The Combined Usage of
β-Cyclodextrin and Milk Proteins
in Microencapsulation of
Bifidobacterium bifidum BB-12
Sultan Arslan-Tontul1
# Springer Science+Business Media, LLC, part of Springer Nature 2019
Abstract
The present study aimed to determine the effects of combined usage ofβ-cyclodextrin with whey protein isolate and sodium caseinate on the microencapsulation of Bifidobacterium bifidum-BB12 by spray drying.
From the results, the highest count of B. bifidum was provided by whey protein isolate as 8.62 log CFU/g. The increasing concentration ofβ-cyclodextrin considerably increases gastric and intestinal resistance to B. bifidum cells. In the gastric and intestinal test, the highest protection was determined in whey protein isolate substituted with 10%β-cyclodextrin with reduction rates of 0.98 and 3.30%, respectively. Moreover, free cells did not survive in the same gastric conditions. The lowest hygro-scopicity was determined in whey protein isolate as 8.57%. It must be noted that increasingβ-cyclodextrin concentration in carrier material combination led to an increase in hygroscopicity of microcapsules. In general, substitution withβ-cyclodextrin increased the particle size of microparticles, and microcapsules produced with whey protein isolate had a smaller size than that of sodium caseinate.
Keywords Probiotic . Cyclodextrin . Sodium caseinate . Whey . Microencapsulation
Introduction
In recent years, there has been an increasing interest in the studies of probiotic microorganisms and Bifidobacterium is a major area of interest within the field of probiotic microorgan-isms. Moreover, 80% of natural intestinal microflora in breast-fed infants is comprised of Bifidobacterium [1]. Many health-promoting effects have been attributed to Bifidobacterium such as decreasing of ammonia levels, improving immune system, lowering of lactose intolerance and prevention of gas-trointestinal diseases [2]. Showing these health-promoting ef-fects of Bifidobacterium should be viable during passing through the gastrointestinal system.
Recent attempts to increase the survivability of probiotics have led to a renewed interest in microencapsulation which has been accepted as a powerful method to protect probiotics from harsh conditions [3,4]. Microencapsulation is defined as a process to entrap the active material within starch, protein or
lipid carrier matrix [5–7]. Among the microencapsulation methods, spray drying is one of the oldest and commonly used techniques for microencapsulation of probiotic microorgan-isms [8–10].
Recently, production of biopolymer (polysaccharides, pro-teins and their combinations) based probiotic-loaded micro-particles, and protection and release mechanisms of these mi-crocapsules have been paid attention [9,11]. Among different biopolymers, milk proteins such as whey protein and sodium caseinate are the widely used ones in probiotic microencapsu-lation [3,7,9,12]. Additionally, sodium caseinate and whey proteins play an important role in human nutrition due to their amino acid content, vitamins and minerals [10,13]. In a pre-vious study, probiotic Lactobacillus rhamnosus GG was mi-croencapsulated by spray drying using sodium caseinate and denatured whey proteins, and cell counts were detected as 7.8 and 8.9 log10 cfu/g, respectively [14]. In another study, the protective effect of sweet whey as a carrier agent of L. acidophilus LA-5 was also observed by Maciel et al. [15]. It was also reported that whey protein improved the surviv-ability rate of probiotics during spray drying and simulated gastric digestion [16]. Milk proteins are the favourable mate-rials for use singly or in combination with other biomatemate-rials in the encapsulation of probiotics, but no cited study gave
* Sultan Arslan-Tontul sultan.arslan@selcuk.edu.tr 1
Agricultural Faculty, Department of Food Engineering, Selçuk University, 42130 Konya, Turkey
https://doi.org/10.1007/s12602-019-09621-x
special emphasis on microencapsulation of probiotics both milk proteins (sodium caseinate and whey protein) and β-cyclodextrin.
Cyclodextrins are cyclic oligosaccharides, composed from a-(1-4)-D-glucopyranose units, and they have a ring formation with the hydrophobic cavity and hydrophilic groups [17,18]. It is produced from starch degradation via intramolecular transglycosylation reactions, induced by cyclodextrin glucanotransferase enzyme [5,19, 20]. Cyclodextrins are grouped asα, β and γ-cyclodextrins according to their com-prised glucose molecules [5,20]. Cyclodextrins was reported as a useful and effective protectant for probiotics due to in-creasing the effectiveness of carrier material [5, 21]. Moreover, cyclodextrins have been reported as a prebiotic substance since it is not digested in the upper gastrointestinal tract and metabolised by the probiotic microflora of the colon [17]. For these reasons, they could provide additional advan-tages during the microencapsulation of probiotic microorgan-isms. However, very limited research has focused on the usage of cyclodextrins as a carrier in probiotic encapsulation. To the best of our knowledge, no paper was published on research in the interactions of milk proteins (whey and casein) and β-cyclodextrin in microencapsulation of probiotic microorgan-isms using by spray drying. Therefore, the main objective of this study was to determine the effects of substitution of whey protein isolate and sodium caseinate withβ-cyclodextrin on the microencapsulation of B. bifidum and the stability of mi-crocapsules in gastrointestinal conditions. Moreover, some physical properties of the microcapsules were also determined.
