Contents lists available atScienceDirect
Ceramics International
journal homepage:www.elsevier.com/locate/ceramint
Development and characterization of low temperature metallic glazes in
Na
2
O-B
2
O
3
-SiO
2
(NBS) system
Nergis Kilinç Mirdali
∗,a, Mustafa Daday
b, Mine Taykurt Daday
caCukurova University, Department of Ceramic, 01330, Adana, Turkey
bEskişehir Technical University, Department of Material Science and Engineering, 26480, Eskişehir, Turkey
cAdana Alparslan Türkeş Science and Technology University, Department of Materials Engineering, 01250, Adana, Turkey
A R T I C L E I N F O Keywords: Metallic glaze Crystal formation Reductive atmosphere A B S T R A C T
This paper presents the results of an investigation into crystal formation in NBS system for glaze with metallic appearance experimentally produced by embedding metallic substances as AgNO3,BaCl2, CuSO4and CuCO3and their characterizations. Composition of NBS was calculated by using Seger Method. According to the quantity of components, crystal phases were marked on Na2O-B2O3-SiO2ternary diagrams. The amount of AgNO3(2 wt%) was kept constant that other metallic substances (BaCl2, CuSO4and CuCO3) were added in an amount of 5 wt% as had an impact on the mechanism of crystalline phase growth. These glaze compositions were applied over the surface of the commercial bodies and fired at 1040 °C, cooled to 750 °C and then reduced in the reductive atmosphere in order to obtain metallic layer on glaze surfaces for 20 min. These glaze layers characterized under mineralogical, microstructural and chemical points of view by means of X-Ray Diffraction (XRD), optical mi-croscopy (OM), Scanning Electron Mimi-croscopy coupled with Energy Dispersive X-ray (SEM-EDX) and Ulltraviolet Visible Spectroscopy (UV–Vis) techniques. The results showed that silver and copper particles/crystals devel-oped near/on the glaze surfaces creating multicolored metallic shine. Diffusion of metallic particles/crystals shown on the glazed layers as Ag and Cu followed by nucleation and growth in a reductive atmosphere.
1. Introduction
A remarkable number of materials, with magnificent optical and me-chanical properties, have been enhanced to fulfil the needs of varied fields of applications using the glass and glass-ceramic materials over many years. A glass-ceramic or decorative glaze system composes of a non-crystalline glassy phase and one or more non-crystalline phases. Crystallization in glass or glaze has two essential stages which are nucleation and crystal growth. When the crystallization process commences, some rearrange-ments are undergone in the structure of the glass. Especially in borosilicate systems, the structure is built up by three-dimensional networks formed by [SiO4] and [BO4] structural units. Other components (alkalines) act as network modifiers. Even Na2O and BaCl2can be incorporated into glass formation to some extent [1].
Macroscopic crystals generate a particularly decorative effect of scintillation that is caused by light rays falling on the surface of the glazes. The sparkling impression in these glazes is owing to the differ-ence in the light-reflection coefficients of the crystalline structures and the glass itself, and depends on the size, shape, quantity, and arrange-ment of these crystals and on the observation angle [2,3].
It is essential to create crystals in which the iridescent colors are desired. Therefore, an understanding of crystal formation is extremely substantial. It is thought that the formation of crystals probably begins most intensely at the phase boundary between glass melt and furnace atmosphere when the energy barrier of seed formation becomes lower. The decorative properties of glazes are related with not only the crys-tals’ size, but also the quantity of crystals in the surface layer of coatings [2].
The studies are carried out on crystalization in the Na2O–B2O3–SiO2 (NBS) system with addition of nitrates, chlorates, sulfates and carbo-nates of Ag, Ba and Cu, respectively.
If the furnace atmosphere and regime are placed onto one side, it is thought that the crystal formation develops depending on the amount of Na2O and B2O3in the glaze systems [2]. Na2O and B2O3significantly affect the physicochemical properties of silicate melts including melting temperature, diffusion, viscosity, crystallization, thermal expansion coefficient, etc. [4]. Their content in glassy matrix enhances the release of free oxygen from the silicate structure [5]. An increase in the amount of Na2O from 2.5 to 12.5 wt% also enlarges the surface area of crys-talline particles and provides the largest sizes of crystals at a lower
https://doi.org/10.1016/j.ceramint.2019.07.164
Received 29 May 2019; Received in revised form 13 July 2019; Accepted 14 July 2019 ∗Corresponding author. Department of Ceramic, Sariçam, Adana, 01330, Turkey.
E-mail addresses:nkilinc@cu.edu.tr,nergismirdali@gmail.com(N. Kilinç Mirdali).
