On/Off Rhodamine-BODIPY-Based Fluorimetric/
Colorimetric Sensor for Detection of
Mercury (II) in Half-Aqueous Medium
Ahmed Nuri Kursunlu, Mehmet Oguz, and Mustafa Yilmaz
Abstract— A turn on/off rhodamine-BODIPY (RB) fluorescent
sensor has been designed for selective and sensitive detection of Hg (II) cation in half-aqueous solution. For this purpose, a BODIPY derivative having dual-cores is bilaterally bound to a rhodamine containing dual-amine moieties. The newly prepared sensor reacts with mercury (II) salt to generate a product with different optical parameters. These are changes confirmed by absorption, emission, and excitation measurements. A blue shift is observed as the “Turn-off” change the pink color to transparent upon the addition of Hg (II) ions which is assigned to the suppression of fluorescence resonance energy transfer upon mercury (II) ion affinity. Selectivity toward the Hg (II) ion is found to depend upon the cavity size created of the amino moieties of rhodamine and the carbonyls of BODIPY to the metal ion. The sensor RB is selective and sensitive to Hg (II) with a detection limit [1.94 (±0.2) 10−8 M]. The three-way fluorescent probe can be selectively applied for the detection of mercury (II) ion in real water samples.
Index Terms— Rhodamine, BODIPY, mercury, absorption,
emission, fluorescent.
I. INTRODUCTION
N
OWADAYS, detections for hazardous heavy metal ions in water sourcing from geochemical cycling and industrial waste have received widespread attention due to their abstruse high toxicity for the environment and bio-logical effects in living systems even at extremely low concentrations [1]–[3]. Among these metal ions, mercury has been considered as highly toxic and widespread global pol-lutant because of their highly poisonous character [4]–[8]. It can cause a lot of human health problems when it goes into the body such as kidney failure, brain damage, motion and central nervous system disorders, vision loss, cardiovascular system destruction and endocrine systems [9], [10]. When these ions accumulated in plants, it can reduce the rate of photosynthesis and transpiration [11], [12]. This serious Manuscript received October 15, 2018; accepted November 29, 2018. Date of publication December 12, 2018; date of current version February 15, 2019. This work was supported by the Research Foundation of Selcuk Univer-sity (BAP). The associate editor coordinating the review of this paper and approving it for publication was Dr. Chang-Soo Kim. (Corresponding author:Mustafa Yilmaz.)
A. N. Kursunlu and M. Yilmaz are with the Department of Chemistry, Selçuk University, 42075 Konya, Turkey (e-mail: ankursunlu@gmail.com; myilmaz42@gmail.com).
M. Oguz is with the Department of Chemistry, Selçuk University, 42075 Konya, Turkey, and also with the Department of Advanced Mater-ial and Nanotechnology, Selçuk University, 42031 Konya, Turkey (e-mail: m.oguz2011@gmail.com).
Digital Object Identifier 10.1109/JSEN.2018.2886383
situation has accelerated the development of Hg (II) detec-tion techniques [13]–[15]. To date, different methods have been reported such as inductively coupled plasma mass spec-trometry (ICP-MS), cold vapor atomic absorption spectrom-etry (CVAAS), inductively coupled plasma optical emission spectrometry (ICP-OES), ultraviolet and visible spectropho-tometry (UV–vis), atomic fluorescence spectrometry (AFS), X-ray fluorescence, electrochemical sensing and high perfor-mance liquid chromatography (HPLC) and electrochemical methods for the detection of mercury ions [16]–[19]. These conventionally used techniques have a low detection limit and good sensitivity. However, some methods have some disad-vantages such as time-consuming, long sample pre-treatment, the need for expert staff, expensive and require complex sample preparations, specific equipment, and operational man-agement challenges. Therefore, it is essential to improve new analytical methods that are cheap, easy, convenient, rapid and efficient to use for the detection of environmental and physi-ologically important heavy and transition metal [20]–[22].
The chemosensors are very important for a lot of practices such as food analysis and medical diagnosis process control. They have to contain both the signaling of fluorophore units and the detection of ionophore. The signaling units can trans-form into a signal expressed as the differences in the photo-physical characteristics of the chemosensor energy transfer or electron charge [23], [24]. In addition to these chemosensors can contain some aromatic groups such as anthracene, dansyl, naphthalene, pyrene, rhodamine and BODIPY as a fluorescent probe [25]–[28].
