3475
Received: 3 October 2018 Revised: 25 December 2018 Accepted article published: 8 January 2019 Published online in Wiley Online Library: 11 February 2019 (wileyonlinelibrary.com) DOI 10.1002/jsfa.9566Design and effectiveness of pulsed electric
fields towards seed disinfection
Gulsun A Evrendilek,
a,b*
Berna Karatas,
a
Sibel Uzuner
a
and Igor Tanasov
c
Abstract
BACKGROUND: Seeds harbor different microorganisms on their surfaces that degrade seed quality, thus causing an economic loss. Even though different approaches are available for the disinfection of seed surfaces, there is a need to develop environmentally friendly and sustainable technologies. A bench-scale pulsed electric field (PEF) unit was designed to inactivate microflora of eight seeds after which the resultant vigor of the treated seeds was determined.
RESULTS: Significant reductions were obtained in endogenous natural and inoculated pathogenic (Alternaria brassica and
Xanthomonas campestris pv. campestris, Drechslera graminea and Fusarium graminearum) microflora of seeds. The survival ratios
of total aerobic mesophilic bacteria and of total mold and yeast decreased significantly for winter wheat and barley, parsley, onion, lettuce, tomato, and garden rocket with the PEF treatments of 240 and 960 J. A significant increase in germination ratio was observed for winter wheat and barley, lettuce, and tomato with 960 J. Germination energy increased for parsley with 240 J and for winter wheat and barley, lettuce, tomato, and garden rocket with 960 J. A better root development and seedling were found for winter barley.
CONCLUSION: PEFs are a viable option to both disinfect seed surfaces and improve seed vigor. © 2019 Society of Chemical Industry
Keywords: pulsed electric fields; microbial inactivation; seed disinfection; seed vigor; seed germination
INTRODUCTION
Seed-borne pathogens reduce crop quantity and quality, thus causing important economic losses annually. Various disinfec-tion treatments have been applied to seed surfaces to kill their pathogens, prevent their transmission to crops, and enhance the storage life of seeds. For example, the use of agricultural bio-cides (e.g. herbibio-cides, insectibio-cides, fungibio-cides, and rodentibio-cides) has enabled farmers to harvest increased yields but, at the same time, has posed public health risks, such as soil and water pollution and the presence of pesticide residues in foods.1,2
Another common application practiced for the surface disinfec-tion is the use of chemical products such as peroxyacetic acid, hydrochloric acid, and sodium hypochlorite.3However, their use generally fails to eradicate bacteria from the infected seeds.4Hot water immersion, and hot humid air were also explored, with no satisfactory outcomes towards the enhanced germination, dor-mancy, and disease resistance of seeds. Physical methods such as ionizing radiation, radioisotopes, thermal effects, electron flow, laser, and space-flight breeding are considered to be more envi-ronmentally friendly but involve a highly complex and expensive processing. Also, such treatments of higher doses may be highly radioactive, ineffective, or destructive.5,6
Pulsed electric fields (PEFs) involve application of short bursts of high electric field pulses, and the applied electric field is transmit-ted to a material to be processed by ions conducting the electric fields.