Available online 31 July 2019
0272-8842/ © 2019 Elsevier Ltd and Techna Group S.r.l. All rights reserved.
firing temperature. An increase up to 25 wt% in the content of B2O3 introduced instead of SiO2significantly decreases the crystal sizes and at the same time increases their quantity [2].
The metallic shining glassy layer, saturated with metal oxides such as Ag, Cu and Ba, changes color dramatically when directed to any light source and produces a wide range of colors. This metallic shining glassy layer was achieved via low temperatures between 700-750 °C with controlled reduction of copper, silver and barium compounds. In a re-duced atmosphere, the temperature, kiln atmosphere and time control are essential for success in order to achieve the glaze in metallic shining. Although the thermal paths and furnace atmosphere are ex-tremely important factors for obtaining metallic thin layered films, the chemical structure and glaze thickness of the metal-containing glassy phase to produce metallic shining are effective.
When the crystal-forming materials used in glaze are to be con-sidered, Ag oxides occur in two different stoichiometries, Ag2O (cubic) and AgO (monoclinic). Oxygen atoms occupy the tetrahedral interstitial sites in Ag2O and an off-center position of the octahedral ones in AgO. Regular Ag structure is only aligned extremely low oxidative atmo-sphere (vacuum condition) where no oxide formation is found [6].
BaCO3, most frequently used to produce a satin matt surface. With boron, it makes free-flowing glazes, producing a smooth and glossy finish. BaCl2is a water soluble material. It used to develop iridescent surfaces by fuming at low temperatures, subsequent to the glaze firing [7]. The low form of BaB2O4appeares in the BaO-B2O3system that is confirmed by phase equilibrium information for the system BaO-B2O3 -SiO2and the subsystem BaO-B2O3. Phase equilibrium indicates a two-liquid region (two-liquid immiscibility): in the ternary BaO-B2O3-SiO2 system, one ternary phase, (Ba3B6Si2O16) and a large two-liquid region. In the region labeled as ‘solid solutions’, several new compounds be-tween BaO·2SiO2and 2BaO·3SiO2are found for the system of BaO-SiO2 [8].
By changing the base glaze compositions and firing conditions, the chemical equilibrium of CuO could be transformed into Cu+, Cu2+, Cu°, CuO and Cu2O, and up to five states in silicate glass besides, one or more states would also dissolve and cause change of glaze colors. While the formation of CuO enhances with an increase in the copper content and oxygen partial pressure, it decreases with an increase in the firing temperature. Meanwhile, at high temperatures, the copper in glaze reveals blue or blue-green color either in oxidation or reduction at-mosphere, it also creates copper red (oxblood), reddish purple and bluish purple colors under reducing atmosphere. In addition, on the oxidizing or reducing components in the raw materials and the atmo-sphere, the redox ratios would be different and thus the colors would change along a wide spectrum [9].
In this study, glazed ceramics with metallic shining effect were produced in reductive atmosphere and characterized. These metallic shining glazes were provided by metallic compounds such as AgNO3, BaCl2, CuSO4and CuCO3and dispersed in an alkaline-borosilicate rich glassy matrix, within low alumina content.
During the firing process, carbonates decompose earlier than sul-fates.
The glaze microstructure is dramatically affected due to decom-position of the sulphates at temperatures of 500 °C and it is faster above this temperature. For that reason, this increases potency of mechanism of blistering and bloating [10]. Therefore, the size and distribution of the grains formed on the surface shows homogeneous distribution of sulphate containing glazes.
2. Experimental procedure 2.1. The glaze preparation process
The Seger formula which is designed to classify and identify glazes was used to prepare basic borosilicate glaze. The formulation of Glaze A and amount of dominant glass modifier and formers are marked at
ternary Na2O-B2O3-SiO2system inFig. 1.
The raw materials which are calcined borax, marble, quartz and kaolin were weighed at certain amounts and milled in a porcelain jar for 30 min for homogenization. Glaze B, Glaze C and Glaze D were prepared by using additives to Glaze A according toTable 1. AgNO3was added to obtain metallic appearance. BaCl2, CuSO4and CuCO3were used to form lusters under reduction atmosphere in Glaze B, Glaze C and Glaze D, respectively. Any silver or copper compound, or a mixture of both, forms lusters under reduction atmosphere. However, not all of these compounds have the same sensitivity to reduction. Therefore, different effects emerge. For example; sulfides, nitrates and chlorides are more easily reduced than oxides and carbonates.