In these days, among the numerous classes of highly fluorescent dyes, the family of difluoroborondipyrromethene (BODIPY) have been received a considerable attention due to their excellent photophysical properties such as large molar extinction coefficients, high fluorescence quantum yield, appropriate redox potential, excellent stability, easy functionalization and high solubility in common organic solvent [29]–[31]. In fact, rhodamine and BODIPY are extremely convenient for advancing the platform. So, the rhodamine has an emission band at 580 nm if the spirocyclic ring open, and the BODIPY can be developed into detection units. Both rhodamine and BODIPY are easy to synthesize functionalization [32], [34]. Owing to their excellent charac-teristics, novel fluorescence probe based on rhodamine and BODIPY attracted much interest due to their potential use as a chemosensor for detecting metal ions [34], [35].
1558-1748 © 2018 IEEE. Personal use is permitted, but republication/redistribution requires IEEE permission. See http://www.ieee.org/publications_standards/publications/rights/index.html for more information.
as Sigma-Aldrich, Acros Organics, Merck used without a further purification. Fourier Transform Infrared (FTIR-ATR) spectra in cm-1 were recorded on a Bruker spectrometer. NMR (1H, 13C-NMR) spectra were obtained by a Varian (400 MHz) spectrometer with tetramethylsilane used as a stan-dard. All absorption and emission measurements were carried out on a Perkin Elmer Lambda 25 UV–vis spectrophotometer and a PerkinElmer LS 55 fluorescence spectrophotometer in a fixed excitation in a 1 cm quartz cell, respectively. All experiments were carried out at 298 K under a nitrogen atmosphere.
B. The Preparing of Compounds
1) The Preparing of 3,5-{bis[4,4-difuoro, 8-(2,6-diethyl, 1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene)]}benzoyl-chloride (Compound B): Compound B is synthesized just as [29] and purified by column chromatography. 1,3,5-benzenetricarbonyl chloride (0.442 g, 1.17 × 10−3 mol) is added dropwise to a solution of kryptopyrrole (0.9 mL, 6.6 mmol) in dry dichloromethane (50 mL) at r. t. and under a nitrogen atmosphere. The reaction is refluxed at 50◦C for 4 h. The mixture is cooled, 8 equiv. of triethylamine (TEA) is dropped and stirred at r. t. for 1h. Finally, boron trifluoride diethyl etherate (15 equiv.) is poured to this solution and stirred at 55 ◦C for 2 h. The reaction is monitored by TLC. The resultant material is purified in column (ethyl acetate-cyclohexane in 3:1 ratio). A red solid. M.p.: 318◦C.1H-NMR (400 MHz, CDCl3): 1.01 (t, 12H, CH3), 1.49 (s, 12H, CH3), 2.28 (q, 8H, CH2), 2.55 (s, 12H, CH3), 7.57 (dd, 1H, ArH), 7.79 (d, 2H, ArH). 13CNMR (100 MHz, CDCl3): d (ppm) 167.9, 147.9, 140.7, 132.9, 131.2, 129.9, 127.0, 126.3, 119.6, 17.5, 15.1, 12.9, 12.4. molecule formula: C41H47B2ClF4N4O; found: C, 66.24; H, 6.75; N, 7.41. ESI-TOF-MS [+H+]; m/z: 744.1.
2) The Preparing of Compound R: This compound is
prepared according to a minor modification of the literature procedure [33]. Under nitrogen, an ethanolic solution of rho-damine B (0.40 g, 0.84 mmol), and tris(2-aminoethyl)amine (0.48 g, 3.4 mmol) are refluxed for 20 h and the color of solution turn toward red. After cooling to the room temper-ature, the solvent was evaporated. The solution is extracted with chloroform/H2O for several times. The all-organic com-binations are dried by MgSO4. After filtration of magnesium sulfate, the solvent is evaporated, obtained a pale-yellow solid.
Scheme 1. The synthesis procedure of RB.
