Dyes and Pigments 173 (2020) 107974
Available online 16 October 2019
0143-7208/© 2019 Elsevier Ltd. All rights reserved.
Low-cost and environmentally sensitive fluorescent cellulose paper for
naked-eye detection of Fe(III) in aqueous media
Mehmet Oguz
a,b, Ahmed Nuri Kursunlu
b, Mustafa Yilmaz
b,* aDepartment of Advanced Material and Nanotechnology, Selcuk University, 42031, Konya, Turkey bDepartment of Chemistry, University of Selcuk, Campus, 42031, Konya, TurkeyA R T I C L E I N F O Keywords: Cellulose Bodipy Iron Filter paper Emission Fluorescent A B S T R A C T
The design of eco-friendly materials to detect heavy metals is today’s demand to decrease pollutions in natural waters. This paper demonstrates low-cost and environmentally sensitive material for removal and detection of Fe (III) ions in polluted waters. For this, raw cellulose with powder form was firstly fabricated via immobilizing hexamethylene diisocyanate. Following the activation of cellulose, it was modified with a Bodipy for useful spectroscopic measurements to effectively detect and remove Fe(III) ion from aqueous medium. The Bodipy- based cellulose (Cell-BODIPY) exhibited exceptional performance for detecting Fe(III) ion under both long-wave light and daylight. For a better visualization in practical applications, the same modification process was also carried out for filter paper. To the best of our knowledge, this paper will inspire alternative methods in the selective detection and elimination of heavy metal ions in polluted waters.
1. Introduction
With the increase in population and the rapid development of the industrial system, the world is rapidly undergoing a booming process of industrialization [1,2]. As this industrialization keep going, diverse species of pollution have steadily enhanced, which has led to prevalent health and environmental problems [3–6]. Among this pollution, water pollution, contaminated due to industrial discharges, has been increasing most particularly with improved industrialization [7].
Environmental pollution with toxic metal contamination is one of the substantial problems to be addressed in the refinement of wastewater. These heavy metals emerge largely from industrial wastewater and consist of different forms [8–12]. It is well known that heavy metal ions cause serious harm to human health because of their highly poisonous character [13]. Once these metal ions entered the human aquatic food chains, they cannot be degraded and can cause serious health problems such as nervous system disorders, vision loss, cardiovascular system destruction, endocrine systems, the kidney, liver, bones, and teeth [14, 15].
In addition to this, they tend to accumulate in microorganisms and plants and they can be decreasing the rate of transpiration and photo-synthesis although low concentrations of heavy-metal ions may not consist immediate danger [16]. Among metals, iron and its compounds
have extensive implementations in daily life such as the construction materials, drinking-water pipes, food colors, coagulants in water treat-ment and pigtreat-ments in plastics and paints [17,18]. Iron is not only a necessary element for the creation of hemoglobin of red cells but also it is crucial for the transport and storage of oxygen to tissues [19]. At low concentrations, iron (III) has a substantial role in fermentation processes and metabolic, as an enzyme activator, stabilizer and functional component of proteins. Iron (III) is a moderately toxic ion as compared to other transition metals, but it is one of hazardous environmental polluter at high concentrations. Thus, excessive iron ions can cause significant health problems such as depression, rapid and shallow respiration, coma, convulsions, and cardiac arrest [20,21].
In view of this, the development of highly selective and sensitive material for iron ions has dire importance to use in the aqueous envi-ronment [22]. At present, number of techniques are reported for the removal and detection of Fe(III) ion, including redox, colorimetric, fluorescent sensors, and various nanomaterials [23,24]. Moreover, UV–visible and fluorometric techniques are one of the most common and simple methods among all the techniques for the detection of iron ions in aqueous solutions [25,26]. Some authors have reported use of biopolymer-based adsorbents (cellulose, chitosan, pectin, and lignin)for the removal of metal ions [27]. Cellulose has been widely chosen during the past few decades, due to cheap, biodegradable and renewable * Corresponding author. Department of Chemistry, University of Selcuk, Campus, 42031, Konya, Turkey.
E-mail address: myilmaz42@gmail.com (M. Yilmaz).
Contents lists available at ScienceDirect
Dyes and Pigments
journal homepage: http://www.elsevier.com/locate/dyepig
https://doi.org/10.1016/j.dyepig.2019.107974
Dyes and Pigments 173 (2020) 107974
properties [28,29]. Cellulose is the most abundant polysaccharide and include a number of hydroxyl groups, which can be used for easily functional group modification in order to improve the chemical and physical properties [30–32]. Due to these reasons, cellulose-based ma-terials have attracted attention as one of the alternatives to the con-ventional heavy metal sensors [33–39]. To the best of our knowledge, there is no other paper on the use of cellulose and filter papers, as the scaffold for the colorimetric and fluorescent detection of Fe (III), there exist no paper on the use of Bodipy based compounds immobilization on cellulose.