PEF treatment at different magnitudes of electric field strength, frequency, and duration is mainly applied to biological cell mem-branes to induce electrical breakdown, defined as electroporation
or electropermeabilization.7,8 Formation of pores on the mem-branes of cells and organelles is also explained by electropora-tion. Electroporation can be explained as mechanical, hydrody-namic, osmotic, and viscoelastic instabilities in cell structure.9,10 The magnitude of applied electric field intensity may induce the formation of transient or permanent pores, causing a reversible or an irreversible electroporation respectively, causing inactivation of the biological membrane.8–10This application is a useful tool for injecting exogenous molecules, such as drugs, proteins, RNA, or DNA, into cells without causing deterioration of cellular functions. A higher magnitude of electric field strength, on the other hand, causes an intense electroporation so that electropermeabilization might be irreversible or the cell membrane and other structures might even be disintegrated, and PEF would cause permanent cell membrane damage or cell lysis, which is the basis for the food pro-cessing method for the food industry as an alternative to thermal processing.10
The earliest report on the application of electricity to a biological entity was reported in 1746 by Maimbray (Edinburgh).11 The
∗ Correspondence to: GA Evrendilek, Department of Food Engineering, Ardahan
University, 75002 Ardahan, Turkey. E-mail: gevrendilek@yahoo.com a Department of Food Engineering Faculty of Engineering, Bolu Abant Izzet
Baysal University, Bolu, Turkey
b Department of Food Engineering, Ardahan University Faculty of Engineering, Ardahan, Turkey
3476
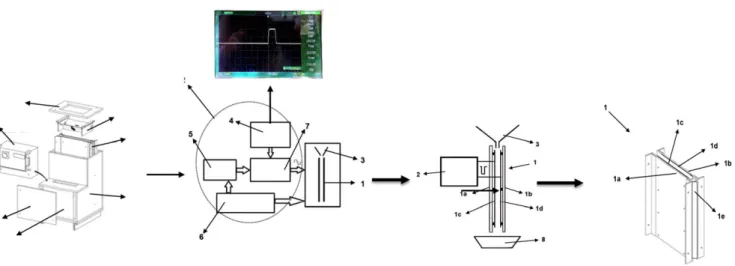
Figure 1. A scheme of a bench-scale PEF system for seed treatment with a parallel plate electrode system, a hopper doser unit, and a monopolar square
wave pulse used in seed treatment. (Numbering explained in text.)
growth stimulation (by 70%) of crops such as potatoes, carrots, and celery was observed as a function of the electrical discharge treatment in 1885.12 A recent effort has been made to treat seeds through PEFs.13 Despite the various approaches already mentioned, to the best of our knowledge there is a limited amount of information in related literature about the application of PEFs to the disinfection of seed surfaces. Therefore, the objective of this study was to design, construct, and test a bench-scale PEF unit and to determine the effectiveness of the PEF unit constructed on the disinfection and vigor of eight seeds.
MATERIALS AND METHODS
Chemicals, seeds, and culturesPeptone, potato dextrose agar (PDA), and plate count agar (PCA) were obtained from Fluka (Seelze, Germany). Tartaric acid was acquired from Sigma Chemical Co. (Stockholm, Sweden). Seeds of winter wheat (Triticum aestivum) and winter barley (Hordeum vulgare) were obtained from a local producer in Kiev (Ukraine). Seeds of cabbage (Brassica oleracea), parsley
(Pet-roselinum crispum), onion (Allium cepa), lettuce (Lactuca sativa),
tomato (Solanum lycopersicum), and garden rocket (Eruca
vesi-caria) were acquired from a local producer in Bolu (Turkey). No
chemical treatment was applied to the seeds. Drechslera graminea and Fusarium graminearum cultures were obtained from the D. K. Zabolotny Institute of Microbiology and Virology (Ukraine).
Alternaria brassica and Xanthomonas campestris pv. campestris
(Xcc) were provided by Wageningen Seed Centre (Netherlands) in petri plates.
Preparation of microbial cultures
The cultures of D. graminea, F. graminearum, and A. brassica were stored at 4 ∘C, sub-cultured once a month, and spore suspen-sion was prepared from 2-week-old PDA culture. The spores were removed from the surface of the culture, suspended in 10 mL ster-ilized distilled water, and used to contaminate seeds. Spore con-centration was determined using a hemocytometer and adjusted to 105–106spores per gram.
Xcc was cultured on yeast extract–dextrose–calcium carbonate
(YDC) plate medium at 25 ∘C for 3 days. Colonies of Xcc were picked and suspended in distilled water to prepare the bacterial suspen-sion. Aliquots of 10 mL of suspension were used to contaminate
cabbage seeds. The inoculated number of bacteria was measured by counting colonies of Xcc on YDC plate medium after incubation for 3 days at 25 ∘C.
Design and application of PEF unit
A bench-scale PEF unit designed for the disinfection consists of a loading unit, a seed treatment chamber, and a high-voltage pulse generator (Fig. 1). The main system components included (1) a treatment chamber, (2) a system control unit, (3) a hopper doser unit, (4) a high-voltage power pulse generator, (5) a pulse generator, (6) a control PC, and (7) a high-voltage switch unit. The electrode system and doser unit included (1) a parallel plate electrode made up of (1a) first and (1b) second plates, (1c) first and (1d) second electrodes, and (1e) an ultraviolet light source, (2) a modulator, and (3) a hopper doser unit. The PEF seed disinfection unit was designed as a compact one with (1) a parallel plate electrode, (2) a treatment chamber, (3) a hopper doser unit, (4) a control unit, (5) front and side covers, and (6) a high-voltage power pulse modulator.