Materials and Method
Material
Probiotic microorganism strain of Bifidobacterium bifidum BB-12 were obtained from Chr. Hansen (Horsholm, Denmark). The carrier materials ofβ-cyclodextrin and sodi-um caseinate (SC) were obtained from Sigma (Taufkirchen, Germany), and whey protein isolate (WPI) was purchased from Davisco (Minnesota, USA). The Man Rogosa Sharpe (MRS) agar and broth used in the enumeration of B. bifidum were purchased from Merck (Darmstadt, Germany), and the other microbiological reagents were obtained from Sigma (Taufkirchen, Germany).
Preparation of
B. bifidum Culture
The B. bifidum was grown in sterile MRS broth (pH, 6.5), enriched with 0.05% L-cysteine. B. bifidum was cultured in 50 mL MRS broth for 12 h at 37 °C. After the first incubation, 5 mL of culture suspension was transferred to 50 mL MRS
broth, and it was re-incubated at same conditions. After the second incubation, culture suspension was centrifuged at 7500 rpm for 10 min. The supernatant was removed, and the pellet was washed twice with sterile ringer solution. B. bifidum concentration was diluted with 20% glycerol and stored at–18 °C until further use.
Production of
B. bifidum Microcapsules by Spray
Drying
In the spray drying, sodium caseinate (SC) and whey protein isolate (WPI) were used as carrier materials, and to determine the effects of β-cyclodextrin, it was substituted with each material at the ratio of 0, 5 and 10%. For the preparing of feeding solutions, 10 g of carriers were dissolved in 100 mL water by Ultraturrax (IKA, Staufen, Germany) at 12200 rpm for 5 min. After homogenisation, 0.5 mL B. bifidum culture was added (109cfu/mL) into the carrier solution, and it was homogenised at the same conditions. Prepared feeding solu-tion was microencapsulated using a laboratory scale spray dryer (Buchi-290, Flawil, Switzerland) at a flow rate of 17 mL/min. The drying was performed at 130 °C inlet and 53 °C ± 3 outlet temperatures. The two-fluid nozzle was operated by compressed air at 0.41 bar, and the aspiration rate was about 38 m3/h during spray drying. The microcapsules of B. bifidum collected from the vessel were stored at–18 °C for further analysis.
Enumeration of
B. bifidum
B. bifidum was enumerated by MRS agar enriched with 0.1% lithium chlorite, 0.05% L-cysteine and 0.01% aniline blue. The pH value of agar was adjusted to 6.5 with 0.1 N NaOH before sterilisation. Serial dilutions were prepared with sterile Ringer solution. The plates were inoculated from appropriate dilutions according to pour plate technique and incubated at 37 °C for 72 h in anaerobic conditions [22].
Survival of Microencapsulated
B. bifidum
in Simulated Gastric Conditions
For the determination of in vitro gastric resistance of micro-encapsulated B. bifidum cells, gastric fluid was prepared using 9 g/L sodium chloride and 3 g/L pepsin. The pH of the gastric solution was adjusted to 2 with 0.1 N HCl. 1 g microcapsule was weighted into a glass tube, and 9 mL gastric solution was added into tube incubated at 37 °C for 180 min.
The intestinal fluid was prepared from 9 g/L sodium chlo-ride, 10 g/L pancreatin, 10 g/L trypsin and 3 g/L bile salts. The pH value of the solution was adjusted to 8 with 0.1 N NaOH. 1 g microcapsule was added with 9 mL solution and incubated at 37 °C for 180 min. B. bifidum enumeration was done by taking samples at specific times [22,23].
Water Activity and Moisture Content
The water activity and moisture content of microcapsules were determined using water activity metre (Aqualab 4TE, Decagon Devices, Pullman, Washington, USA) at 25 °C and moisture analyser (Kern DBS, Balingen, Germany) at 105 °C, respectively.
Hygroscopicity
For the determination of hygroscopicity of microcapsules, 0.25 g sample was weighed into a preconditioned beaker. The beakers were placed into a desiccator conditioned with saturated NaCl and held at 25 °C for 7 days. After incubation, hygroscopicity value was calculated from weight increase and expressed as a percentage [24].
Powder Yield
Powder yield of microcapsules was calculated as the ratio of dry matter weight of obtained powder to the dry matter of feed solution.
Tapped Bulk Density
The tapped bulk density value of microcapsules was deter-mined after 1 g sample weighed into 10 mL cylindrical mea-sure and tapped for 30 times. It was calculated by dividing the sample weight by volume measured in the cylinder [25].
Particle Size Distribution
Particle size distribution of produced microcapsules was de-termined by particle size analyser (Mastersizer 2000, Malvern, Worcestershire, UK) equipped with a wet sample unit (Hydro 2000S). 2-propanol was used as a dispersant, and refractive index of microcapsules was set to 1.52. The particle size dis-tribution of the microcapsules was calculated as surface and volume mean diameter [26].
Differential Scanning Calorimetry (DSC) Analysis
The thermal properties of microcapsules were measured by DSC analyser (6000, Perkin Elmer, Massachusetts, ABD). 15–25 mg microcapsule was weighed in an aluminium pan and gradually heated (20 °C/min) to temperatures between 0 and 200 °C in under constant nitrogen flow.
Morphological Properties
Morphological properties of microcapsules were determined by scanning electron microscope (SEM) (Leo-1430, Zeiss,
Oberkochen, Germany) with a digital camera (DS-FI2, Nikon, Tokyo, Japan).