2.2. The sample preparation process
The commercial bodies were fired at 900 °C. These bodies were fa-vorable for making well conjunction with glazes and adequately porous. Glazes were applied onto commercial biscuit fired porcelain plates with 10 cm diameter and fired at 1040 °C for 6 h and cooled to 750 °C in oxidizing atmosphere. Then, in order to obtain reducing at-mosphere, they were taken with the help of tongs and thrown into the wood chip-filled tin bucket for 20 min. Finally, they were cleaned down with hard brush under water in order to remove carbon layer on surface of glazes.
2.3. Characterization
The crystalline phases in the glazes were carried out by X-ray dif-fraction (XRD) 40 kV and 30 mA with Rigaku Rint 2000 using Cu Kα radiation at a scan speed of 2°/min.
Polarized light microscope (Olympus SC50, Kyowa Corporation, Tokyo, Japan) was used to capture the micrographs.
The chemical characterization and microstructural investigation of the surface of samples were made by using two different scanning electron microscope (SEM). These were FEI Quanta 650 SEM (USA) equipped with Ametek EDAX Octane Plus EDS detector and Zeiss Supra 50VP SEM (Zeiss–Germany) equipped with Oxford Instruments INCA Energy EDS detector. The first could detect the light elements (Be, B, C, N, O and F) but the second could not detect them. In order to determine the crystals formed by silica and boron which are used as glass maker, micro structural and micro chemical features were analyzed with these two separate detectors.
Ultraviolet Visible (UV–VIS) Spectrophotometer measurements were obtained from the surface of the glazes using Universal Measurement Spectrophotometer (Agilent, Cary 700) applied with wavelength from 400 to 700 nm.
3. Result and discussion
Presence of silver and copper in glaze composition make production of crystalline phases possible. Crystallization is the combination of nucleation and crystal growth. Size and shape of crystals are related with both amount and diffusivity of atoms at certain temperature and atmosphere.
Dispersion of silver and copper particles in glassy matrix produce countless number of nucleus which will grow in the form of metal particles. However, the process may result in the precipitation of the silver and the dissolution of the copper in glaze [12].
Silver particles collapse in glaze when copper and silver collocate in glass composition. In addition, copper dissolves in the form of Cu+and Cu2+in the glaze [12].For this reason, it generates countless nucleus and grows in the form of metal particles when copper and silver enter the glaze.In these conditions, metal ions were reduced to metal, which remained trapped within the first layer of the glaze [13,14]. Copper and silver cations are converted into metal particles by a reduction process and spread to the glassy surface. The mechanism liable for the
penetration of Cu+and Ag+is ionic replacement with Na+and K+ions and diffusion to glassy matrix [14–16].
The metallic shining decoration on ceramics depends on a con-trolled scattering of the metallic Ag, Ba and Cu particles within the upper layer of the glaze.
3.1. X-ray diffraction studies
In this study, X-ray diffractometry, optical and scanning electron microscopy, Energy Dispersive X-ray spectroscopy and Ultraviolet Visible Spectroscopy studies were carried out to understand how crystal formation phenomenon occurs.
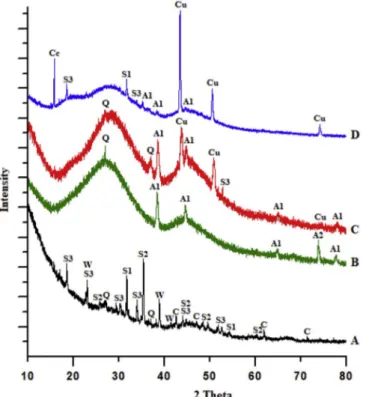
The XRD patterns of the four glazes showed the crystallization of various phases which were identified and given inFig. 2for low firing temperatures.
In the Glaze A, sodium phases occur as sodium silicon (S1), sodium silicate (S2) and sodium borate (S3) owing to aggressive reductive kiln process. In addition, carbon (C) was determined because of the reduc-tion by using sawdust and paper. It was tought that calcium from sawdust combined with silicate to form wollastonite (W). The sawdust includes high amounts of calcium in the ashes [17,18]. When compared with others, Glaze A possesses significant differences in terms of surface crystallinity.
In the Glaze B, as the BaCl2added, the crystalline formations de-creased and only silver phases such as metallic silver (A1) and silver oxide (A2) acquired the form of micro crystals.
The apparent metallic silver (A1) and copper (Cu) peaks were ob-served in the x-ray patterns of Glazes C and D. Because of the high boron and sodium ratio, it was expected that the sodium borate (S3) phase would be detected besides these phases in Glaze C and D and
sodium silicon (S1) in Glaze D.