Herein, we report the design and preparation of a cellulose and filter paper fluorimetric/colorimetric material for the detection of Fe (III) ion. For cellulose and filter paper activation, Bodipy derivative was cova-lently bonded to the filter paper and cellulose powder and used for the detection of Fe(III) as mentioned above, Bodipy-based cellulose and filter paper supply most advantages of using iron (III) detection component due to their excellent properties such as viewable, low cost, environment conscious, non-toxic.
2. Experimental section
2.1. Materials, methods, and instruments
1H NMR spectral measurements were carried out on a Varian 400 MHz spectrometer in CDCl3 at 298 K. The 13C NMR spectra were recorded at 100 MHz and chemical shift values are reported in ppm relative to the solvent peak. The melting points of Bodipy compounds were measured on a Gallenkamp melting point apparatus. The vibra-tional spectra of compounds were recorded on a Bruker Fourier Trans-form Infrared FTIR (ATR). The emission curves of compounds, modified cellulose/metal ion mixtures were recorded on a PerkinElmer LS 55 fluorescence spectrophotometer at 25 �C and the emission slits were set as 6 nm (fixed excitation). The elemental analysis data of organic com-pounds were obtained on a TruSpec E. A. The spectroscopic measure-ments were performed by metal chloride in half aqueous medium. The
synthesis of three Bodipy compounds was carried out at 25 �C under an inert atmosphere. Dichloromethane was used in dry form. Merck plates (silica gel 60 F254 on aluminum) was used for TLC (thin layer chro-matography) observations.
2.2. The preparing of compounds 2.2.1. The synthesis of compound 1
8-{4-(chloromethyl)phenyl}-2,6-diethyl-4,4-difluoro-1,3,5,7-tetra-methyl-4-bora-3a,4a-diaza-s-indacene was prepared following the syn-thesis procedure in our previous papers. p-(chloromethyl)benzoyl chloride (7.5 g, 0.04 mol) was added to a solution (in DCM, 200 mL) of Kryptopyrrole (10.8 mL, 0.08 mol) in Ar atmosphere at room tempera-ture. Then, the stirring of the solution was allowed for 4 h. After cooling of the solution, 10 mL of triethylamine was added to the mixture, it was stirred at r. t. for half hour and boron trifluoride diethyl etherate (8 mL) was injected by syringe. The mixture was heated to 65 �C for 3 h and the residue was purified by a solution of ethylacetate-cyclohexane in 1:8 ratio (3.47 g, Yield 40%). Mp: 189 �C. 1H NMR [400 MHz, CDCl 3]: 7.42 (PhH, J ¼ 8.2 Hz, d, 2H), 7.20 (PhH, J ¼ 8.2 Hz, d, 2H), 4.65 (CH2, s, 2H), 2.46 (CH3, s, 6H) 2.23 (CH2, J ¼ 7.5 Hz, q, 4H) 1.29 (CH3, s, 6H) 0.91 (CH3, J ¼ 7.5 Hz, t, 6H). 13C NMR [100 MHz, CDCl3]: δ (ppm); 154.03, 139.49, 138.66, 135.88, 136.11, 133.02, 130.77, 128.99, 128.38, 45.66, 16.98, 14.66, 12.54, 11.66. Analytical Cal. for (%) C24H28N2F2ClB: H, 6.58; C, 67.22; N, 6.53; Found: H, 6.97; C, 66.98; N, 6.13.
2.2.2. The synthesis of compound 2 (8-{4-(azidomethyl)phenyl}-2,6- diethyl-4,4-difluoro-1,3,5,7-tetra methyl-4-bora-3a,4a-diaza-s-indacene)
Compound 1 (0.44 mmol) and NaN3 (0.344 g, 0.53 mol) were dis-solved in N,N-dimethylformamide (20 mL) and mixed for 24 h at room temperature. Under inert atmosphere. The residue was extracted with water/chloroform. The organic phases were collected, dried with Na2SO4. Following evaporation of chloroform, raw product was purified in column (only dichloromethane) (0.56 g, 96%). Caution: 3 must be Scheme 1. The preparing route of Bodipy derivatives.