The seeds are fed from the hopper with an adjustable gap through the electrodes. The gap distance between the electrodes at 30 cm in height was set as 1.1 cm, which serves to determine the treatment time of the free fall of the seeds from the hopper doser. An oscillator microprocessor with an integrated frequency counter, a low-voltage power pulse generator to the (dis)charge of the capacitor, a high-voltage transformer (transformer ratio of 1 : 45), and a control unit are included in the high-voltage pulse generator unit. The secondary winding of the transformer pulses was applied to the electrodes disposed into the treatment cham-ber. The high-voltage pulse generator provides a unipolar rectan-gular pulse of 1.2 μs in width (Fig. 1). The treatment modes were determined using an oscillator frequency in the range 50–300 Hz and an electric field strength in the range 12–18 kV cm−1. An ultra-violet source (1b) to enhance the electron emission was placed to provide better air ionization into the treatment chamber (Fig. 1).
The magnitude of total energy during the PEF treatment was calculated thus:
E = Pt (1)
where E (J) is energy, P (W) is power, and t (s) is treatment time.
P = cf V 2
3477
where V (V) is voltage, c (μs) is pulse width, and f (Hz) is frequency.The treatment time was estimated as follows:
t =√2h∕g (3)
where h (m) is the height of the treatment chamber, and g (m s−2) is the acceleration due to gravity.
The PEF treatments of seeds were first performed by apply-ing 12 kV cm−1with a total energy of 240 or 960 J to determine the survival ratio (as a percentage), germination rate, and germi-nation energy. Then, the PEF treatments were performed using 100–400 μs treatment times and 100–300 Hz frequencies with moisture contents of 6–16% to determine the inactivation lev-els of total aerobic mesophilic bacteria (TAMB), total mold and yeast (TMY), and the inoculated cultures of Xcc and A. brassica for the cabbage seeds, and D. graminea and F. graminearum for winter barley, as well as their vigor.
Microbiological analyses
Once diluted with 90 mL of 0.1% (w/v) peptone water and prepa-ration of serial dilutions, 10 g of the seed samples was plated onto PCA to count TAMB and onto YDC to count Xcc culture using the surface plating method. The same method was applied onto PDA acidified with 10% (w/v) tartaric acid to count TMY and
D. graminea, F. graminearum, and A. brassica cultures separately.
PCA plates were incubated at 35 ± 2 ∘C for 24–48 h, whereas the YDC and PDA plates were incubated at 22 ± 2 ∘C for 3–5 days. Results were expressed in log(cfu g−1).
Germination rate
Germination rate was estimated using the standard laboratory methods for the operational conditions of 4 × 100 seeds at 25 ∘C under 95% humidity for 10 days seeds.14 Filter paper rolls and sterile sand were used as the substrate.
Germination energy
Germination energy was determined using the standard laboratory method described by the International Seed Testing Association.15 According to this protocol, 4 × 100 seeds were planted and tested in wet sterilized sand used as the medium. Seeds were incubated in a germination chamber at 25 ∘C and 95% relative humidity. Germination energy was assessed counting the number of typical seedlings on the fourth day.
