problem that can be associated with mortality and disability
(1). TBI is an important public health problem in the United
States with an annual incidence of approximately 1.7 million,
of which 50,000 injuries result in death (2). According to the
literature, the incidence of TBI is estimated to be 939 cases
per 100,000 individuals (3).
TBI occurs as a result of various mechanical traumas due
traffic accidents and falls (4). TBI can be classified as primary
and secondary damage. Primary TBI occurs after impact. In the
primary TBI phase, contusions, rupture of blood vessels, and
intracranial hemorrhage constitute ongoing brain damage (5).
Secondary TBI occurs a few seconds after the primary injury.
This phase is characterized by neuroflamation, oxidative stress,
and increased intracranial pressure due to brain edema (6). Niu
X et al. have demonstrated the role of tumor necrosis factor
alpha (TNF-α) and other proinflammatory cytokines in the
pathogenesis of TBI. In addition, increased free oxygen radical
(FOR) and malondialdehyde (MDA) production and decreased
glutathione peroxidase (Gpx), glutathione (GSH) and
superoxide dismutase (SOD) levels have been associated with
TBI (7). Nuclear factor kappa B (NF-ĸB) is also known to
mediate the transcription of inflammatory genes in TBI (8).
Furthermore, it has been reported that NF-ĸB is responsible for
brain edema developing in TBI (9). All this information
suggests that antioxidant, anti-inflammatory and anti-edema
factors might be useful for the treatment of TBI and
TBI-related brain edema (TBIE).
In this study, we investigated the effect of rutin
(3,3,4,5,7-pentahydroxyflavone-3-rhamnoglucoside), a vitamin P1
flavonoid, against TBI in rats (10). Rutin has been found to be
effective against edema that develops as a result of increased
capillary permeability and plasma leakage into tissue (11). It has
been demonstrated that rutin suppresses the production of
NF-ĸB and TNF-α, two proinflammatory factors (12), indicating
that rutin can be useful in preventing TBIE development.
However, no studies have investigated the effect of rutin on
brain injury and edema induced by mechanical trauma in rats.
Therefore, the aim of this study was to evaluate the effect of
rutin on TBI in rats biochemically and histopathologically.
This article is freely accessible online.Correspondence to: Selcuk Ozdogan, MD, Ph.D., Department of Neurosurgery, Beykent University School of Medicine, Beykent Universitesi Tıp Fakultesi Beyin Cerrahisi Anabilim Dalı, 34000 Beylikduzu/Istanbul, Turkey. Tel: +90 5067637173, e-mail: selcukozdogan@beykent.edu.tr
Key Words: Traumatic brain injury, rutin, brain edema.
The Effect of Rutin on Experimental Traumatic
Brain Injury and Edema in Rats
SAHIN YUCELI
1, GULCE NAZ YAZICI
2, RENAD MAMMADOV
3,
HALIS SULEYMAN
3, MUSTAFA KAYA
4and SELCUK OZDOGAN
5 1Department of Neurosurgery, Neon Hospital, Erzincan, Turkey;
2
Department of Histology and Embryology,
Erzincan Binali Yildirim University School of Medicine, Erzincan, Turkey;
3
Department of Pharmacology, Erzincan Binali Yildirim University School of Medicine, Erzincan, Turkey;
4Department of Neurosurgery, Sakarya Training and Research Hospital, Sakarya, Turkey;
Materials and Methods
Rats were obtained from the Ataturk University Medical Experimental Application and Research Center. Eighteen male albino Wistar rats weighing 285-296 grams were used in the experiment. The animals were fed and kept for one week at room temperature (22˚C, pressure of 1 atm) for adaptation to the environment. After one week, the experiment was performed on. The procedures and protocols were approved by the Ataturk University Animal Experimentation Ethics Committee (Date: 28.03.2019 Meeting No:4).