Dyes and Pigments 173 (2020) 107974
handled with special care due to its explosive character, mp: 140 �C1H NMR [400 MHz, CDCl3]: 7.45 (PhH, J ¼ 8.0 Hz, d, 2H), 7.33 (PhH,
J ¼ 8.1 Hz, d, 2H,) 4.48 (CH2, s, 2H,), 2.57 (CH3, s, 6H) 2.32 (CH2,
J ¼ 7.5 Hz, q, 4H), 1.28 (CH3, s, 6H,), 0.99 (CH3, J ¼ 7.5 Hz, t, 6H). 13C NMR [100 MHz, CDCl3]: δ (ppm); 154.04, 138.99, 138.03, 135.98, 135.62, 132.95, 130.81, 128.29, 129.03, 55.34, 17.44, 15.02, 12.32, 11.99. Analytical Cal. for (%) C24H28BF2N5: H, 6.48; C, 66.22; N, 16.09; Found: H, 6.67; C, 66.38; N, 16.15. MS for C24H28N5F2B: 436.2461 [MþH]þ.
2.2.3. The synthesis of compound 3 (8-{4-(aminomethyl)phenyl}-2,6- diethyl-4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene) (see Scheme 1)
2 (0.02 mol, 1.78 g) and triphenylphosphine (0.02 mol, 0.524 2 g)
were solved in 20 mL dry THF. After 6 h of mixing, 4 drops of distilled water were injected. The solution was stirred for overnight. The product was purified by a difficult procedure in column (methanol/dichloro-methane 1:10). Yield is calculated as 73% (0.6 g). Mp � 170 �C.
1H NMR [400 MHz, CDCl3]: 7.45 (PhH, J ¼ 8.0 Hz, d, 2H), 7.27 (PhH, J ¼ 8.1 Hz, d, 2H) 4.04 (CH2, s, 2H), 2.57 (CH3, s, 6H) 2.37 (CH2,
J ¼ 7.5 Hz, q, 4H,) 1.24 (CH3, s, 6H) 1.02 (CH3, J ¼ 7.6 Hz, t, 6H). 13C NMR [100 MHz, CDCl3]: δ (ppm); 154.35, 142.12, 140.01, 137.01, 135.01, 133.11, 130.99, 128.22, 127.87, 46.33, 17.53, 14.91, 12.56, 11.97. Analytical Cal. for (%) C24H30BF2N3: H, 7.39; C, 70.42; N, 10.27; Found: H, 7.68; C, 70.55; N, 10.12. MS for C24H30N3F2B: 410.2516 [MþH]þ.
2.2.4. Treatment of cellulose powder with hexamethylene diisocyanate (Cell-HMDI)
A mixture of 1.0 g cellulose powder and 20 mL of hexamethylene diisocyanate (HMDI) were dissolved in 30 mL of dry DMF and stirred at 70 �C for 8 h. The reaction was checked using IR spectrum. When the reaction finished, the white precipitated material was filtered and cleaned by using acetone for the excess of HMDI several times. The final residue was dried under vacuum and obtained a white solid. Hexam-ethylene diisocyanate was chemically anchored on raw cellulose surface and named as Cell-HDMI. The infrared specifics: 2263 cm 1 isocyanate N––C––O), 1733 cm 1 (C ¼ 0), 1681 cm 1 (amide C––O).
2.2.5. Fabrication of BODIPY-Immobilized cellulose powder (Cell- BODIPY)
0.2 g of 3 based compound was mixed in dry DMF (30 mL). After 1 g of Cell-HMDI was added to the solution. The mixture was stirred for 48 h maintaining the temperature at 70 �C. The experiment was monitored by FTIR spectroscopy. When the corresponding band for isocyanate bond disappeared, the reaction is stopped. After cooling to room temperature, that mixture was filtered off and purified with CH2Cl2 and acetone to remove excess of 3, obtained a pink solid. Bodipy-based cellulose was dried under vacuum. So, compound 3 was chemically immobilized on Cell-HMDI surface and named as Cell-BODIPY (The IR spectral data is as ATR per centimeter: 1731 cm 1 (C––O), 1679 cm 1 (amide C––O).
2.2.6. Preparation of hexamethylene diisocyanate-modified filter paper (filter paper -HMDI)
Prior to immobilization of the HMDI, the clean filter paper was cut in to 20 � 20 mm size per piece. The papers were washed with 0.5 mol/L H2SO4 and 0.5 mol/L NaOH respectively. Then, papers were cleaned with ethanol, acetone, and tetrahydrofuran (THF), and were also ultrasonicated for 10 min in distilled water, and then the papers were dried in the oven. In a 50 mL flask, paper pieces were immersed into 40 mL dry DMF. The isothiocyanate was added into this solution and heated at 70 �C under N
2 for 15 h. After cooling to room temperature, the resulting filter papers-HMDI was washed with ethanol and acetone respectively prior to being dried vacuum oven. The IR spectral data is as ATR per centimeter: 2264 cm 1 (isocyanate N––C––O), 1616 cm 1 (amide C––O).