Yield 79 %. 1H NMR (400 MHz, CDCl3). 1.09 (bs, 12H, NCH2CH3), 2.08-1.99 (m, 2H, NCH2CH2N), 2.29-2.27 (m, 2H, NCH2CH2N), 2.59-2.52 (m, 2H, NCH2CH2N), 2.89-2.83 (m, 2H, NCH2CH2N), 3.09 (bs, 2H, NCH2CH2N), 3.25 (bs, 8H, NCH2CH3), 5.01-4.05 (bs, 4H, NH2), 5.25 (bs, 2H, NCH2CH2N), 6.31-6.21 (m, 6H, ArH), 6.99 (s, 1H, ArH), 7.39 (s, 2H, ArH), 7.83- 7.79 (m,1H, ArH).
3) The Synthesis of RB: To a solution of R (0.057g,
0.1 × 10−3 mol) and trimethylamine (0.38 mL) in chloro-form (20 mL) at 0 °C, is added a solution of B (0.149 g, 0.2 × 10−3 mol), and after 1 h, the mixture is warmed to r.t. and stirred for overnight, then heated at reflux for further 8 h. The solution is extracted with chloroform/H2O for 3 times. The chloroform phases are assembled and dried with MgSO4. The heterogenic mixture is filtrated and, chloroform is evaporated. The result residue is puri-fied on column (chloroform/hexane in 1:1 ratio) and obtained a red solid. Yield: 54%. 1H NMR (400 MHz, CDCl3): δ (ppm). 1.03 (t, 24H, CH3), 1.50-1.51 (bs, 12H, NCH2CH3), 1.57 (s, 24H, CH3), 2.35 (q, 16H, CH2), 2.10 (m, 4H, NCH2CH2N), 2.50 (s, 24H, CH3), 2.60-2.63 (m, 4H, NCH2CH2N), 3.34 (q, 8H, NCH2CH3), 3.65 (m, 4H,
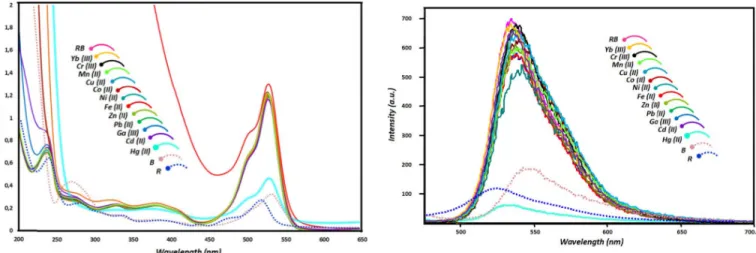
Fig. 1. The absorption spectra of B, R and RB in the absence and presence of different metal ions (Yb (III), Cr (III), Co (II), Zn (II), Mn (II), Cu (II), Ni (II), Fe (II), Pb (II), Ga (III), Hg (II), Cd (II), (20 equiv.)) (1× 10−6M in acetonitrile/H2O, 4:1). NCH2CH2N), 5.27 (bs, 2H, NH) 6.33-6.24 (m, 6H, ArH), 7.05 (m, 2H, ArH), 7.42 (s, 2H, ArH), 7.57 (dd, 2H, ArH), 7.98 (d, 4H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) 168.1, 167.2, 153.1, 149.1, 148.1, 141.0, 132.7, 132.1, 131.2, 131.9, 130.2, 129.1, 128.7, 127.2, 126.0, 123.9, 122.9, 119.9, 108.5, 105.9, 97.3, 72.9, 56.8, 49.3, 41.5, 39.1, 17.7, 15.2, 12.8, 12.6, 12.3. Anal. Calc. C116H138B4F8N14O4; 70.09; H, 7.00; N, 9.87; Found: C, 69.95; H, 7.13; N, 9.67. ESI-TOF-MS [+H+]; m/z: 1988,1.