Chemicals. Ketamin was obtained from Pfizer (Istanbul, Turkey) and Rutin was obtained from Solgar (Leonia, NJ, USA).
Experimental groups. Rats were divided into three groups: Sham group (SG) with no trauma or medication, mechanical brain trauma (BT) group, and 50 mg/kg rutin administered mechanical brain trauma (RBT) group.
Experimental procedure. Surgical procedures were performed under sterile conditions in an appropriate laboratory environment. The Marmarou's impact acceleration model was modified and used to create the brain trauma model (13). In this model, a cylindrical tube with an inner diameter of 18 mm and an outer diameter of 19 mm was used. Ketamine hydrochloride was administered intraperitoneally at a dose of 60 mg/kg to the rats. Subsequently, rats were placed in prone position on cork blocks. In order to prevent collapse fractures in the skull, a 10 mm-diameter and 3 mm-thick steel disc was inserted following skin incision between the coronal and lamdoid sutures. Head trauma was created by applying a mass of 300 g via free fall from a height of one meter. BT group and SG group were orally administered with 0.5 ml normal saline (0.9% NaCl) as solvent. Fifty mg/kg rutin was administered orally to the RBT group. Rutin and distilled water were administered once a day for three days. Animals were fed with standard rat feed, each in a separate cage at an appropriate environment and room temperature (22˚C). On the fourth day, rats were sacrificed by high-dose ketamine (120 mg/kg) anesthesia and brain tissues were removed. MDA, tGSH, NF-ĸB, and TNF-α levels were measured in the extracted brain tissues. Brain tissues were also examined histopathologically. MDA analysis. Tissue samples were placed in 2 ml 1.15% potassium chloride solution for MDA analysis or in pH=7.5 phosphate buffer for other measurements and homogenized on ice. Then, the homogenates were centrifuged at +4˚C for 10 min at 9000 ×g. The supernatants were used for the analysis.
MDA levels were measured according to the Ohkawa H et al. method (14). This method is based on the spectrophotometric measurement (at 532 nm) of the absorbance of the pink colored complex formed by thiobarbituric acid (TBA) and MDA at high temperature (95˚C). The homogenates were centrifuged at 5000×g for 20 min and these supernatants were used to quantify MDA. One hunderd μl of 8% sodium dodecyl sulfate (SDS), 250 μl of homogenate, 750 μl of 20% acetic acid, 750 μl of 0.08% TBA and 150 μl of pure water were pipetted into capped tubes and vortexed. The mixture was incubated at 100˚C for 60 min, and then 2.5 ml of n-butanol were added and spectrophotometrically measured. The resulting red color was measured using 3 ml cuvettes at 532 nm and the MDA levels were determined by using a standard curve generated using various concentrations of MDA.
tGSH analysis. DTNB [5,5’-Dithiobis (2-nitrobenzoic acid)] in the measuring medium is a disulfide chromogen and is easily reduced by compounds with sulfhydryl groups. The resulting yellow color was measured spectrophotometrically at 412 nm (15). The homogenates were centrifuged at 12,000×g for 10 min and the supernatants were used to determine the amount of GSH. 1500 μl measuring buffer (200 mM Tris-HCl containing 0.2 mM EDTA, pH=8.2), 500 μl supernatant, 100 μl 5,5
’
-Dithio-bis (2-nitrobenzoic acid) (DTNB), and 7,900 μl methanol were pipetted into capped tubes and vortexed. The mixture was incubated at 37˚C for 30 min and then the absorbance was measured at 412 nm. The GSH levels in the samples were determined by using a standard curve generated using various concentrations of GSH.TNF-α and NF-ĸB analysis. Tissue-homogenate TNF-α and NF-ĸB concentrations were investigated using rat-specific sandwich enzyme-linked immunosorbent assay Rat Tumor Necrosis Factor α ELISA kit (Cat no: YHB1098Ra, Shanghai LZ, PR China) and Rat Nuclear Factor- Kappa B ELISA immunoassay kit (Cat. No:201-11-0288, SunRed, Shanghai, PR China). Analyses were performed according to the manufacturers’ instructions. Monoclonal antibody specific for rat TNF-α or NF-ĸB were coated onto the wells of the micro plates. The tissue homogenate, standards and streptavidin-HRP and biotinylated monoclonal antibody were pipetted into these wells and then incubated at 37˚C for 60 min. Following the addition of chromogen A and chromogen B, the samples were incubated at 37˚C for 10 min to develop the color. Than the stop solution was added. The intensity of the colored product is directly proportional to the concentration of rat TNF-α and NF-ĸB present in the original specimen. At the end, the absorbance was measured at 450 nm and the concentrations of TNF-α and NF-ĸB were calculated using the corresponding standard curves.