2.2.7. Chemical modification of filter paper using BODIPY (filter paper- BODIPY)
HMDI-based filter papers were immersed into 40 mL dry DMF. 3 was added and then the mixture was mixed at 70 �C for 50 h. The experiment was followed by infrared spectroscopy. When the corresponding band for isocyanate bond disappeared, the reaction is stopped. After the cooling to r.t., papers were washed by using acetone and dichloro-methane to remove excess of 3. At last, the sample was dried in a vac-uum oven. So, compound 3 was chemically immobilized on filter paper and named as Filter Paper-BODIPY (The IR spectral data are as ATR per Scheme 2. The preparing route of Cellulose&Filter Paper modified with BODIPY.
Dyes and Pigments 173 (2020) 107974
centimeter: 1626 cm 1 (amide C––O).
3. Results and discussion
3.1. Synthesis and characterization of BODIPY-Immobilized Cellulose&Filter paper
The main object of this study was to design and synthesis of BODIPY immobilized cellulose powder (Cell-BODIPY) and BODIPY-based filter paper (Filter Paper-BODIPY), and these materials were applied in the detection of Fe(III) ions in aqueous media. All materials have been characterized through SEM, EDS 1H NMR, 13C NMR and FT-IR techniques.
The spectral data (NMR and FTIR) of organic compounds were pre-sent in supporting information. The proton signals of CH2 and CH3 fragments on pyrrole moieties compound 1, 2 and 3 appeared between 2.57 and 0.99 ppm like given data. The proton signals of CH2 attached to benzene ring in compound 1, 2 and 3 raised at 4.65, 4.48 and 4.04 ppm in singlet form, respectively. As expected, the protons on phenyl units overlapped as multiple forms around 7–8 ppm. 13C NMR results of 1, 2 and 3 all presented similar data that carbon atoms in phenyl and pyrrole fragments gave multiple signals between 110 and 165 ppm whereas
carbon signals of aliphatic groups were observed between 11.66 and 17.44 ppm. Otherwise, the 13C atom signals of CH2 attached to benzene ring appeared at 45.66, 55.34 and 46.33 ppm for compound 1, 2 and 3, respectively.
In the infrared spectrum of compound 2, the bands appeared at 1473, 1541, 1651, 2869 and 2962 cm 1, which can be referred to, alkene C––C, C––N pyrrole imine, aliphatic C–H, aromatic C–H, respectively. For compound 3, the most unique band was observed at 2091 cm 1 and the peak attributed to the N––N stretching of N3 fragment. This peak disappeared in the spectrum of compound 3 following the amino con-version. The new peak appeared at 3407 cm 1 assigned to N–H stretching band and other peaks were observed in similar frequencies.
To create a convenient binding site onto materials (Cellulose and filter paper) for BODIPY, cellulose powder or filter paper were treated with HMDI. The formations of Cell-HMDI and Filter Paper-HMDI were confirmed by the presence of the specific isocyanate band and amide bands at about 2263, 1733 and 1681 cm 1 for Cell-HMDI, and at 2264 and 1637 cm 1 for Filter Paper-HMDI in the FT-IR spectrum. The cor-responding Cell-HMDI or Filter Paper-HMDI was reacted with BODIPY [40] to give the corresponding Cell-BODIPY or Filter Paper-BODIPY (see Scheme 2). The formations of Cell-BODIPY and Filter Paper-BODIPY were approved by the presence of the specific carbonyl bands at about Fig. 1. Comparative FTIR spectra of a-) Cell-HMDI and Cell-BODIPY and b-) Comparative FTIR spectra of Filter Paper-HMDI and Filter Paper-BODIPY. M. Oguz et al.
Dyes and Pigments 173 (2020) 107974
Fig. 2. SEM photographs of a-) Cell-HMDI, b-) Cell-BODIPY c-) Filter Paper-HMDI and d-) Filter Paper-BODIPY.
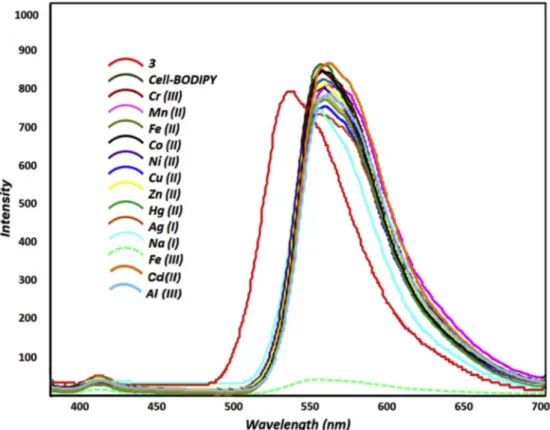
Fig. 3. Fluorescence spectral changes of Cell-BODIPY upon addition of various metal ions (Cr(III), Fe(II), Co (II), Cu (II), Fe(III), Ni (II), Zn (II), Ag (I), Hg(II), Cd (II), Al (III) and Na(I)) (λex ¼ 350 nm).