III. RESULTS ANDDISCUSSION
To explore cation sensing properties of RB, twelve kinds of competitive metal ions including Yb (III), Cr (III), Co (II), Zn (II), Mn (II), Cu (II), Ni (II), Fe (II), Pb (II), Ga (III), Hg (II), Cd (II) were monitored in acetonitrile/H2O (4:1, v:v) by UV–vis and fluorescence spectrophotometer. As shown in Fig. 1, the characteristic band of R and B appeared around 525 nm and 550 nm respectively while the relation band appeared at 545 nm in the absorption spectrum of RB. It has been found that upon addition of metal ions into the RB (1× 10−6 M), there is a change in the specific band of the RB, depending on the metal ion. The emission intensity at 545 nm has not changed for the used metal ions except Hg (II) ion among all the tested metal ions (Fig. 1). As it is explicit from Fig. 1 a wide range of metal cations were evaluated to explore the selectivity of RB toward different metal ions. To our surprise, upon addition of Hg(II) ion (20 equiv.) to RB in solutions, an important decrease in absorbance at about 545 nm was observed. Moreover, the multi-transitions assigned π–π∗ and n–σ∗ between 275-450 slightly shifted to red or blue are. Among the various metal ions, Hg (II) ion and color change from purple to colorless, which was visible to the naked eyes under natural light. All of the above observations demonstrate that RB sensor has an excellent colorimetric selectivity to Hg(II) ions. All results indicate that this alteration can be explained by the complexation of RB with mercury ion and RB sensor has an excellent colorimetric selectivity to Hg(II) ions.
Fig. 2. The emission spectra of B, R and RB in the absence and presence of different metal ions (Yb (III), Cr (III), Co (II), Zn (II), Mn (II), Cu (II), Ni (II), Fe (II), Pb (II), Ga (III), Hg (II), Cd (II) (20 equiv.)) (1× 10−6M in acetonitrile/H2O, 4:1) (λex: 470 nm, slit:3).
Fig. 3. The emission spectra of RB in the absence and presence of different metal ions (Yb (III), Cr (III), Co (II), Zn (II), Mn (II), Cu (II), Ni (II), Fe (II), Pb (II), Ga (III), Hg (II), Cd (II), (20 equiv.)) (1×10−6M in acetonitrile/H2O,
4:1) (λem: 560 nm, slit:3.).
The emission maximums of R, B and RB are observed around 525, 550 and 545 nm, respectively. In addition to, the fluorescence intensity of RB is larger than both R and B (almost eight times). The shifting and broadening can be depending on an effective FRET from rhodamine derivative to Bodipy fragments. In order to investigate the selective binding of sensor RB, the fluorescence measurements are also carried out against same cations such as Yb (III), Cr (III), Co (II), Zn (II), Mn (II), Cu (II), Ni (II), Fe (II), Pb (II), Ga (III), Hg (II), Cd (II) by the addition of metal ions (20 equiv.) in an acetonitrile/water (4:1) solution (Fig. 2). Among these ions, the fluorescence intensity of RB is only quenched with a concomitant blue shift (7-8 nm) in presence of Hg (II) ion, whereas no a significant change for all other cations. The amines of alkyl chain in R and carbonyl fragments of B generated a chelating cage and the obtained construction easily interacted with mercury ions. These results clearly reveal that RB shows a high affinity towards Hg(II).
Further, we studied the influence of cations on the value of the signal change in the excitation spectrum of sensor RB (1× 10−6M). The excitation experiments are carried out with some metal ions. As shown in Fig. 3, the noticeable changes are not
Fig. 4. (a) and (b) Hg (II)-induced FRET ON→OFF process of RB along with visual color changes upon irradiation at 365 nm and daylight (pink bottles: RB and RB+other ions). (c) Overlap between the spectra of the donor emission and acceptor absorption.
observed after the addition of all metal ions except the solution containing Hg (II) ion. Hg (II) ions caused a dwindling in the excitation curve of RB due to a potentially complex reaction. These observations are further photographed under near-ultraviolet light (365 nm) and daylight (Fig. 4). Under the near-ultraviolet light, the solutions including RB and metal ion are bright green due to their excellent fluorescent character, however, the color of RB-Hg(II) mixture is observed in a quenched form. Similarly, mercury (II) ion converted the color of RB from pink to a transparent in daylight. These results show the selective binding of sensor RB to Hg(II) in a half-aqueous medium when compared with other metal ions. These two photographs are consistent with the absorption, emission, excitation spectra and consequently, an effective energy transfer mechanism. Bodipy groups as an acceptor act by rhodamine derivative in a system exhibiting excellent FRET processing (Fig. 4(c)). However, after the complex reaction with mercury ion, it gives a lower emission and FRET mechanism “off”.