Cold test
A hundred seeds counted per box were evenly distributed in a plant growth container with a soil depth of 2.5 cm placed above and below the seed layer. Each box was watered up to 60% sat-uration and covered with a lid. The boxes were stored at 10 ∘C for 7 days in dark and then moved to 25 ∘C under daylight for 7 days. Seedlings were evaluated on the 15th day. Results were reported as a percentage, which represents the number of seedlings categorized as ‘normal’ out of the 100 seeds tested.14
Electrolyte leakage
Fifty seeds counted were weighed and placed into a beaker that contained 250 mL of distilled water. After the closure of each beaker with a lid, they were placed into an incubator at 23–25 ∘C. Their conductivity was measured after 2 and 24 h in dark. Electrical
conductivity was measured using a conductivity meter (Model T
able 1. Sur v ival ratio (N /N o ), germination rat e (G R), a nd germination e ner g y (GE) o f d iff er en t seeds tr e at ed b y PEF (n = 3) Sur v ival ratio o f TAMB (%) Sur v ival ratio o f T MY (%) G R (%) GE (%) S eed C o ntr o l 240 J 960 J C ontr ol 240 J 960 J C ontr ol 240 J 960 J C ontr ol 240 J 960 J W in ter wheat 100 a 0. 84 ± 0. 02 b 0. 60 ± 0. 01 c 100 a 8. 7 ± 0. 0 b 1. 4 ± 0. 0 c 82 ± 3. 46 a 88 ± 2. 2 a 89 ± 2. 3 b 78 ± 6. 1 a 81 ± 3. 3 a 93 ± 3. 0 b W in ter barley 100 a 22 .1 ± 0. 06 b 16 .7 ± 0. 04 c 100 a 16 .1 ± 0. 1 b 9. 2 ± 0. 3 c 80 ± 2. 12 a 93 ± 3. 0 b 95 ± 3. 5 b 89 ± 5. 2 a 93 ± 3. 5 ab 98 ± 3. 3 b P a rsley 100 a 65 .9 ± 0. 26 b 52 .4 ± 0. 09 c 100 a 24 .2 ± 0. 0 b 1. 6 ± 0. 0 c 78 ± 6. 0 a 82 ± 3. 6 a 86 ± 4. 2 a 74 ± 3. 6 a 86 ± 3. 2 b 93 ± 2. 9 c Onion 100 a 35 .7 ± 0. 42 b 1. 48 ± 0. 01 c 100 a 19 .2 ± 0. 0 b 12 .3 ± 0. 0 c 86 ± 4. 2 a 86 ± 2. 5 a 88 ± 3. 7 a 89 ± 3. 5 a 91 ± 4. 0 a 93 ± 2. 8 a Le ttuc e 100 a 62 .9 ± 0. 01 b 51 .3 ± 0. 01 c 100 a 31 .1 ± 0. 2 b 5. 9 ± 0. 0 c 80 ± 3. 7 a 84 ± 2. 7 ab 89 ± 4. 0 b 82 ± 3. 4 a 88 ± 2. 5 a 89 ± 3. 1 b To mat o 100 a 3. 29 ± 0. 00 b 0. 40 ± 0. 00 c 100 a 8. 5 ± 2. 6 b 6. 4 ± 0. 0 c 82 ± 4. 1 a 87 ± 3. 0 ab 89 ± 3. 6 b 85 ± 5. 0 a 89 ± 3. 1 ab 92 ± 3. 3 b G a rd e n ro ck e t 1 0 0 a 1. 67 ± 0. 0 b 0. 33 ± 0. 00 c 100 a 92 .5 ± 0. 0 b 89 .6 ± 0. 0 c 87 ± 3. 8 a 90 ± 2. 9 a 91 ± 3. 6 a 83 ± 5. 2 a 90 ± 2. 8 ab 91 ± 2. 7 b
3478
0 1 2 0 0 1 200 300 5 400 10 15 3 5 0 1 2 3 5 4 5 1 00 15 4 0 0 3 0 20 4 400 4 5 0 10 0 0 2 300 -1 0 1 100 4 00 0 0 2 0 3 0 1 2 0 0 1 200 0 0 3 . 0 15 0 . 0 2 5 . 0 2 0 0 1 0 0 4 0 0 2 300 5 0 2 0 3 . 0TMY log reduction
Moisture (%) Treatment time (µs) n oit c u de r g ol B M A T Moisture (%) Treatment time (µs) Xcc log reduction Treatment time (µs) Frequency (Hz) A.brassica n oit c u de r g ol Treatment time (µs) Frequency (Hz) (A) (B) (C) (D)
Figure 2. Inactivations of (A) total aerobic mesophilic bacteria (TAMB) and (B) total mold and yeast (TMY), (C) Alternaria brassica and (D) Xanthomonas
campestris pv. campestris (Xcc) for cabbage seeds as a function of treatment time and frequency. Sension 5; HACH Company, Loveland, CO, USA). Electrolyte leakage
(EL) results were calculated thus:
EL =
Conductivity of seed − water mixture −Conductivity of distilled water
Seed weight (4)
Statistical analyses
Statistical data analyses were performed using Minitab 17 (Minitab, Inc., State College, PA, USA). Tukey’s multiple compar-ison test following one-way analysis of variance was conducted to determine the significant differences among mean responses to the treatments at P< 0.05. A paired t-test was performed to determine the significant difference before and after the PEF treatments.