Dyes and Pigments 173 (2020) 107974
1677 and 1624 cm 1, respectively, and by the absence of isocyanate band at 2263 cm 1 of Cell-HMDI and 2264 cm 1 of Filter Paper-HMDI in the FT-IR spectrum (see Fig. 1(a and b)).
The surface morphology of the cellulose and filter papers before and after surface functionalization was analyzed by scanning electron mi-croscopy (SEM). Smooth morphology of the cellulose with a clear and
uniform pore structure can be seen in Fig. 2a. SEM image of the BODIPY based cellulose (Fig. 2b) on high magnification reveals that the BODIPY is predominantly localized at inside of the fiber of cellulose. Fig. 2c shows the SEM image of Filter Paper. Following the immobilization of BODIPY onto filter paper, the expected surface cavity of filter paper was filled with BODIPY (Fig. 2d). These results confirm the immobilization Fig. 4. Fluorescent emission response around 570 nm of Cell-BODIPY& Fe(III) ion mixture in the presence of a series of metal ions (Cr(III), Fe(II), Ni (II), Fe(III), Co (II), Hg(II), Cu (II), Zn (II), Ag (I), Cd (II), Al (III) and Na(I)).
Fig. 5. SEM micrographs and EDS spectral data of a-) Cell-BODIPY in the presence of Fe(III) ion and b-) Filter Paper-BODIPY in the presence Fe(III) ion at high magnification (50 μm). Digital photographs of c-) Filter Paper-BODIPY in daylight d-) Cell-BODIPY in daylight e-) Filter Paper-BODIPY in longwave light (365 nm) f-) Cell-BODIPY in longwave light (365 nm) h-) Filter Paper-BODIPY&Fe(III) mixture in longwave light (365 nm) g-) Cell-BODIPY&Fe(III) mixture in longwave light (365 nm).
Dyes and Pigments 173 (2020) 107974
[40].
3.2. Selectivity and sensitivity studies of BODIPY-immobilized cellulose&filter paper
Bodipy-based cellulose composite developed under optimum condi-tions was applied for the detection of metal cacondi-tions. A stock suspension of Cell-BODIPY (0.2 g) was prepared in methanol (100 mL). The solu-tions of different metal ions (Cr(III), Co (II), Fe(II), Fe(III), Ni (II), Zn (II), Cu (II), Hg(II), Ag (I), Cd (II), Al (III) and Na(I) with 2.5 � 10 5 M concentration (in water) were also prepared for selectivity tests. 2 mL of these solutions was respectively added to 2 mL of the stock suspension of Bodipy-anchored cellulose and allowed to interact for about 5 min to investigate the metal ion selectivity at room temperature. The spectro-scopic properties of compound 3 were previously reported that it had an emission maximum at 538 nm 33. Following the binding of 3 on the activated cellulose surface, the emission maximum shifted dramatically toward red and appeared at 570 nm.
As shown in Fig. 3, the emission intensity of Cell-BODIPY around 570 nm was selectively quenched in the presence of Fe(III). On the other hand, tested metal ions have not any change in the fluorescence in-tensity and wavelength of Cell-BODIPY. The quenching effect could be attributed paramagnetic Fe(III) ions binding to the donor atoms (such as O and N) close to the Bodipy moiety, along with the hydroxyl groups from the cellulose backbone. In addition to this, hard-soft acid-base interaction plays an important role in binding Fe(III) by increasing its charge density that leads to its hard acid character.
Cations such as Cr(III), Fe(II), Co (II), Cu (II), Fe(III), Ni (II), Zn (II), Ag (I), Hg(II), Cd (II), Al (III) and Na(I) are commonly present in the groundwater. A competitive ion measurement was carried out for selectivity of Cell-BODIPY, Results revealed that these metal ions did not affect the emission band and intensity of Cell-BODIPY/Fe (III) mixture in the studied concentration range. (Fig. 4). This clearly indicates that Cell- BODIPY has a good selectivity toward Fe(III) cation over other studied competitive metal ions in the groundwater samples.
In ordered to check the purity and elemental content of Cell-BODIPY and Filter-Paper-BODIPY after Fe(III) absorption, the scanning electron microscopy (SEM) was combined with energy dispersive spectroscopy (EDS). EDS sampling was done on two different material grains i.e., Cell- BODIPY and Filter Paper-BODIPY. Fig. 5a and b presents the SEM mi-crographs and EDS spectra with the elemental composition for Cell- BODIPY and Filter Paper-BODIPY, respectively after absorption of Fe (III) ion. Cellulose and filter paper are mainly composed of hydrogen, carbon, and oxygen, however, they do not contain iron, boron, fluorine, nitrogen. Following the immobilization of BODIPY, the EDS results of cellulose and filter paper demonstrate 4.38% boron, 11.72% nitrogen, 3.93% fluorine in Fig. 5a and 1.15% boron, 4.97% nitrogen, 3.99% fluorine in Fig. 5b. Also, after absorption of Fe(III), Cell-BODIPY and Filter Paper-BODIPY, the EDS results show that 8.59% and 15.50% presence of the iron, respectively in Fig. 5a and b.