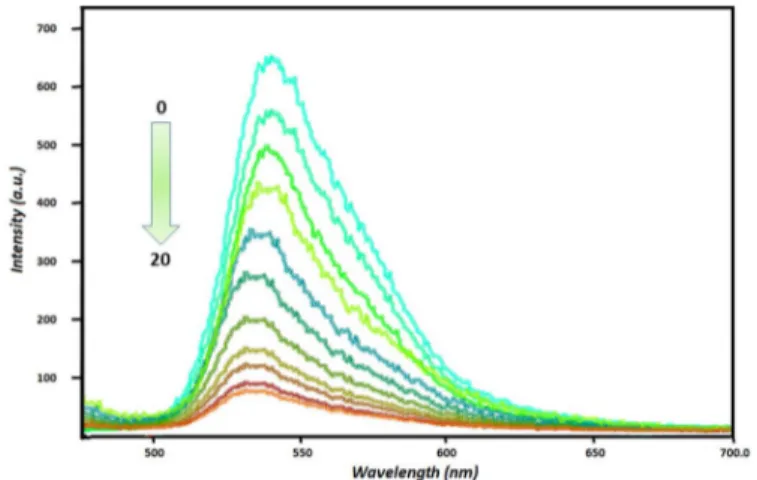
In order to prove the selective binding of Hg(II) over other metal cations to sensor RB, emission titrations are carried out in an acetonitrile/water (4:1) solution. It is shown that the fluorescence intensity of sensor RB (1 μM) is gradually quenched when titrated with Hg ions (0-20 μM), and the color of the mixture turned from pink to colorless. Moreover, the wavelength of RB slightly shifted from 545 nm to 538 nm depending on the increasing concentration of Hg (II) ion. The blue-shift and the quenching effect can be explained by an effective energy transfer.
The binding constant of mercury (II) with sensor RB are evaluated in acetonitrile/water (4:1) solution, in which Hg (II) bound strongly to sensor RB. I0/I ratio increased gradually by increasing concentration of mercury (II) ion and the highest binding constant value reached in 2× 10−5 M concentration. The Stern-Volmer equation was used for the binding constant and calculated and found as 3.32 × 105 M (λemmax:545 nm).
Fig. 5. The emission intensities of RB (1× 10−6 M) in the presence of different concentrations of Hg (II) (2× 10−6, 4× 10−6, 6× 10−6, 8× 10−6, 1× 10−5, 1, 2 × 10−5, 1, 4 × 10−5, 1, 6 × 10−51, 6 × 10−5, 2× 10−5M) (λex: 470 nm, slit:3).
Fig. 6. The binding constant of RB-Hg (II) using Stern-Volmer plot at
λemmax:545 nm (λex: 470 nm, slit:3).
The detection limit (LOD) is calculated by the analysis of samples with known concentrations of Hg (II) and by estab-lishing the minimum level at which the Hg (II) can be reliably detected. So, LOD is evaluated by the fluorescence intensities get from the solutions of RB (1× 10−6 M) and Hg (II) ion in various concentrations.
oD= 3STANDARTDEVIATION/FALSE
LOD of RB towards Hg(II) calculated to be 1.94(±0.2) × 10−8M. This value suggests that RB can be used as a selective sensing sensor for the analysis of Hg(II) in real environmental water samples.
To determinate complex stoichiometry, Job’s plot is per-formed by the method of continuous variation and used the emission values at 545 nm against mole fraction. Fig. 7 shows a maximum at a molar fraction of 0.5 that the ligand:metal ratio in RB-Hg (II) complex is 1:1. The interaction between RB and mercury (II) ions can be attributed a typical chelating effect of RB including multi-dentate such as amine and carbonyl. Namely, the cavity made up of two Bodipy and rhodamine is compatible with the big radius of mercury and this created trap encapsulates to mercury (II) ions by a complexation principle.
Fig. 7. Job plot of the RB/Hg (II) complex in acetonitrile/H2O, keeping the
total concentration of RB and Hg (II) [acetonitrile/H2O (4:1)].