Residual quartz (Q) was also found in A, B and C glazes. 3.2. Optical and SEM microscopy and EDX spectroscopy
Optical microscopy (OM) was used in order to obtain surface ap-pearance by reflectance mode. Copper and silver compounds form a decorative layer with a wide range of colors. When the OM images are examined, it is observed that the metallic-looking glazes consist of complex and striking colors showing two, three and sometimes four reflected colors.
Chemical and micromorphological analysis corresponding to dif-ferent metallic areas of the glazes were obtained by SEM-EDX on the glaze surfaces. InTable 2, images of optical microscope (OM) and SEM, Fig. 1. Ternary diagram of Na2O-B2O3-SiO2system [11] and the red mark indicates the composition of studied glaze. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1
Molecular formula of glazes A, B, C and D.
Sample Code Basic Oxides Neutral Oxides Acid Oxides Additives
A 0.9 Na2O 0.1 CaO 0.05 Al2O3 1.25 SiO1.8 B2O32 – B 0.9 Na2O 0.1 CaO 0.05 Al2O3 1.25 SiO1.8 B2O32 2% AgNO3 5% BaCl2 C 0.9 Na2O 0.1 CaO 0.05 Al2O3 1.25 SiO1.8 B2O32 2% AgNO3 5% CuSO4 D 0.9 Na2O 0.1 CaO 0.05 Al2O3 1.25 SiO1.8 B2O32 2% AgNO3 5% CuCO3
Table 2
The OM, SEM images and EDX analysis of glazes (Left: Polarized light microscope images; Middle: SEM and EDX not detect light elements (wt%); Right: SEM and EDX detect light elements (wt%)).
and results of EDX of Glaze A, B, C and D are shown respectively. In the EDX analysis, all sample surfaces have carbon and nitrogen remaining from the plants used during the reduction process, and also gold, pal-ladium and platin elements in the coatings for sample preparation phases were eliminated.
Glaze A has large cracks, leather like appearance and light colored iridescent light effect. The light colored crystals displayed by optical microscope were not observed by SEM. The fact that there is varied light diffraction and reflection on the surface in OM images suggest that these crystals are formed at different depths of the glaze layer. This provided a pearl-like color transition to glaze.
In EDX analysis taken from various point of surface, element con-tents changed by depending on detectors' ability to detect boron. When the detector could not measure it, high content of SiO2and Na2O were detected. However, as long as it could, high content of boron were detected. There were some high CaO content grains which are not bound to glaze surface. Considering the literature and in the light of the findings, these grains are suggested to be the residue from calcium-rich plants.
Glaze B has small cracks, leather like appearance and gives a light-colored iridescent light effect similar to Glaze A. The fact that the cracks are small suggests that BaCl2reduces high thermal expansion caused by high amount of Na2O, resulting in a more crack-free surface formation. OM images show that the glaze contains entire colors from visible region and these demonstrate a color harmony. The iridescent effect on the surface provided by small and heterogeneously dispersed metallic silver particles were observed on the surface of Glaze B. There was no other crystal structure except a small size and small amount of Ag crystallites on the surface of Glaze B. The absence of barium-containing crystals were associated with dissolution of Ba cations into glassy NBS system. The color reflection intensities are as low as Glaze A.
Small grains formed on the surface viewed by SEM images were identified and these grains were found to be Ag rich grains by EDX. The grains were very small in size, on the glaze surface, disperse state and SiO2and Na2O rich structure. In addition, the matrix has SiO2, B2O3, Al2O3, Na2O, CaO and BaO rich content.
The Glaze C has a cracked surface containing blue, green and yellow rich colors. The grains with an average particle size of 0,5 μm were distributed on a regular basis on the surface. It was determined by EDX that the grains were rich in CuO. In addition, Ag2O was also deposited less than CuO. It is thought that a special grain examined on the surface was sodium borate crystal which was also determined from XRD.
In the Glaze C, after reduction process, Ag and Cu particles were transported to the glaze surface and formed crystals in metallic ap-pearance. It was observed that particles of different sizes detected on the surface contain a relatively large amount of Cu and a smaller amount of Ag. This is related to smaller amount of silver content and is greater ionic exchange capacity of silver than that of copper. Hence, displacement of silver into the glassy matrix made the copper crystals predominantly on surface. As seen in micrograph, CuO crystals are homogeneously distributed as a layer on near-surface region of glaze surface shown in Table 2. These results are also supported by X-ray difractograms.