For better visualization, filter paper-BODIPY was cut in small pieces and the pieces were immersed in the solutions of test metal ions such as Cr(III), Fe(II), Cu (II), Fe(III), Co (II), Ni (II), Zn (II), Ag (I), Hg(II), Cd (II), Al (III) and Na(I)), respectively. Cell-BODIPY was then triturated for more effective interaction with test metal ions. Just as fluorometric applications, all these metal cations except Fe(III) did not show any visual change in both Cell-BODIPY and Filter Paper-BODIPY (Fig. 5c–g). All changes on Bodipy-anchored cellulose and filter paper photographed in absence/presence Fe(III) ion under both daylight and longwave light (365 nm), respectively. By the immobilization of Bodipy, the pink- orange color was observed whereas the white tone of cellulose and fil-ter paper. By addition of Fe(III) ions, it is seen that the shiny yellow color turn clearly to light-brown color in longwave light.
For detection with better performance, the fluorescence intensities of the Cell-BODIPY to Fe(III) complex forms at various pH (3–9) values were collected to examine the optimum conditions.
As shown in Fig. 6, F0 and F were defined as the fluorescent in-tensities of Cell-BODIPY Cell-BODIPY/Fe(III) mixtures, respectively, in the presence of pH ranging systematically from 3 to 9. It is clearly indicated that the fluorescence intensity ratios (F0/F) reduced gradually Fig. 6. Effect of pH on Cell-BODIPY/Fe(III) complexation in aqueous system (pH:3–9). (λemmax ¼570 nm).
Dyes and Pigments 173 (2020) 107974
for pH ranging from 3 toward 7.5, however, higher pH conditions re- increased to this ratio. The functional groups of cellulose’ surface might be protonated in lower pH values and positively charged surface. Hence, it difficult to achieve binding of iron cations to the surface of Cell-BODIPY. Following the increase of pH, the deprotonation process caused the more effective interaction between Cell-BODIPY and Fe(III) cation depending on the high chelating effect.
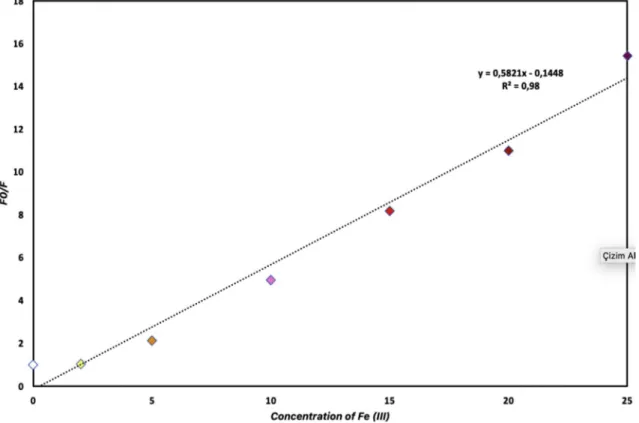
The binding constant of iron (III) to Cell-BODIPY was investigated by using the Stern-Volmer equation in an aqueous medium, in which Fe (III) bound strongly to Cell-BODIPY. The fluorescence intensity ratio (F0/F) increased gradually by enhancing the concentration of iron (III) cation and the highest binding constant value was obtained in 2.5 � 10 5 M concentration. The limit of detection (LOD) for the inter-action of iron (III) ions with Cell-BODIPY was calculated by using fluorescent measurement (Fig. 7). The titration process was applied by adding different concentrations of iron solutions (1.0 � 10 4 - 2.5 � 10 4 M) to Cell-BODIPY suspension and then allowed to interact for 10 min. Following these experiments, the calibration curve/slope was obtained, the limit of detection was calculated using Limit of
Detection ¼ K (α/s) formula (where α; standard deviation, K; 3, s; slope).