Fig. 8. The change of emission intensities of RB (1 × 10−6 M acetonitrile/H2O (4:1)) in the presence of both Hg (II) and the competing
metal ion (20.0 equiv.)λemmax:545 nm.
The competing ion measurements to test the practical applicability and interference of metal cations to sensor RB for the selective fluorimetric and colorimetric determination of Hg (II) ions is also carried out. So, the effect of foreign ion is illuminated for strong or weaker complexation nature of RB towards mercury (II) ion. For this, Hg (II) (2× 10−5 M) and RB (1× 10−6 M) solutions was mixed in an Erlenmeyer and following, added the interfering metal ions, (Yb (III), Cr (III), Co (II), Zn (II), Mn (II), Cu (II), Ni (II), Fe (II), Pb (II), Ga (III), Hg (II), Cd (II), (2× 10−5 M)) (Fig. 8). However, no remarkable change in the fluorescence character is observed, which causes non-reacting of the added metal ions to the quenching property of Hg (II) in presence of metal ions used in this study. Although a very little response with other metal ions is observed, sensor RB showed a clear Hg(II) ion-dependent quenching behavior.
The influence of acidity and basicity on the affinity of Hg (II) ion is investigated in the pH range of 3-12 and λem is preferred 545 nm as a reference. As shown in Fig. 9, the fluorescence intensity of RB is slowly quenched between pH:3-6 and any change was found in the more basic medium. This difference in a high acidic medium can be explained to the interaction of a lot atom capable of forming hydrogen bonding and thus, the decreasing amount of energy transfer. On the contrary, the emission intensity of RB-Hg (II) complex raised dramatically after pH:5 whereas it is constant in lower pH values. A noticeable increasing/quenching is not observed
Fig. 9. The effect of pH (range of pH:3-12) to the emission intensity of RB (pink line) (1× 10−6 M acetonitrile/H2O (4:1)) in the absence and
presence of Hg (II) ion (turquoise line) (λemmax:545 nm).
Fig. 10. The effect of response time (0-30 minutes) to the emission intensity of RB (1× 10−6 M acetonitrile/H2O (4:1)) by the addition of Hg (II) ion
(λemmax:545 nm).
in pH:8 and higher values. The sensitivity of sensor RB towards Hg (II) ion is determinate in the range of 6-12, which will be useful in a lot of environmental applications.
The reaction response time on the RB- Hg (II) is studied by monitoring the emission intensity of sensor (1× 10−6 M) reacting with Hg (II) solutions (2× 10−5 M) atλex: 570 nm. As it is given in Fig. 10, the response of sensor RB is continued for the first eight minutes. Next minutes, no important change is not observed and almost stopped after ten minutes. The multi-dentate nature of RB extended to the interaction duration of the metal ion. Moreover, it can be considered that most intramolecular and intermolecular interaction as hydrogen bonding of macromolecule RB caused to a complicating in a complex reaction. However, the response time is an acceptable value for sensor compounds.
IV. CONCLUSION
We designed and prepared novel rhodamine- BODIPY based-on fluorescent sensor that it showed a highly sensitive and selectivity for Hg(II) ion over other metal ions in half-aqueous solution. Furthermore, the affinity of the synthesized sensor against Hg (II) supported by the concentration, foreign ion, response time and pH experiments. The detection limit of
mechanism,” J. Fluoresc. vol. 25, no. 2, pp. 319–325, Mar. 2015. [3] I. Samanta and S. Bandyopadhyay, Pet Bird Diseases and Care, vol. 339.
Cham, Switzerland: Springer, 2017, pp. 253–262.
[4] K. P. Carter, A. M. Young, and A. E. Palmer, “Fluorescent sensors for measuring metal ions in living systems,” Chem. Rev., vol. 114, no. 8, pp. 4564–4601, 2014.
[5] H. J. Peng et al., “A fluorescent probe for fast and quantitative detec-tion of hydrogen sulfide in blood,” Angew. Chem., Int. Ed., vol. 50, pp. 9672–9675, 2011.
[6] R. V. Burg, “Inorganic mercury,” Appl. Toxicol., vol. 15, pp. 483–493, 1995.