Statistical analysis. Descriptive statistics were used for each group. The results obtained from the experiments were expressed as “mean value±standard deviation” (x±SD). Outlier analysis was performed using the Tukey test. Differences between groups were compared by one-way analysis of variance (ANOVA). All statistical analyses were performed with “SPSS Statistics Version 18” statistical software (IBM Statistics, USA) and p-values<0.05 were considered significant.
Histopathological examination. First, all tissue samples were placed in 10% formaldehyde solution for light-microscope investigation. Then, tissue samples were washed with water for 24 h and serially treated with increasing concentrations of alcohol (70%, 80%, 90%, and 100%) to remove water from the tissues. The tissues were incubated with xylol and embedded in paraffin. Forty-five micron sections were cut from the paraffin blocks and were stained with hematoxylin-eosin. The photos were taken using the Olympus DP2-SAL microscope (Olympus®Inc. Tokyo, Japan). Histopathological
examination was performed blindly by the pathologist .
Results
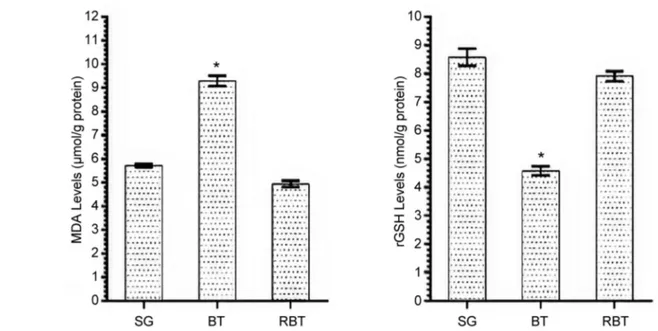
MDA and GSH analysis results. While the amount of MDA
in brain tissue of the BT group was significantly higher
compared to the SG and RBT groups (p<0.001), the amount
of tGSH was found to be (p<0.001) (Figure 1). The
in vivo
34: 2453-2460 (2020)difference between the amount of MDA and tGSH in RBT
and SG animal groups was statistically insignificant.
TNF-α and NF-ĸB analysis results: TNF-α and NF-ĸB levels
in brain tissue of BT group were significantly higher
compared to SG and RBT groups (p<0.001). However, the
difference between TNF-α and NF-ĸB levels in the RBT and
SG groups was not statistically significant (Figure 2).