The fluorescence intensity of Cell-BODIPY quenched upon the addition of different concentrations of Fe(III) ion. The limit of detection was found as 1.72 μM by using the fluorescence intensities in Fig. 7. 4. Conclusion
In brief, a fluorometric and colorimetric module was presented for selective recognition of Fe(III) ions using Bodipy-immobilized cellulose and filter paper. The fluorescent material is obtained by a reaction be-tween the amine moiety of Bodipy and the cellulose activated by hex-amethylene diisocyanate. Upon the interaction with Fe (III) ions, the fluorescence intensity of Cell-BODIPY is highly quenched and illumi-nated by using the fluorometric method. Moreover, for the practical applications, Bodipy derivative was also anchored on the modified filter paper using similar procedure and pink color of Bodipy-anchored filter
paper turn to light-brown in a naked-eye detectable form. A wide scope is open to further investigations on the visual change of functionalized filter paper in metal ion detection. Consequently, the improved natural- sourced materials (cellulose and filter paper) are selectively adequate for the detection and reliable monitoring of Fe (III) ions.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank the Research Foundation of Selcuk University for financial support of this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi. org/10.1016/j.dyepig.2019.107974.
References
[1] Taha AA, Wu Y-n, Wang H, Li F. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr (VI) ion removal from aqueous solution. J Environ Manag 2012;112:10–6. [2] Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM.
Science and technology for water purification in the coming decades. In: Nanoscience and technology: a collection of reviews from nature journals. World Scientific; 2010. p. 337–46.
[3] Zhang X, Zhao J, Cheng L, Lu C, Wang Y, He X, et al. Acrylic acid grafted and acrylic acid/sodium humate grafted bamboo cellulose nanofibers for Cu 2þ adsorption. RSC Adv 2014;4(98):55195–201.
[4] Kurniawan TA, Chan GY, Lo W-h, Babel S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 2006;366(2–3): 409–26.
[5] Gavrilescu M. Removal of heavy metals from the environment by biosorption. Eng Life Sci 2004;4(3):219–32.
[6] Reddad Z, Gerente C, Andres Y, Le Cloirec P. Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 2002; 36(9):2067–73.
Fig. 7. The fluorescence intensities ratio (F0/F) around 570 nm plotted against iron (III) cation concentrations (1.10 6-2.5 � 10 5 M) (λemmax ¼570 nm). M. Oguz et al.
Dyes and Pigments 173 (2020) 107974 [7] Chitpong N, Husson SM. Polyacid functionalized cellulose nanofiber membranes
for removal of heavy metals from impaired waters. J Membr Sci 2017;523:418–29. [8] Feng L, Li H, Niu L-Y, Guan Y-S, Duan C-F, Guan Y-F, et al. A fluorometric paper- based sensor array for the discrimination of heavy-metal ions. Talanta 2013;108: 103–8.
[9] Nordberg G, Fowler B, Nordberg M, Friberg L. Handbook of the toxicology of metals. third ed. London: Academic; 2007, ISBN 978-0-12-369413-3.
[10] Çimen A, Bilgiç A, Kursunlu AN, Gübbük _IH, Uçan H_I. Adsorptive removal of Co (II), Ni(II), and Cu(II) ions from aqueous media using chemically modified sporopollenin of Lycopodium clavatum as novel biosorbent. Desalin Water Treat 2014;52(25–27):4837–47.
[11] Çimen A, Torun M, Bilgiç A. Immobilization of 4-amino-2-hydroxyacetophenone onto silica gel surface and sorption studies of Cu(II), Ni(II), and Co(II) ions. Desalin Water Treat 2015;53(8):2106–16.
[12] Kursunlu AN, Oguz M, Yilmaz M. On/off rhodamine-BODIPY-based fluorimetric/ colorimetric sensor for detection of mercury (II) in half-aqueous medium. IEEE Sens J 2018;19(6):2009–15.
[13] Lv P, Yao Y, Li D, Zhou H, Naeem MA, Feng Q, et al. Self-assembly of nitrogen- doped carbon dots anchored on bacterial cellulose and their application in iron ion detection. Carbohydr Polym 2017;172:93–101.
[14] McRae R, Bagchi P, Sumalekshmy S, Fahrni CJ. In situ imaging of metals in cells and tissues. Chem Rev 2009;109(10):4780–827.
[15] Orvig C, Abrams MJ. Medicinal inorganic chemistry: introduction. Chem Rev 1999; 99(9):2201–4.
[16] Kumari S, Chauhan GS. New cellulose–lysine schiff-base-based sensor–adsorbent for mercury ions. ACS Appl Mater Interfaces 2014;6(8):5908–17.
[17] Kumar SA, Thakur N, Parab HJ, Pandey SP, Shinde RN, Pandey AK, et al. A visual strip sensor for determination of iron. Anal Chim Acta 2014;851:87–94. [18] Bhatti AA, Oguz M, Memon S, Yilmaz M. Dual fluorescence response of newly
synthesized naphthalene appended calix [4] arene derivative towards Cu 2þ and I . J Fluoresc 2017;27(1):263–70.
[19] Alemdaro�glu T, Onur E, Akgün H. Determination of major and trace elements in sediments of lake E�gridir, Turkey. Int J Environ Stud 2000;57(2):157–66. [20] Ba�g H, Lale M, Türker AR. Determination of iron and nickel by flame atomic
absorption spectrophotometry after preconcentration on Saccharomyces cerevisiae immobilized sepiolite. Talanta 1998;47(3):689–96.