Statistical Analysis
The spray drying experiments and analyses were duplicated. The data was subjected to analysis of variance, and appropri-ate mean separation was conducted using Duncan’s Multiple Range test (p < 0.05). All statistical calculations were per-formed by SAS statistical software (SAS Institute Inc., Cary, NC, USA).
Results and Discussions
B. bifidum Viability After Spray Drying
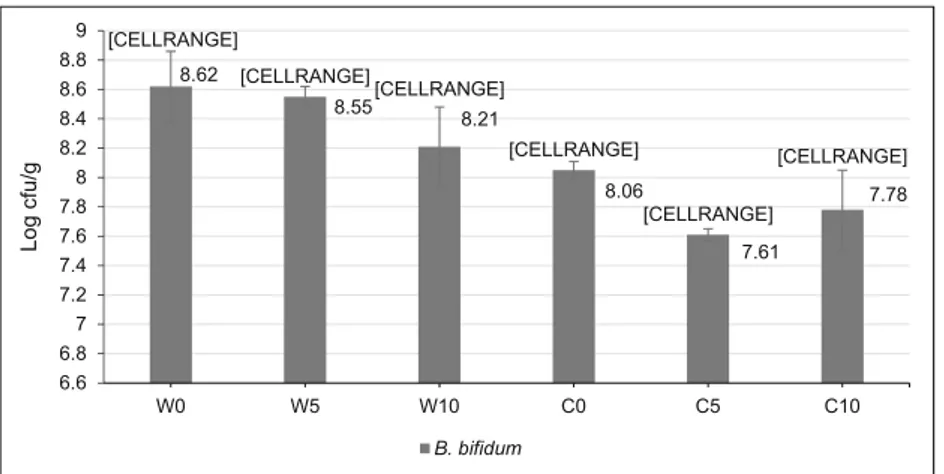
The used carrier medium is a very important factor for maintaining high protection on probiotic microorganism cells since there is a high correlation between thermal conductivity and diffusivity of the carrier and cell viabil-ity [27]. It was shown that the carrier composition signif-icantly (P < 0.05) affected B. bifidum viability during the spray-drying process. The highest B. bifidum was achieved in WPI containing 0 and 5%β-cyclodextrin that of 8.62 and 8.55 log cfu/g, respectively (Fig. 1). It was clearly seen that SC ensured lower protection to B. bifidum cells compared to WPI during the spray-drying process. The low encapsulation capacity of sodium caseinate could arise from the interaction between the car-rier solution and B. bifidum cell. Indeed, Burgain et al. [28] reported that probiotic strain could develop specific interactions with micellar casein and whey proteins in spray drying experiment and physical properties of strain could affect the viability [29]. Another possible explana-tion for this result might be lactose content of used WPI since it may limit the diffusion of heat and thereby pro-vide higher viability [8]. It was also reported that lactose played a dominant role by direct interaction with sensitive biomolecules of bacteria [30]. Similar results were also reported by Soukoulis et al. [31] who found that the sur-vivability rate of L. acidophilus was lower in SC micro-capsules than that of whey protein concentrate.
WPI containing 0 and 5% β-cyclodextrin had lower reduction rates when compared to other carrier formula-tions. It has been revealed that the type of milk protein and pH affects the survivability during spray drying as strain specific [28]. However, in all microcapsules, B. bifidum count was detected higher than 7 log cfu/g than the accepted limit for food to show its probiotic benefits. Similar probiotic counts were reported by Guerin et al. [14] who microencapsulated L. rhamnosus by dairy ma-trices composed of sodium caseinate and denatured whey
proteins. In another study, B. animalis was microencapsu-lated using whey protein concentrate by the spray-drying process, and bacteria count was reported as 1.48 × 108 and 1.28 × 108cfu/g [8].
The spray-drying process of probiotics takes place in the two stages. In the first stage, it is usually called a constant drying rate, and continuous evaporation occurs from the surface of the atomized wet droplet. Thermal inactivation in the cell is generally very limited in this stage because evaporation of water prevents increasing the temperature of the droplet. The most detrimental ef-fects are shown in the second stage of drying. In this time, the surface of droplets is completely dried, and continuous temperature rise is shown depending on dryer configura-tion [32–35]. Spray drying experiment carried at low tem-peratures cause only dehydration inactivation on probiotics. In all spray drying experiments, a decrease of less than 2 log was observed that was accepted as a good survival for B. bifidum BB-12. In this study, the low outlet temperature more than the lethal temperature of probiotics (65 °C) could allow a high survivability rate of B. bifidum.