RESULTS AND DISCUSSION
Inactivation of endogenous and pathogenic microflora The PEF treatments with an electric field strength of 12 kV cm−1 and a total energy of 240 or 960 J resulted in different survival ratios for the indigenous microflora of TAMB and TMY of the seven seed types (parsley, onion, lettuce, wheat, garden rocket, tomato, and barley). The group treated at 960 J had a lower TAMB sur-vival ratio than did the control group and the treatment group of 240 J (P< 0.05). For the total applied energy level of 240 J, the lowest TAMB survival ratios were 0.84 ± 0.02%, 3.29 ± 0.001%, and 1.67 ± 0.01% for winter wheat, tomato, and garden rocket respectively. In response to 960 J, the lowest TAMB survival ratios were estimated at 0.60 ± 0.01%, 1.48 ± 0.01%, 0.40 ± 0.001%, and 0.33 ± 0.001% for the winter wheat, onion, tomato, and garden
rocket seeds respectively (P< 0.05) (Table 1). The winter wheat and tomato seeds exhibited the lowest TMY survival ratios of 8.7 ± 0.0% and 8.5 ± 2.6% respectively with 240 J. The PEF treat-ment at 960 J provided the lowest TMY survival ratio of 1.4 ± 0.0%, 1.6 ± 0.0%, 5.9 ± 0.0%, and 6.4 ± 0.0% for the winter wheat, pars-ley, lettuce and tomato seeds respectively (P< 0.05) (Table 1). The energy level of 960 J increased the germination rate for the winter wheat, winter barley, lettuce, tomato, and garden rocket seeds (P< 0.05). A significant increase in the germination energy occurred with 240 J for parsley and with 960 J for winter wheat, winter barley, parsley, lettuce, tomato, and garden rocket (Table 1). Though obtained from the same plot and phenological state, the moisture content differed significantly among the seed types, and thus this was factored in the microbial inactivation (Fig. 2). For example, the moisture content of the cabbage seeds ranged from 6 to 16% under the same conditions. For the maximum inac-tivation levels of TAMB (Fig. 2(A)) and TMY (Fig. 2(B)), the optimum operational conditions were estimated at 10.5% (median) as the moisture content and 400 μs as the treatment time (P< 0.05). The inactivation of TMY increased with the increased moisture con-tent in that the maximum inactivation was achieved with 16% moisture and 100 μs treatment time (P< 0.05) (Fig. 2(B)). A. brassica and Xcc, as two of the most important pathogens, were inocu-lated into cabbage seeds at levels of 5.98 ± 0.28 log(cfu g−1) and 7.83 ± 0.42 log(cfu g−1) respectively. The maximum inactivation for both A. brassica (Fig. 2(C)) and Xcc (Fig. 2(D)) was obtained with 400 μs treatment time holding 10.5% moisture constant (P< 0.05). Seed vigor
Germination rate, as one of the most important factors that deter-mine seed quality, is affected by seed storage conditions and seed
3479
0 0 1 200 0 2 4 0 0 1 00 3 4 0 0 3 00 2 4 400 4 6 25 0 3 100 0 0 2 0 0 3 100 0 0 4 200 0 0 3 35 100 0 0 2 0 0 3 0 0 1 120 100 0 0 4 200 0 0 3 0 4 1 µ( yti vit c u d n oc h 4 2S c m -1) Treatment time (µs) Frequency (Hz) mc S µ( yti vit c u d n oc h 2 -1) Treatment time (µs) Frequency (Hz) ) %( et ar n oit a ni mr e G Frequency (Hz) Treatment time (µs) (A) (B) (C)Figure 3. Effect of PEF on (A) cabbage seed germination and conductivity
measured (B) 2 h and (C) 24 h after cold test as a function of frequency and treatment time.
age. The control group of the cabbage seeds had an average ger-mination rate of 1.99 ± 0.67%. All the PEF treatments enhanced the seed germination rate (10.0 ± 0.4%) (P< 0.05). Their germina-tion rate was boosted with increased PEF treatment time up to 400 μs and with the frequencies between 100 and 200, and 250 and 300 Hz (Fig. 3(A)).