[21] Ram B, Chauhan GS. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem Eng J 2018;331:587–96. [22] Xu LQ, Neoh K-G, Kang E-T, Fu GD. Rhodamine derivative-modified filter papers
for colorimetric and fluorescent detection of Hg 2þ in aqueous media. J Mater Chem 2013;1(7):2526–32.
[23] Sevgi F, Bagkesici U, Kursunlu AN, Guler E. Fe (III), Co (II), Ni (II), Cu (II) and Zn (II) complexes of schiff bases based-on glycine and phenylalanine: synthesis, magnetic/thermal properties and antimicrobial activity. J Mol Struct 2018;1154: 256–60.
[24] Li C-Y, Zou C-X, Li Y-F, Tang J-L, Weng C. A new rhodamine-based fluorescent chemosensor for Fe3þ and its application in living cell imaging. Dyes Pigments 2014;104:110–5.
[25] Wang Q, Kim D, Dionysiou DD, Sorial GA, Timberlake D. Sources and remediation for mercury contamination in aquatic systems—a literature review. Environ Pollut 2004;131(2):323–36.
[26] Bhatti AA, Oguz M, Yilmaz M. One-pot synthesis of Fe 3 O 4@ Chitosan-pSDCalix hybrid nanomaterial for the detection and removal of Hg 2þ ion from aqueous media. Appl Surf Sci 2018;434:1217–23.
[27] Yang B, Wu W. Fabrication of a novel natural cellulose-based paper
chemodosimeter via grafting-to of Rhodamine B moieties for detection of Hg2þ. React Funct Polym 2013;73(11):1553–8.
[28] Fakhre NA, Ibrahim BM. The use of new chemically modified cellulose for heavy metal ion adsorption. J Hazard Mater 2018;343:324–31.
[29] Nagata E, Ara T, Nakano H. Mechanochromic luminescence of 1-alkanoylamino-pyrenes adsorbed onto cellulose papers. Dyes Pigments 2017;141:48–52. [30] Ozyilmaz E, Sayin S. Preparation of new calix [4] arene-immobilized biopolymers
for enhancing catalytic properties of Candida rugosa lipase by sol–gel encapsulation. Appl Biochem Biotechnol 2013;170(8):1871–84.
[31] Stephen M, Catherine N, Brenda M, Andrew K, Leslie P, Corrine G. Oxolane-2, 5- dione modified electrospun cellulose nanofibers for heavy metals adsorption. J Hazard Mater 2011;192(2):922–7.
[32] Li M, Liu Z, Wang H-C, Sedgwick AC, Gardiner JE, Bull SD, et al. Dual-function cellulose composites for fluorescence detection and removal of fluoride. Dyes Pigments 2018;149:669–75.
[33] d’Halluin M, Rull-Barrull J, Bretel G, Labrugère C, Le Grognec E, Felpin Fo -X. Chemically modified cellulose filter paper for heavy metal remediation in water. ACS Sustainable Chem Eng 2017;5(2):1965–73.
[34] Zhou D, Zhang L, Zhou J, Guo S. Cellulose/chitin beads for adsorption of heavy metals in aqueous solution. Water Res 2004;38(11):2643–50.
[35] Zhou D, Zhang L, Guo S. Mechanisms of lead biosorption on cellulose/chitin beads. Water Res 2005;39(16):3755–62.
[36] Wang X, Bai H, Yao Z, Liu A, Shi G. Electrically conductive and mechanically strong biomimetic chitosan/reduced graphene oxide composite films. J Mater Chem 2010;20(41):9032–6.
[37] Aliabadi M, Irani M, Ismaeili J, Piri H, Parnian MJ. Electrospun nanofiber membrane of PEO/Chitosan for the adsorption of nickel, cadmium, lead and copper ions from aqueous solution. Chem Eng J 2013;220:237–43.
[38] Aliabadi M, Irani M, Ismaeili J, Najafzadeh S. Design and evaluation of chitosan/ hydroxyapatite composite nanofiber membrane for the removal of heavy metal ions from aqueous solution. Journal of the Taiwan Institute of Chemical Engineers 2014;45(2):518–26.
[39] Kang S-M, Jang S-C, Huh YS, Lee C-S, Roh C. A highly facile and selective Chemo- Paper-Sensor (CPS) for detection of strontium. Chemosphere 2016;152:39–46. [40] Kursunlu AN, Baslak C. A Bodipy-bearing pillar [5] arene for mimicking
photosynthesis: multi-fluorophoric light harvesting system. Tetrahedron Lett 2018; 59(20):1958–62.