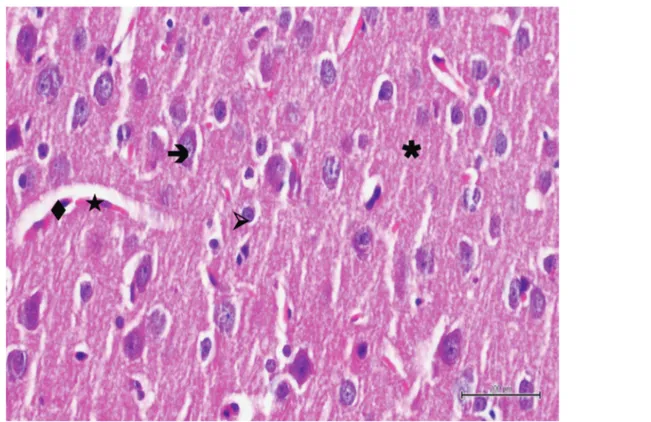
Histopathological findings. Histopathological examination
showed normal histological architecture of the brain tissue
in the sections of the control group. Neurons had normal
oval soma shape and stained basophilic, centered
euchromatic nuclei and prominent nucleoli. Glial cells,
especially astrocytes were normal in shape and glial cells
were located was also normal and dense in appearance
(Figure 3). In the BT group, most of pyramidal neurons were
Figure 1. MDA and tGSH levels of SG, BT and RBT groups. *p<0.0001 compared to SG and RBT groups (n=6).in vivo
34: 2453-2460 (2020)Figure 3. Hematoxylin-eosin staining in brain tissue in the control group; : neuron, : astrocyte, : blood vessel, : endothelial cell, ✱: neuropile ×400.
Figure 4. Hematoxylin-eosin staining of brain tissue in the experimental trauma group; : edematous neuron, : degenerated neuron, : swollen astrocyte, : dilated and congested blood vessel, : swollen endothelial cell, ✱: vacuolized neuropile, ×400.
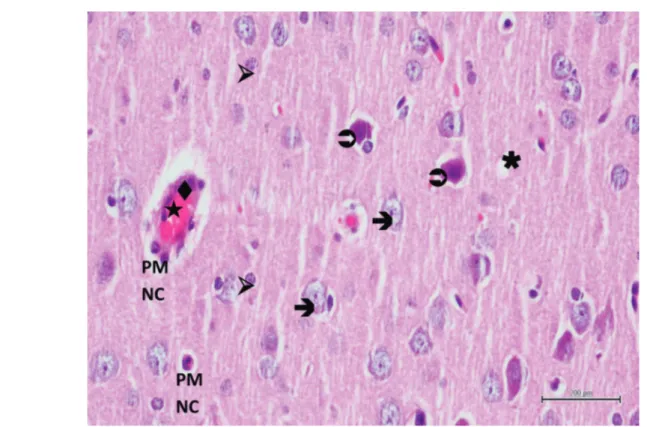
Figure 5. Hematoxylin-eosin staining of brain tissue in the experimental trauma group; : edematous neuron, : degenerated neuron, : swollen astrocyte, : dilated and congested blood vessel, : swollen endothelial cell, PMNC: polymorphonuclear cell, ✱: vacuolized neuropile, ×400.
Figure 6. Hematoxylin-eosin staining of brain tissue in the rutin applied group after the experimental trauma; : neuron, : degenerated neuron, : astrocyte, : blood vessel, : endothelial cell, ✱: neuropile, ×400.
irregular in shape and had dark stained nuclei. Other neurons
had pericellular halos and their nuclei had lost euchromatic
appearance and their nucleoli had become subtle. Neuron
blebbing and edema were also observed. Some neurons were
degenerated and in the process of apoptosis. These findings
were accompained by activation of astrocytes. Astrocytes
had pericellular halos and the surrounding neuropile was
intensely vacuolized. The blood vessels were dilated and
congested and, in addition, endothelial cells were swollen
(Figure 4). In some blood vessels polymorphonuclear cells
were remarkable (Figure 5). In RBT group, neurons were
generally normal in shape, they had euchromatic nuclei and
clear nucleoli. However, pyramidal neurons still degenerated.
Blood vessels, endothelial cells and astrocytes were normal
in shape but the surrounding neuropile was still vacuolized
in some regions (Figure 6).
Discussion
Primary TBI occurs immediately after impact. Secondary
TBI occurs a few seconds after the first injury. The
secondary phase is characterized by neuroinflammation,
oxidative stress, and increased intracranial pressure due to
brain edema (7). In this study, the effect of rutin on the
secondary phase of TBI was evaluated. Treatment of head
trauma usually aims at preventing secondary damage;
prevention of secondary brain injury significantly reduces
morbidity and mortality (16). Identification of novel
therapeutic targets for the treatment of cellular injury would
be beneficial to eliminate or reduce disability from nervous
system trauma (17).