Simulated Gastric Resistance of
B. bifidum
Microcapsules
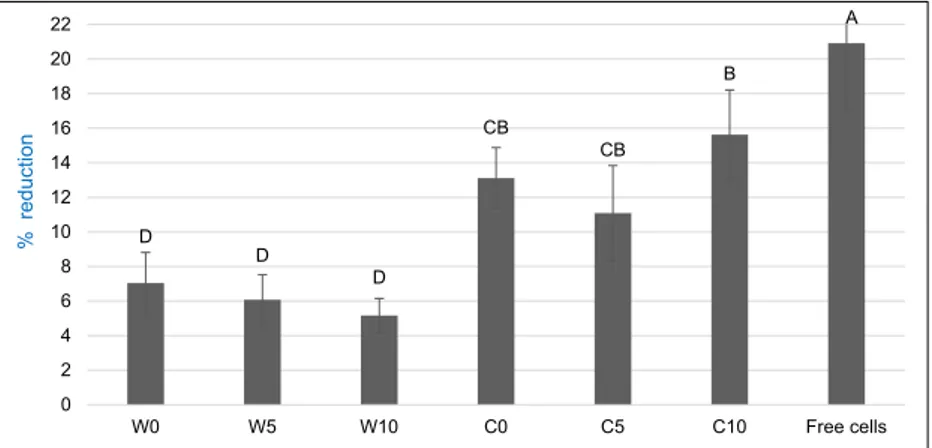
The reduction rate (%) of microencapsulated B. bifidum cells during simulated gastric digestion test was shown in Fig.2. The carriers and test time had significant effects (P < 0.01) on gastric resistance of B. bifidum cells. The highest protection against low pH and pepsin determined in W10, and the reduc-tion rate in this microcapsule was only 0.98% at the end of 180 min. Moreover, increasing the concentration of β-cyclodextrin in carrier agents supported the gastric resistance of B. bifidum cells. This protective effect of β-cyclodextrin might be related to its physical structure with the hydrophobic cavity and hydrophilic groups. Duchene et al. [36] reported that cyclodextrin increases the capacity of carriers. Moreover, the mix usage of proteins and polysaccharides has been rec-ommended for high protection of probiotics [37].
WPI provided more protection to the B. bifidum compared to SC during gastric digestion test. The physical properties of microcapsules may cause this result. WPI may ensure a good entrapment of probiotic cells and limit the physical interaction of cell and gastric medium. However, microcapsules produced
C D D B B CB A 0 5 10 15 20 25 30 35 40 45 50 W0 W5 W10 C0 C5 C10 Free cells %r e d u c ti o n
Fig. 2. Reduction rate (%) of B. bifidum viability under simulated gastric conditions. W = whey protein isolate and C = sodium caseinate with 0, 5, 10% β-cyclodextrin (w/w) [CELLRANGE] [CELLRANGE] [CELLRANGE] [CELLRANGE] [CELLRANGE] [CELLRANGE] 6.6 6.8 7 7.2 7.4 7.6 7.8 8 8.2 8.4 8.6 8.62 8.55 8.21 8.06 7.61 7.78 8.8 9 W0 W5 W10 C0 C5 C10 Lo g cfu /g B. bifidum
by SC caused low protective effect since sodium caseinate led to bigger particles and lower gel elasticity [13]. It was reported that the presence of whey increased the resistance of probiotic Streptococcus thermophilus and L. delbrueckii ssp. bulgaricus against acid and bile salts [3].
In gastric digestion test, nearly 88% of the microencapsu-lated B. bifidum cells survived. However almost all of the free cells were lost in 180 min. The present findings of the current study were consistent with De Castro-Cislaghi et al. [7] who determined that the viability of the free Bifidobacterium cells was lower than microencapsulated cells with whey protein. Additionally, a sharp decrease in the count of free L. plantarum count was reported compared to microencapsu-lated cells with whey when exposed to simumicroencapsu-lated gastrointes-tinal test [10]
Simulated Intestinal Resistance of
B. bifidum
Microcapsules
The decrease rate of B. bifidum viability during simulated intestinal digestion test was given in Fig.3. According to the results, the carriers and test time had significant effects (P < 0.01) on intestinal resistance of B. bifidum cells. Similar to the gastric digestion test results, the highest protection was de-termined in the microcapsule of W10 that the reduction rate was only 3.30%. The substitution withβ-cyclodextrin in the microcapsules resulted in higher survivability.
The reduction rate was higher in the intestinal test than gastric test since bile salts had a more negative effect than low pH on probiotic microorganisms. Additionally, carrier combination containing WPI provided higher B. bifidum via-bility in comparison with the SC during the intestinal test. De Castro-Cislaghi et al. [7] reported that the count of microen-capsulated B. bifidum with whey protein was decreased by 2.71 ± 0.51 log cfu/g when exposed to 1% bile after 6 h. During the test procedure, as expected, the viability lost was higher in free cells than microencapsulated B. bifidum cells. As a note, intestinal medium was less detrimental for free cells
than gastric medium. This is ultimately proving to be the main factor in the loss of probiotic viability of the stomach. As a conclusion, milk proteins offer several functional and techno-logical advantages for microencapsulation of probiotics and in most cases increase their survival during digestion [3].
Water Activity and Moisture Content (%)
of Microcapsules
Water activity and moisture content were important phys-icochemical properties in spray-dried microcapsules. It was pointed out that especially moisture content strongly influences the product stability and probiotic viability dur-ing storage which is one of the quality parameters to take into account for powders containing cells [38]. In this present study, obtained results were presented in Table 1
(P > 0.05). Water activity and moisture content results changed between 0.42–0.47 and 9.33–10.0%. These re-sults were higher than reported critic water activity, and moisture content results in the literature. It was noted that for a good storage stability, water activity and moisture content of microcapsules should have been 0.3 and 5%, respectively [39]. This may be attributed to low drying capacity of WPI and SC. The higher hydrogen-bonding capacity of proteins causes difficulties in evaporation. Therefore, microcapsules contain more moisture. Soukoulis et al. [31] were referred that the moisture con-tent of spray-dried microcapsules depends on processing conditions and carrier composition. Moreover, they re-ported that protein-based carriers caused high moisture content due to the presence of hygroscopic materials such as lactose and mineral salts (Soukoulis et al., 2014). Similar to the present study, Guerin et al. [14] determined the moisture content of probiotic-loaded microcapsules in the range of 5.8–12.0%, and they also determined that the desired moisture content value recommended for probiotic microcapsules (4–7%) was only achieved for the highest outlet air temperature (85 °C).