[7] H. H. Harris, I. J. Pickering, and G. N. George, “The chemical form of mercury in fish,” Science, vol. 301, no. 5637, p. 1203, 2003. [8] C. M. L. Carvalho, E. H. Chew, S. I. Hashemy, J. Lu, and A. Holmgren,
“Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity,” J. Biol. Chem., vol. 283, no. 18, pp. 11913–11923, 2008.
[9] M. Jonaghani and H. Zali-Boeini, “Highly selective fluorescent and colorimetric chemosensor for detection of Hg2+ion in aqueous media,”
Spectrochim. Acta A, Mol. Spectrosc., vol. 178, pp. 66–70, May 2017.
[10] D. Liu, X. Wang, Z. Chen, H. Xu, and Y. Wang, “Influence of mercury on chlorophyll content in winter wheat and mercury bioaccumulation,”
Plant Soil Environ., vol. 56, pp. 139–143, Mar. 2010.
[11] A. A. Bhatti, M. Oguz, and M. Yilmaz, “One-pot synthesis of Fe3O4@Chitosan-pSDCalix hybrid nanomaterial for the detection and removal of Hg2+ ion from aqueous media, Appl. Surf. Sci., vol. 434, pp. 1217–1223, Mar. 2018.
[12] M. Ghaedi, M. R. Fathi, A. Shokrollahi, and F. Shajarat, “Highly selec-tive and sensiselec-tive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy,” Anal. Lett., vol. 39, no. 6, pp. 1171–1185, May 2006.
[13] V. K. Gupta, A. K. Singh, M. R. Ganjali, P. Norouzi, F. Faridbod, and N. Mergu, “Comparative study of colorimetric sensors based on newly synthesized Schiff bases,” Sens. Actuators B, Chem., vol. 182, pp. 642–651, Jun. 2013.
[14] S. Malkondu and S. Erdemir, “A novel perylene-bisimide dye as ‘turn on’ fluorescent sensor for Hg2+ ion found in DMF/H2O,” Dyes
Pig-ments, vol. 113, pp. 763–769, Feb. 2015.
[15] X. Zhang, W. Shi, X. Chen, and Z. Xie, “Isocyano-functionalized, 1,8-naphthalimide-based chromophore as efficient ratiometric fluores-cence probe for Hg2+in aqueous medium,” Sens. Actuators B, Chem., vol. 255, no. 3, pp. 3074–3084, Feb. 2018.
[16] C. Baslak, H. Bingol, A. Coskun, and T. Atalay, “Synthesis of a novel thiadiazine derivative and electrochemical properties for Pb2+transfer across water/1,2-dichloroethane interface,” Acta Phys. Chim. Sinica, vol. 27, no. 8, pp. 1859–1862, Aug. 2011.
[17] K. Farhadi, M. Forough, R. Molaeia, S. Hajizadeha, and A. Rafipourb, “Highly selective Hg2+colorimetric sensor using green synthesized and unmodified silver nanoparticles,” Sens. Actuators B, Chem., vol. 161, no. 1, pp. 880–885, Jan. 2012.
[18] C. Zhang, B. Gao, Q. Zhang, G. Zhang, S. Shuang, and C. Dong, “A simple schiff base fluorescence probe for highly sensitive and selective detection of Hg2+and Cu2+,” Talanta, vol. 154, pp. 278–283, Jun. 2016.
[19] M. Oguz, A. A. Bhatti, S. Karakurt, M. Aktas, and M. Yilmaz, “New water soluble Hg2+selective fluorescent calix[4]arenes: Synthesis and application in living cells imaging,” Spectrochim. Acta A, Mol.
Spectrosc., vol. 171, pp. 340–345, Jan. 2017.
mimicking photosynthesis: Multi-fluorophoric light harvesting system,”
Tetrahedron Lett., vol. 59, no. 20, pp. 1958–1962, May 2018.
[26] C. Baslak and A. N. Kursunlu, “A naked-eye fluorescent sensor for cop-per(II) ions based on a naphthalene conjugate Bodipy dye,” Photochem.
Photobiol., vol. 17, no. 8, pp. 1091–1097, 2018.