Head trauma has been shown to result in subsequent
neurotrauma as a result of elevations in nervous system
oxidative stress and free radical levels (18). Our
experimental results showed that the levels of MDA in
untreated brain tissue following mechanical trauma was
higher, whereas the levels of tGSH were lower compared to
the healthy group and rutin administered group. These results
demonstrate that the oxidant-antioxidant balance in untreated
damaged brain tissue is disrupted in favor of oxidants. The
results also indicate that ROS production in brain tissue is
induced. It has been shown that ROSs oxidize cell membrane
lipids and produce toxic products such as MDA, which is in
agreement with our biochemical findings. It has also been
argued that oxidative damage occurs when the endogenous
antioxidant system fails to neutralize excess ROS, which
cause cellular damage (19). In our study, it was found that
the oxidant-antioxidant balance was maintained in the brain
tissue of animals that were treated with rutin following
mechanical trauma. Rutin suppressed TBI-induced MDA
increase and tGSH decrease. GSH is an endogenous
antioxidant molecule that eliminates the harmful effects of
radicals (20). Previous studies have also reported that MDA
levels increase and those of tGSH decrease following TBI
(21). It has been reported that rutin protects brain tissue cells
from oxidative damage caused by increased MDA and
decreased tGSH (22, 23).
In our study, mechanical trauma applied to the rat brain
also caused an increase in the production of
proinflammatory cytokines such as TNF-α and NF-ĸB.
However, rutin suppressed the increase of Tα and
NF-ĸB in TBI. TNF-α, and NF-NF-ĸB have been documented to be
cytokines responsible for the inflammatory process of
secondary TBI injury (24, 25). Gugliandolo et al. have
shown that TNF-α and NF-ĸB production increased
following TBI, and also injury regressed in brain tissue
where TNF-α and NF-ĸB production was suppressed (26).
There are no studies showing that rutin inhibits the
overproduction of TNF-α and NF-ĸB in damaged brain
tissue. However, there are studies reporting that the
anti-inflammatory effect of routine is associated with
proinflammatory cytokine inhibition (27).
The results of our biochemical experiments were
consistent with the histopathological findings. There was
marked dilated and conjunctival blood vessels,
polymorphonuclear leukocyte (PMNL) accumulation,
hemorrhage, and edema in traumatic brain tissue where
oxidative stress and proinflammatory markers were
increased. Degeneration, apoptosis, euchromatic nuclei, and
pericellular halos were observed in pyramidal and other
neurons. Halos and swelling were also detected in
astrocytes. It has also been reported that secondary injury
due to brain trauma is characterized neuroflamation,
oxidative stress and increased brain edema (7). The role of
NF-ĸB in the development of trauma-related brain edema
has been reported (9). Rutin administration reduced
pyramidal neuron degeneration and PMNL accumulation
due to brain tissue trauma, while eliminated edema and
other histopathological symptoms. Previous studies have
reported that rutin suppresses proinflammatory TNF-α and
NF-ĸB production (12). Also, Moutinho et al. reported that
rutin reduced cyclo-oxygenase-2 expression in the dermis
and tumor-associated inflammation in HPV16-induced
lesions on mice (28). It has also been reported that rutin
prevents edema developing as a result of increased capillary
permeability and plasma leakage into the tissue (11). In
addition, it has been shown that rutin prevents PMNL
adhesion and migration and maintains vascular barrier
integrity (12).
In conclusion, mechanical impact applied using an
established method caused oxidative and proinflammatory
damage in rat brain tissue. In addition, mechanical trauma
caused significant brain edema. Rutin alleviated oxidative
and proinflammatory brain damage and prevented brain
injury. Therefore, rutin might be useful in the treatment of
TBI and TBI-related edema.