D D D CB CB B A 0 2 4 6 8 10 12 14 16 18 20 22 W0 W5 W10 C0 C5 C10 Free cells % reduction
Fig. 3. Reduction rate (%) of B. bifidum viability under simulated intestinal conditions. W = whey protein isolate and C = sodium caseinate with 0, 5, 10 % β-cyclodextrin (w/w)
Hygroscopicity of Microcapsules
Hygroscopicity was defined as water holding a capacity of microcapsules and one of the main factors for good stability in microcapsules. Water mobility causes high rate of enzymat-ic or chemenzymat-ical reactions and results in some quality deteriora-tions and changes in the physical properties in the microcap-sules [31].
It was determined that the carrier had a significant effect (P < 0.05) on the hygroscopicity value of microcapsules (Table1). Hygroscopicity of W0 microcapsule was the lowest among the different carriers (8.57%) although it was statisti-cally similar to W5, C0 and C5. It must be noted that increas-ingβ-cyclodextrin concentration in carrier combination led to higher hygroscopicity of microcapsules. This result might be attributed to the hydrophilic group contained in the structure of theβ-cyclodextrin. In a previous study, the hygroscopicity of microcapsules containing sodium caseinate and whey pro-tein was determined as 0.100 and 0.107 g water/g powder, respectively [31].
Powder Yield and Bulk Density of Microcapsules
The powder yield and bulk density results were given in Table 1. The powder yield of microcapsules had signifi-cantly (P < 0.01) affected by the carrier combination and substitution of milk proteins with β-cyclodextrin in-creased powder yield. In general, WPI combinations en-sured higher powder yield than those of SC. The main discrepancy between bulk density results was observed in WPI and SC. According to the results, microcapsules prepared with WPI had higher bulk density than those prepared with SC. The substitution with β-cyclodextrin did not change the bulk density of microcapsules.
Particle Size Distribution of Microcapsules
Particle size is an important factor since high volume–surface ratio has increased the protective effect and small capsules are preferred due to sensorial properties [40].
The surface and volume mean diameters of microparticles were significantly (P < 0.05) affected by the carrier (Table1). The surface and volume mean diameter of microparticles were determined as 2.85–3.65 μm and 7.31–12.15 μm, respective-ly. This detected size range was advantageous to avoid nega-tive sensorial impact when added to food [14]. In a previous study, the particle size of microcapsules produced using milk proteins was ranged in 9.38–13.37 μm [31].
In general,β-cyclodextrin addition increased the particle size of microparticles. Pasrija et al. [41] determined that en-capsulation with cyclodextrin resulted in bigger particle size. However, this finding was not supported by Fareez et al. [42] who determined that the addition ofβ-cyclodextrin did not influence average diameter of beads microencapsulated by extrusion. This difference might be related to the microcapsule production techniques. Generally, particle size distribution un-der 100 μm has been recommended to avoid any sensory negativity [43]. Therefore, particle size results of this study were in acceptable range.
Microcapsules produced with WPI had smaller size than those of SC. These results might be indicated from agglomer-ation of SC during spray-drying process. Additionally, high water-binding capacity of SC could led to increase particle size. Indeed, using of high-molecular-weight carrier agents or binding of high amount of water by microcapsules was reported to increase particle size of microcapsules [31]. Similar result to the present study was also observed in previ-ous studies. It was determined that particle size obtained using casein (69μm) was bigger than whey protein (56 μm) because
Table 1 Physical properties of B. bifidum loaded microcapsules Carrier Water activity Moisture content (%) Hygroscopicity (%)
Yield (%) Tapped bulk density (g/mL) Surface mean diameter (μm) Volume mean diameter (μm) Melting point (°C) W0 0.43a± 0.04 9.42a± 0.48 8.57b± 0.31 62.80b± 2.00 0.35a± 0.02 2.85c± 0.10 7.56b± 0.14 176.63a± 10.32 W5 0.42a± 0.02 9.33a± 0.07 8.69ba± 0.05 72.30a± 0.70 0.34a± 0.01 3.10bc± 0.01 7.89b± 0.10 163.50ba± 2.50 W10 0.45a± 0.01 9.96a± 0.07 9.35a± 0.07 71.70a± 0.40 0.35a± 0.01 3.01bc± 0.14 7.31b± 0.78 161.64ba± 0.32 C0 0.45a± 0.02 9.67a± 0.34 8.84ba± 0.23 42.40d± 0.10 0.27b± 0.02 3.45ba± 0.03 12.08ba± 0.31 156.96ba± 10.72 C5 0.47a± 0.01 9.51a± 0.25 8.90ba± 0.26 48.55c± 0.45 0.29b± 0.01 3.64a± 0.19 12.15a± 0.55 154.73ba± 11.05 C10 0.46a± 0.01 10.0a± 0.18 9.40a± 0.17 51.90c± 2.10 0.27b± 0.01 3.65a± 0.19 12.10a± 0.95 136.75b± 7.25 W = whey protein isolate and C = sodium caseinate with 0, 5, 10 %β-cyclodextrin (w/w)
high amount of micellar casein led to significantly bigger par-ticles and lower gel elasticity [13]. L. rhamnosus was encap-sulated with the carrier formulation of denatured whey pro-teins and sodium caseinate at spray drying, and particle size of powders was reported as smaller than 18μm [14].