The electrical conductivity of the control group after 2 h (38 ± 8.67 μS cm−1) and 24 h (113.6 ± 10.8 μS cm−1) rose up to 48 ± 0.28 μS cm−1 and 156 ± 2.68 μS cm−1 respectively (P< 0.05) with the increased treatment time and frequency. The increased frequency and the decreased treatment time increased the elec-trical conductivity 2 h after the PEF treatment under a moisture content of 10.5% (Fig. 3(B)). The electrical conductivity 24 h after the PEF treatment increased significantly compared with the control and 2 h treatment groups (P< 0.05) (Fig. 3(C)). The increased conductivity was significant with the shorter treat-ment time and the higher frequency, whereas the minimum change was observed with the longer treatment time and higher frequency. 0 2 4 6 8 10 12
untreated seeds PEF treated (960 J)
D. graminea F. graminearum
fungal contam
ination
(%)
Figure 4. Inactivation of Drechslera graminea and Fusarium graminearum
inoculated into winter barley.
(A)
(B)
Figure 5. Comparisons of (A) germination and (B) seedlings roots of winter
barley on seventh day after sowing (left: control; right: PEF-treated).
Winter barley as a representative of the cereal seeds was fur-ther subjected to both inactivation and growth tests. The PEF disinfection of the winter barley seeds decreased D. graminea by 28.97% and F. graminearum by 26.67% (Fig. 4). The winter barley seeds treated with 12 kV cm−1and 960 J exhibited a better and faster growth (Fig. 5(A)). On the seventh day after sowing, the PEF-treated barley seeds showed a better root development and seedling (Fig. 5(B)).
Seed disinfection has been recommended as a major inter-vention step in a multiple-hurdle approach to reduce the risk of diseases associated with contaminated seeds and grains. The US Food and Drug Administration recommended 20 000 ppm calcium hypochlorite as the reference standard for seed disinfection treatments. New disinfection treatments, such as biological interventions or competitive exclusions and
3480
high-pressure processing, appear to achieve the safe minimum standard of 20 000 ppm calcium hypochlorite use. Since the application of chemical disinfectants in concentrations above 20 000 ppm calcium hypochlorite can pose environmental and public safety risks, alternative intervention approaches should be considered.5
Owing to their environmentally friendly nature, plant essential oils have been frequently used in seed vigor tests. For example, the effect of Origanum rotundifolium essential oil on Xanthomonas
axonopodis pv. vesicatoria strain (Xcv-761) and Clavibacter michi-ganensis spp. michimichi-ganensis strain (Cmm) in terms of germination
and growth of tomato seeds was tested, and it was found that the highest germination rate in the Cmm-infected seeds and the lowest disease severity in the Cmm- and Xcv-761-infected seeds was obtained with a 250 μL mL−1essential oil treatment.16 Com-bination of savory and thyme essential oils, the antagonistic bac-teria of Pseudomonas spp., and the immersion in 55 ∘C water for 10 min improved the disinfection efficacy more than did the essen-tial oils alone for carrot seeds inoculated with Alternaria radicina.17 It was also reported that the effect of the combined treatments on
A. radicina was not additive.18
Gaseous acetic acid treatment at 4.7 mmol L−1at 55 ∘C for 3 h was found to reduce Escherichia coli O157:H7 and Salmonella
enter-itidis by 5.0 log(cfu g−1), and Bacillus subtilis spores for black pepper and fenugreek seeds by 4.0 log(cfu g−1) and 3.5 log(cfu g−1) respectively (P< 0.05).6The comparison of the antagonist strains of Pseudomonas spp. versus the standard chemical uses of prochlo-raz and thiram against Fusarium oxysporum f. sp. lactucae showed similar but limited efficacy for lettuce seeds (L. sativa) in terms of infected plants and disease index.18 Low-pressure plasma treatments with voltage and argon gas flow rates of 5.5 kV and 0.5 L min−1respectively for 5 min and 40 min reduced X. campestris by 3.9 log(cfu g−1) and 6.6 log(cfu g−1) respectively, and damaged cell membrane after 5 min for cruciferous seeds.19
CONCLUSIONS
This study is the first extensive report about the design and effect of a PEF unit on the germination and vigor of eight seeds. The bench-scale PEF treatment appears to offer promising results for the healthy and organic production of seeds but remains to be scaled up to the industrial scale. Further studies are needed about the combination of multiple green treatments sequentially and/or simultaneously to further improve seed disinfection.