Conflicts of Interest
The Authors have no conflicts of interest to declare regarding this study.
Authors’ Contributions
Sahin Yuceli: Study Design, data collection, writing manuscript; Gulce Naz Yazici: Histological examinations, data analysis; Renad Mammadov: Experimental procedure, data collection; Halis Suleyman: Experimental procedure, data collection, statistical analyses; Mustafa Kaya: Statistical analysis, writing manuscript; Selcuk Ozdogan: Data analyses, writing manuscript, final revisions.
References
1 Kim JM, Kim SC, Lee KH, Kim HJ, Kim H, Lee SW, Na DS and Park JS: Preventive effects of seat belts on traumatic brain injury in motor vehicle collisions classified by crash severities and collision directions. Eur J Trauma Emerg Surg, 2019. PMID: 30798345. DOI: 10.1007/s00068-019-01095-4
2 Coronado VG, Haileyesus T, Cheng TA, Bell JM, Haarbauer-Krupa J, Lionbarger MR, Flores-Herrera J and McGuire LC: Gilchrist trends in sports- and recreation-related traumatic brain ınjuries treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001-2012. J Head Trauma Rehabil 30(3): 185-197, 2005. PMID: 25955705. DOI: 10.1097/HTR. 0000000000000156
3 Dewan, MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG and Rubiano AM: Estimating the global incidence of traumatic brain injury. J Neurosurg 1: 1-18, 2018. PMID: 29701556. DOI: 10.3171/ 2017.10.JNS17352
4 Yattoo G and Tabish A: The profile of head injuries and traumatic brain injury deaths in Kashmir. J Trauma Manag Outcomes 2(1): 5, 2008. PMID: 18570674. DOI: 10.1186/1752-2897-2-5
5 Andriessen TM, Jacobs B and Vos PE: Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J Cell Mol Med 14: 2381-2392, 2010. PMID: 20738443. DOI: 10.1111/j.1582-4934.2010.01164.x 6 Abdul-Muneer PM, Chandra N and Haorah J: Interactions of
oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51(3): 966-979, 2015. PMID: 24865512. DOI: 10.1007/s12035-014-8752-3
7 Niu X, Zheng S, Liu H and Li S: Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol Med Rep 18(5): 4516-4522, 2018. PMID: 30221665. DOI: 10.3892/mmr.2018.9465
8 Kaltschmidt B, Widera D and Kaltschmidt C: Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta 1745: 287-299, 2005. PMID: 15993497. DOI: 10.1016/j.bbamcr. 2005.05.009
9 Xiao G and Wei J: Effects of β-Aescin on the expression of nuclear factor-ĸB and tumor necrosis factor-α after traumatic brain injury in rats. J Zhejiang Univ Sci B 6(1): 28-32, 2005. PMID: 15593388. DOI: 10.1631/jzus.2005.B0028
10 Harborne JB: Nature, distribution and function of plant flavonoids. Prog Clin Biol Res 213: 15-24, 1986. PMID: 3520585.
11 Chen WM, Jin M and Wu W: Experimental study on inhibitory effect of rutin against platelet activation induced by platelet activating factor in rabbits. Zhongguo Zhong Xi Yi Jie He Za Zhi 22(4): 283-285, 2002. PMID: 12584792.