DSC Results
The melting point of microparticles was shown in Table1. The highest melting point was obtained in W0 (176.63 °C). Pinto et al. [9] determined the melting point of microcapsules produced by liquid whey to be 163.7 °C, and they reported that microcapsules with high melting point ensured better thermal stability [9]. It was determined that melting point of microcapsules decreased by substitution withβ-cyclodextrin. In addition, the melting point of microcapsules produced using SC was lower than those of whey protein. Similar results were also measured by Soukoulis et al. [31] that microcap-sules containing sodium caseinate had lower melting point than whey protein.
Morphological Properties
The morphology of microparticles is under the influence of many mechanisms such as expansion, occurred as a result of
desorption of air from the feeding solution after atomization and formation of a steam bubble within the drying droplet; or incorporation [9].
The SEM images of microcapsules (Fig.4) demonstrated that WPI microparticles had smoother and monolith appear-ance than SC microcapsules in low concentration of β-cyclo-dextrin. However, increasing rate of β-cyclodextrin in WPI caused the collapse of microparticle. It was reported that the kind of milk protein affected the morphological properties of microcapsules. For example, whey has been known as smooth, spherical and open hollow powders, while caseins caused more wrinkled, nonspherical and dense powder struc-tures [14]. Additionally, no B. bifidum cells were visible on the surface of microcapsule which proved effective microencap-sulation was carried out.
The substitution withβ-cyclodextrin resulted in no dif-ferences or negative effect on morphology. Microcapsules generally had a round shape, smooth surface, and these properties are common in microcapsules produced by spray drying. The same physical structure was also ob-served by different researchers [7, 9, 31]. Guerin et al. [14] observed that microcapsules produced with whey protein were smooth, spherical and open hollow powders, while sodium caseinate ensured more wrinkled, nonspher-ical and dense powder structures. It was also reported by
W0 W5 W10 C0 C5 C10
Fig. 4. SEM images of B. bifidum loaded microcapsules. W = whey protein isolate and C = sodium caseinate with 0, 5, 10 % β-cyclodextrin (w/w)
other researchers that sodium caseinate-based carriers ex-hibited more deflated surface than whey protein [31].
Conclusion
The main goal of the current study was to determine the pro-tective effect ofβ-cyclodextrin on probiotics when used to-gether with milk proteins in microencapsulation. This study has shown that B. bifidum was successfully encapsulated with sodium caseinate and whey protein isolate by using β-cyclodextrin as a supporter encapsulation agent in the spray drying system. From the results, it could be revealed that β-cyclodextrin did not affect B. bifidum viability and that whey ensured higher B. bifidum count than casein during produc-tion. However, increasing concentration of β-cyclodextrin provided higher viability during gastric and intestinal diges-tion of microcapsules. On the other hand, free cells exhibited a sharp decrease during gastric digestion. Hygroscopicity of mi-crocapsules produced by whey protein was lower compared to sodium caseinate microcapsules and substitution with β-cyclodextrin increased hygroscopicity of microcapsules. Additionally, microparticles produced with whey protein had smaller size than those of sodium caseinate.
This is the first study reporting that the usage of β-cyclodextrin with whey in spray-drying process provided high protection during simulated gastrointestinal digestion of B. bifidum. The results clearly showed that whey protein as carrier agent ensured better protection and physical properties than sodium caseinate. However, further studies along this area are needed for better understanding the mechanisms and factors affecting the interaction of milk proteins and β-cyclodextrin for efficient microencapsulation of probiotics. Therefore, findings of this study have a number of important implications for future practice.
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflicts of interest.
Ethical Approval The author declares that this article does not contain any studies with human participants or animals.