ACKNOWLEDGEMENTS
Financial support was provided by Bolu Abant Izzet Baysal Uni-versity Research fund (BAIBU BAP project no: 2016.09.04.1118). We would like to thank Dr Kubay and Dr Groot for providing the microbial cultures.
REFERENCES
1 Amein T, Wright SAI, Wikström M, Koch E, Schmitt A, Stephan D et al., Evaluation of non-chemical seed treatment methods for control of Alternaria brassicola on cabbage seeds. J Plant Dis Prot 118:214–221 (2011).
2 Koch E and Roberts SJ, Non-chemical seed treatment in the control of seed-borne pathogens, in Global Perspectives on the Health of Seeds and Plant Propagation Material. Plant Pathology in the 21st Century (Contributions to the 9th International Congress), Vol. 6, ed. by Gullino M and Munkvold G. Springer, Dordrecht, Netherlands, pp. 105–123 (2014).
3 Dutta B, Avci U, Hahn MG and Walcott RR, Location of Acidovorax citrulli in infested watermelon seeds is influenced by the pathway of bacterial invasion. Phytopathology 102:461–468 (2012). 4 Wang XQ, Zhou RW, De Groot G, Bazaka K, Murphy AB and Ostrikov KK,
Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci Rep 7:5601 (2017).
5 Kang MH, Pengkit A, Choi K, Jeon SS, Choi HW, Shin DB et al., Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS ONE 10:e0139263 (2015).
6 Nei D, Enomoto K and Nakamura N, A gaseous acetic acid treat-ment to disinfect fenugreek seeds and black pepper inoculated with pathogenic and spoilage bacteria. Food Microbiol 49:226–230 (2015).
7 Zimmermann U, Electric breakdown, electropermeabilization and elec-trofusion. Rev Physiol Biochem Pharmacol 105:196–256 (1986). 8 Zimmermann U, Pilwat G, Beckers F and Riemann F, Effects of external
electrical fields on cell membranes. Bioelectrochem Bioenerg 3:58–83 (1976).
9 Weaver JC and Chizmadzhev YA, Theory of electroporation: a review. Bioelectrochem Bioenerg 41:135–160 (1996).
10 Pakhomov AG, Miklavˇciˇc D and Markov MS, Advanced Electroporation Techniques in Biology and Medicine. CRC Press, Boca Raton, FL, USA, pp. 71–97 (2010).
11 Nollet JA, Recherches sur les Causes Particulières des Phénoménes Elec-triques. Chez les Frères Guérin, Paris, France (1749).
12 Sitzmann W, Vorobiev E and Lebovka N, Applications of electricity and specifically pulsed electric fields in food processing: historical backgrounds. Innov Food Sci Emerg Technol 37:302–331 (2016). 13 Evrendilek GA and Tanasov I, Configuring pulsed electric fields to
process seeds: an innovative method of seed disinfection. Seed Sci Technol 45:72–80 (2017).
14 ISTA, International Rules for Seed Testing. International Seed Testing Association, Bassersdorf, Switzerland (2009).
15 ISTA, International Rules for Seed Testing. International Seed Testing Association, Bassersdorf, Switzerland (2004).
16 Dadasoglu F, Kotan R, Karagoz K, Dikbas N, Cakmakci R, Cakir A et al., Effect of essential oil of Origanum rotundifolium on some plant pathogenic bacteria, seed germination and plant growth of tomato. AIP Conf Proc, Antalya, Turkey 1726:020022 (2016).
17 Lopez-Reyes JG, Gilardi G, Garibaldi A and Gullino ML, In vivo evaluation of essential oils and biocontrol agents combined with hot water treatments on carrot seeds against Alternaria radicina. J Phytopathol
164:131–135 (2016).
18 Lopez-Reyes JG, Gilardi GG, Garibaldi A and Gullino ML, Efficacy of bac-terial and fungal biocontrol agents as seed treatments against Fusar-ium oxysporum f. sp. lactucae on lettuce. J Plant Pathol 96:535–539 (2014).
19 Nishioka Y, Takai Y, Mishima T, Kawaradani M, Tanimoto H, Okada K et al., Low-pressure plasma application for the inactivation of the seed-borne pathogen Xanthomonas campestris. Biocontrol Sci