12 Lee W, Ku SK and Bae JS: Barrier protective effects of rutin in LPS-induced inflammation in vitro and in vivo. Food Chem Toxicol 50(9): 3048-3055, 2012. PMID: 22721984. DOI: 0.1016/j.fct.2012.06.013
13 Ucar T, Tanriover G, Gurer I, Onal MZ and Kazan S: Modified experimental mild traumatic brain injury model. J Trauma 60(3): 558-565, 2006. PMID: 16531854. DOI: 10.1097/01.ta. 0000209172.75637.db
14 Ohkawa H, Ohishi N and Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358, 1979. PMID: 36810. DOI: 10.1016/0003-2697(79)90738-3
15 Sedlak J and Lindsay RH: Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25: 192-205, 1968. PMID: 4973948. DOI: 10.1016/0003-2697(68)90092-4
16 Korkmaz Dilmen O, Akçıl EF and Tunalı Y: ıntensive care treatment in traumatic brain ınjury. Turk J Anaesth Reanim 43: 1-6, 2015. DOI: 10.5152/TJAR.2014.26680
17 Chong ZZ, Li F and Maiese K: Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol 75: 207-246, 2005. PMID: 15882775. DOI: 10.1016/j.pneurobio.2005.02.004 18 Cernak I, Savic VJ, Kotur J, Prokic V, Veljovic M and Grbovic
D: Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma 17: 53-68, 2000. PMID: 10674758. DOI: 10.1089/neu.2000.17.53
19 Suleyman H, Gül V and Erhan E: Oxidative stress and tissue damage. Erzincan Med J 1: 1, 2018.
20 Urso ML and Clarkson PM: Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189: 41-54, 2003. PMID: 12821281. DOI: 10.1016/s0300-483x(03)00151-3 21 Zou P, Liu X, Li G and Wang Y: Resveratrol pretreatment
attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep 17(2): 3212-3217, 2018. PMID: 29257276. DOI: 10.3892/mmr.2017.8241
22 Zhai X, Ding Y, Wang Q, Zhang H and Li F: Rutin acid ameliorates neural apoptosis ınduced by traumatic brain ınjury via mitochondrial pathways in mice. Neuroimmunomodulation 23(3): 179-187, 2016. PMID: 27644033. DOI: 10.1159/ 000448716
23 Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastawa P, Khan MB, Raza SS, Javed H, Vaibhav K, Khan A and Islam F: Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292: 123-135, 2009. PMID: 19631195. DOI: 10.1016/j.brainres.2009.07.026
24 Batsaikhan B, Wang JY, Scerba MT, Tweedie D, Greig NH, Miller JP, Hoffer BJ, Lin CT and Wang JY: Post-ınjury neuroprotective effects of the thalidomide analog 3,6
’
-dithiothalidomide on traumatic brain ınjury. Int J Mol Sci 20(3): 502, 2019. PMID: 30682785. DOI: 10.3390/ijms20030502.25 Hu YC, Sun Q, Li W, Zhang DD, Ma B, Li S, Li WD, Zhou ML and Hang CH: Biphasic activation of nuclear factor kappa B and expression of p65 and c-Rel after traumatic brain injury in rats. Inflamm Res 63(2): 109-115, 2014. PMID: 24146067. DOI: 10.1007/s00011-013-0677-1
26 Gugliandolo E, D'Amico R, Cordaro M, Fusco R, Siracusa R, Crupi R, Impellizzeri D, Cuzzocrea S and Di Paola R: Neuroprotective effect of artesunate in experimental model of traumatic brain ınjury. Front Neurol 9: 590, 2018. PMID: 30108544. DOI: 10.3389/fneur.2018.00590
27 Nafees S, Rashid S, Ali N, Hasan SK and Sultana S: Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFĸB/MAPK pathway. Chem Biol Interact 231: 98-107, 2015. PMID: 25753322. DOI: 10.1016/j.cbi.2015.02.021
28 Moutinho MSS, Aragao S, Carmo D, Casaca F, Silva S, Ribeiro J, Sousa H, Pires I, Queiroga F, Colaco B, Medeiros R, Oliviera PA, Lopes C, Bastos MMSM and Da Costa RMG: Curcumin and rutin down-regulate Cyclooxygenase-2 and reduce tumor-associated ınflammation in HPV16-transgenic mice. Anticancer Res 38(3): 1461-1466, 2018. PMID: 29491072. DOI: 10.21873/ anticanres.12371