References
1. Ku S, Park MS, Ji GE, You HJ (2016) Review on Bifidobacterium bifidum BGN4: functionality and nutraceutical applications as a probiotic microorganism. Int J Mol Sci 17(9).https://doi.org/10. 3390/ijms17091544
2. Sarao LK, Arora M (2017) Probiotics, prebiotics, and microencap-sulation: a review. Crit Rev Food Sci Nutr 57(2):344–371.https:// doi.org/10.1080/10408398.2014.887055
3. Abd El-Salam MH, El-Shibiny S (2015) Preparation and properties of milk proteins-based encapsulated probiotics: a review. Dairy Sci Technol 95(4):393–412. https://doi.org/10.1007/s13594-015-0223-8
4. De Prisco A, Mauriello G (2016) Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci Technol 48(Supplement C):27–39.https://doi.org/10.1016/j.tifs.2015.11. 009
5. Watson MA, Lea JM, Bett-Garber KL (2017) Spray drying of pomegranate juice using maltodextrin/cyclodextrin blends as the wall material. Food Sci Nutr 5(3):820–826.https://doi.org/10. 1002/fsn3.467
6. Burgain J, Gaiani C, Linder M, Scher J (2011) Encapsulation of probiotic living cells: from laboratory scale to industrial applica-tions. J Food Eng 104(4):467–483.https://doi.org/10.1016/j. jfoodeng.2010.12.031
7. De Castro-Cislaghi FP, Silva CDE, Fritzen-Freire CB, Lorenz JG, Sant’Anna ES (2012) Bifidobacterium Bb-12 microencapsulated by spray drying with whey: survival under simulated gastrointesti-nal conditions, tolerance to NaCl, and viability during storage. J Food Eng 113(2):186–193.https://doi.org/10.1016/j.jfoodeng. 2012.06.006
8. Rodrigues D, Sousa S, Rocha-Santos T, Silva JP, Lobo JMS, Costa P, Amaral MH, Pintado MM, Gomes AM, Malcata FX, Freitas AC (2011) Influence of L-cysteine, oxygen and relative humidity upon survival throughout storage of probiotic bacteria in whey protein-based microcapsules. Int Dairy J 21(11):869–876.https://doi.org/ 10.1016/j.idairyj.2011.05.005
9. Pinto SS, Fritzen-Freire CB, Benedetti S, Murakami FS, Petrus JCC, Prudencio ES, Amboni RDMC (2015) Potential use of whey concentrate and prebiotics as carrier agents to protect Bifidobacterium-BB-12 microencapsulated by spray drying. Food Res Int 67:400–408.https://doi.org/10.1016/j.foodres.2014.11.038
10. Eckert C, Serpa VG, Felipe dos Santos AC, da Costa SM, Dalpubel V, Lehn DN, Volken de Souza CF (2017) Microencapsulation of Lactobacillus plantarum ATCC 8014 through spray drying and using dairy whey as wall materials. LWT Food Sci Technol 82: 176–183.https://doi.org/10.1016/j.lwt.2017.04.045
11. Matalanis A, Jones OG, McClements DJ (2011) Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll 25(8):1865– 1880.https://doi.org/10.1016/j.foodhyd.2011.04.014
12. Dianawati D, Mishra V, Shah NP (2016) Survival of microencap-sulated probiotic bacteria after processing and during storage: a Review. Crit Rev Food Sci Nutr 56(10):1685–1716.https://doi. org/10.1080/10408398.2013.798779
13. Burgain J, Gaiani C, Cailliez-Grimal C, Jeandel C, Scher J (2013) Encapsulation of Lactobacillus rhamnosus GG in microparticles: influence of casein to whey protein ratio on bacterial survival dur-ing digestion. Innov Food Sci Emerg Technol 19:233–242.https:// doi.org/10.1016/j.ifset.2013.04.012
14. Guerin J, Petit J, Burgain J, Borges F, Bhandari B, Perroud C, Desobry S, Scher J, Gaiani C (2017) Lactobacillus rhamnosus GG encapsulation by spray-drying: milk proteins clotting control to produce innovative matrices. J Food Eng 193:10–19.https://doi. org/10.1016/j.jfoodeng.2016.08.008
15. Maciel GM, Chaves KS, Grosso CRF, Gigante ML (2014) Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J Dairy Sci 97(4):1991–1998. https://doi.org/10.3168/jds.2013-7463
16. Pinto SS, Cavalcante BDM, Verruck S, Alves LF, Prudncio ES, Amboni RDMC (2017) Effect of the incorporation of Bifidobacterium BB-12 microencapsulated with sweet whey and inulin on the properties of Greek-style yogurt. J Food Sci
Tech-Mysore 54(9):2804–2813. https://doi.org/10.1007/s13197-017-2717-2
17. Marques HMC (2010) A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Frag J 25(5):313–326.https:// doi.org/10.1002/ffj.2019
18. Wang YF, Shao JJ, Wang ZL, Lu ZX (2012) Study of allicin mi-crocapsules in beta-cyclodextrin and porous starch mixture. Food Res Int 49(2):641–647.https://doi.org/10.1016/j.foodres.2012.09. 033
19. Paramera EI, Konteles SJ, Karathanos VT (2011) Stability and re-lease properties of curcumin encapsulated in Saccharomyces cerevisiae, beta-cyclodextrin and modified starch. Food Chem 125(3):913–922.https://doi.org/10.1016/j.foodchem.2010.09.071
20. Del Valle EMM (2004) Cyclodextrins and their uses: a review. Process Biochem 39(9):1033–1046. https://doi.org/10.1016/ s0032-9592(03)00258-9
21. Kawakami K, Fujita A, Mikami T, Yoshii H, Paramita V, Neoh TL, Furuta T (2009) Formation of rice flavor powder with alpha-cyclodextrin by spray drying. Eur Food Res Technol 229(2):239–
245.https://doi.org/10.1007/s00217-009-1043-y
22. Pedroso DL, Thomazini M, Heinemann RJB, Favaro-Trindade CS (2012) Protection of Bifidobacterium lactis and Lactobacillus acidophilus by microencapsulation using spray-chilling. Int Dairy J 26(2):127–132.https://doi.org/10.1016/j.idairyj.2012.04.008