[27] Z.-Q. Hu, C.-L. Cui, H.-Y. Lu, L. Ding, and X.-D. Yang, “A highly selective fluorescent chemosensor for fluoride based on an anthracene diamine derivative incorporating indole,” Sens. Actuators B, Chem. vol. 141, no. 1, pp. 200–204, Aug. 2009.
[28] C. Baslak, G. Arslan, M. Kus, and Y. Cengeloglu, “Removal of rho-damine B from water by using CdTeSe quantum dot-cellulose membrane composites,” RSC Adv., vol. 6, no. 2, pp. 18549–18557, 2016. [29] A. N. Kursunlu, M. Ozmen, and E. Guler, “Novel magnetite nanoparticle
based on BODIPY as fluorescent hybrid material for Ag(I) detection in aqueous medium,” Talanta vol. 153, pp. 191–196, Jun. 2016. [30] L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung, and
Q.-Z. Yang, “BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine,”
J. Amer. Chem. Soc., vol. 134, no. 46, pp. 18928–18931, 2012.
[31] X. Qi et al., “New BODIPY derivatives as OFF-ON fluorescent chemosensor and fluorescent chemodosimeter for Cu2+: Cooperative selectivity enhancement toward Cu2+,” J. Organic Chem., vol. 71, no. 7, pp. 2881–2884, 2006.
[32] H. Yu, Y. Xiao, H. Guo, and X. Qian, “Convenient and efficient FRET platform featuring a rigid biphenyl spacer between rhodamine and BODIPY: Transformation of ‘turn-on’ sensors into ratiometric ones with dual emission,” Chemistry, vol. 17, no. 11, pp. 3179–3191, Mar. 2011.
[33] M. J. Culzoni, A. M. Peña, A. Machuca, H. C. Goicoechea, and R. Babiano, “Rhodamine and BODIPY chemodosimeters and chemosen-sors for the detection of Hg2+, based on fluorescence enhancement effects,” Anal. Methods, vol. 5, no. 1, pp. 30–49, 2013.
[34] E. Karaku¸s, M. Ücüncu, and M. Emrullaho˜glu, “A rhodamine/ BODIPY-based fluorescent probe for the differential detection of Hg2+and Au3+,” Chem. Commun., vol. 50, no. 9, pp. 1119–1121, 2014. [35] Y. Jiao, L. Zhang, and P. Zhou, “A rhodamine B-based fluorescent sensor toward highly selective mercury (II) ions detection,” Talanta, vol. 150, pp. 14–19, Apr. 2016.
Ahmed Nuri Kursunlu was born in Karaman,
Turkey. He received the M.Sc. degree from Selçuk University, Konya, Turkey, in 2008, and the Ph.D. degree in 2014 under the supervision of Assoc. Prof. Dr. E. Güler. His research focused on the synthesis of BODIPY-Schiff bases. Following this he spent one year in the U.K., first as a Research Fellow, during his Ph.D., with the University of Hull with Prof. R. W. Boyle. He is currently a Researcher with Selçuk University, where his work focuses on BODIPY-complex formations and applications.
Mehmet Oguz is currently pursuing the Ph.D.
degree. He is also with the Department of Advanced Material and Nanotechnology, Selçuk University, Turkey, working on the synthesis of supramolecules, nanoparticles, biosensors, and drug delivery.
Mustafa Yilmaz received the bachelor’s degree in
chemistry from Ataturk University, Turkey, in 1978, and the Ph.D. degree in organic chemistry from the Institute of Science, Selçuk University, in 1987. His research focuses on the synthesis and charac-terization of calixarene-based compounds and phase transfer-reaction, chiral catalysis, sensors, cation, anion recognition studies, magnetic nanoparticles and enzyme immobilization, and kinetic resolution studies. He has authored over 200 articles in peer-reviewed journals and 5 book chapters.
![Fig. 7. Job plot of the RB/Hg (II) complex in acetonitrile/H 2 O, keeping the total concentration of RB and Hg (II) [acetonitrile/H 2 O (4:1)].](https://thumb-eu.123doks.com/thumbv2/9libnet/4985348.101183/5.918.79.450.90.332/fig-job-complex-acetonitrile-keeping-total-concentration-acetonitrile.webp)