23. Brinques GB, Ayub MAZ (2011) Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J Food Eng 103(2):123–128.
https://doi.org/10.1016/j.jfoodeng.2010.10.006
24. Tontul I, Topuz A, Ozkan C, Karacan M (2016) Effect of vegetable proteins on physical characteristics of spray-dried tomato powders. Food Sci Technol Int 22(6):516–524. https://doi.org/10.1177/ 1082013216629528
25. Beristain CI, García HS, Vernon-Carter EJ (2001) Spray-dried en-capsulation of Cardamom (Elettaria cardamomum) essential oil with mesquite (Prosopis juliflora) Gum. LWT Food Sci Technol 34(6):398–401.https://doi.org/10.1006/fstl.2001.0779
26. Arslan-Tontul S, Erbas M (2017) Single and double layered micro-encapsulation of probiotics by spray drying and spray chilling. LWT Food Sci Technol 81:160–169.https://doi.org/10.1016/j.lwt. 2017.03.060
27. Lian W-C, Hsiao H-C, Chou C-C (2002) Survival of bifidobacteria after spray-drying. Int J Food Microbiol 74(1–2):79–86.https://doi. org/10.1016/S0168-1605(01)00733-4
28. Burgain J, Gaiani C, Francius G, Revol-Junelles AM, Cailliez-Grimal C, Lebeer S, Tytgat HLP, Vanderleyden J, Scher J (2013) In vitro interactions between probiotic bacteria and milk proteins probed by atomic force microscopy. Colloid Surface B 104:153–
162.https://doi.org/10.1016/j.colsurfb.2012.11.032
29. Burgain J, Scher J, Lebeer S, Vanderleyden J, Cailliez-Grimal C, Corgneau M, Francius G, Gaiani C (2014) Significance of bacterial surface molecules interactions with milk proteins to enhance micro-encapsulation of Lactobacillus rhamnosus GG. Food Hydrocoll 41: 60–70.https://doi.org/10.1016/j.foodhyd.2014.03.029
30. Ananta E, Volkert M, Knorr D (2005) Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int Dairy J 15(4):399–409.https://doi.org/10.1016/j.idairyj.2004.08.004
31. Soukoulis C, Behboudi-Jobbehdar S, Yonekura L, Parmenter C, Fisk I (2014) Impact of milk protein type on the viability and stor-age stability of microencapsulated Lactobacillus acidophilus NCIMB 701748 using spray drying. Food Bioprocess Technol 7(5):1255–1268.https://doi.org/10.1007/s11947-013-1120-x
32. Boza Y, Barbin D, Scamparini ARP (2004) Effect of spray-drying on the quality of encapsulated cells of Beijerinckia sp. Process Biochem 39(10):1275–1284.https://doi.org/10.1016/j.procbio. 2003.06.002
33. Huang S, Vignolles M-L, Chen XD, Le Loir Y, Jan G, Schuck P, Jeantet R (2017) Spray drying of probiotics and other food-grade bacteria: a review. Trends Food Sci Technol 63:1–17.https://doi. org/10.1016/j.tifs.2017.02.007
34. Peighambardoust SH, Tafti AG, Hesari J (2011) Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol 22(5):215–224.https://doi.org/10.1016/j. tifs.2011.01.009
35. Schutyser MAI, Perdana J, Boom RM (2012) Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci Technol 27(2):73–82.https://doi.org/10.1016/j.tifs.2012.05.006
36. Duchene D, Ponchel G, Wouessidjewe D (1999) Cyclodextrins in targeting - application to nanoparticles. Adv Drug Deliv Rev 36(1): 29–40.https://doi.org/10.1016/s0169-409x(98)00053-2
37. Garcia AH (2011) Anhydrobiosis in bacteria: from physiology to applications. J Biosci 36(5):939–950.https://doi.org/10.1007/ s12038-011-9107-0
38. Ying DY, Phoon MC, Sanguansri L, Weerakkody R, Burgar I, Augustin MA (2010) Microencapsulated Lactobacillus rhamnosus GG powders: relationship of powder physical properties to probi-otic survival during storage. J Food Sci 75(9):E588–E595.https:// doi.org/10.1111/j.1750-3841.2010.01838.x
39. Avila-Reyes SV, Garcia-Suarez FJ, Jiménez MT, San Martín-Gonzalez MF, Bello-Perez LA (2014) Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the via-bility. Carbohydr Polym 102:423–430.https://doi.org/10.1016/j. carbpol.2013.11.033
40. Anal AK, Singh H (2007) Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Technol 18(5):240–251.https://doi.org/10.1016/ j.tifs.2007.01.004
41. Pasrija D, Ezhilarasi PN, Indrani D, Anandharamakrishnan C (2015) Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food Sci Technol 64(1):289–
296.https://doi.org/10.1016/j.lwt.2015.05.054
42. Fareez IM, Lim SM, Lim FT, Mishra RK, Ramasamy K (2017) Microencapsulation of Lactobacillus sp. using chitosan-alginate-xanthan gum-cyclodextrin and characterization of its cholesterol reducing potential and resistance against pH, temperature and stor-age. J Food Process Eng 40(3).https://doi.org/10.1111/jfpe.12458
43. Hansen LT, Allan-Wojtas PM, Jin YL, Paulson AT (2002) Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol 19(1):35–45.
https://doi.org/10.1006/fmic.2001.0452
Publisher’s Note Springer Nature remains neutral with regard to jurisdic-tional claims in published maps and institujurisdic-tional affiliations.