The surface of Glaze D has butterfly wing pattern. Presence of glassy matrix and heterogeneous distribution of CuO-rich crystals on surface are also detected by SEM images. Result of EDX measurement shows that the surface consists of plenty of copper oxide.
Although Glaze C and D contains equal amounts of silver, presence of sulphate and carbonate as a source of copper makes their crystal structures different from each other.
3.2.1. Ultraviolet Visible (UV–Vis) spectrophotometer measurement UV–Vis spectroscopy measurements were performed directly on the surface to identify the metallic particles existing in the metallic glaze layers. Nearly all glazes exhibit shades of red, yellow, orange, brown, green and blue with different hues. Reflectance spectra of the glazes are
shown inFig. 3in the range of 400–700 nm.
Thickness of glaze, presence of crystalline phases and porosities, refraction index of glassy matrix and crystals may change the re-flectance.
The reflectance curve intensity of A, B, C and D glaze are different, because of different scattering and precipitated crystal from matrix.
Silver ion produces yellow or green, and copper ion produces amber, brown and red. Cu2+diffused on the glassy matrix is green and the silver rich lustre then sometimes appears dark brown.
The reflectance values of A and B are lower than C and D glazes. Due to the opacity of barium, Glaze B has a lower reflectance than Glaze A. No significant differences were observed in the reflectance curves of the Glaze A and Glaze B at every wavelength in the region of 400–700 nm.
Copper rich glazes reflect the light like metal copper and silver rich glazes like metal gold. Glaze C, the reflectance of these glazes increase from blue to green wavelengths (~430 nm–580 nm) and diminishes from yellow to orange wavelengths (~580 nm–600 nm), what justifies the blue-green tonality of this glaze.
The Glaze D contains all colors in visible spectrum and intensities of color are stronger than compared with others. It was observed that the reflectance of Glaze D increased in direct proportion to the wavelength of 500 nm and decreased inversely in the 500–700 nm range. However, Cu crystals were distributed in the form of smaller crystals on the glaze surface and can be explained by the scattering of the incoming light from each particle as compared to the Glaze C. In other words, the reflection intensity due to the small size of the crystal also increased. 4. Conclusion
In this study, glazes with polychrome metallic appearance, in-cluding several combinations of colors and shines, were produced at low temperatures. In the reduction atmosphere, additions of AgNO3, BaCl2, CuSO4and CuCO3were investigated in order to obtain glazes with metallic appearance and crystal formation in NBS system. In this reducing stage, copper and silver ions were converted into metallic form and difused to the glaze surface. OM and SEM observations re-vealed that all glazes had cracks on their surface due to the high thermal expansion of Na+. In addition, nitrogen compounds and carbon residues from the organic materials which were used during the re-duction were found on the surfaces of all glazes.
In Glaze A, crystals were formed at different depths of the glaze layer meanwhile no crystal formation occurred on the surface. In Glaze B, the BaCl2did not form a crystal but instead, dissolved in the glassy
matrix. This structure involves thinner cracks and there were small and heterogeneous dispersed silver crystals formed on the glaze surface. Homogeneously dispersed particles detected on the surface of the Glaze C contains a relatively large amount of Cu and a smaller amount of Ag. The use of CuSO4 produced homogenous grain-sized crystals and caused predominantly green color hues.
In Glaze D, silver and copper crystals are small, irregular and homogeneously distributed. The color due to usage of CuCO3ranged from rusty to brown. Although Glaze C and Glaze D contain the same amount of Ag and Cu, the difference of the crystal shapes is due to the sulphate and carbon compounds of copper. UV/VIS reflectance mea-surements introduced the wavelengths of glazes ranges from 400 nm to 700 nm, indicating mainly purple, green, blue and orange in color. Since copper is transported to the surface and forms crystals, the re-flectance of C and D is stronger than A and B glazes.
References
[1] N. Shasmal, B. Karmakar, Synthesis of transparent chloroborosilicate nanoglass-ceramics: crystallization and growth mechanism of BaCl2nanocrystals, Journal of
Asian Ceramic Societies 3 (4) (2015) 390–401https://doi.org/10.1016/j.jascer. 2015.08.002.
[2] I.A. Levitskii, Mechanism of phase formation in aventurine glaze, Glass Ceram. 58 (Issue 5–6) (2001) 223–226,https://doi.org/10.1023/A:1012351301045. [3] I. LazăuS. Borcănescu, C. Păcurariu, C. Vancea, Kinetic study of the non-isothermal
crystallization process of hematite in ceramic glazes obtained from CRT wastes, J. Therm. Anal. Calorim. 112 (Issue 1) (2013) 345–351.
[4] R. Mathieu, G. Libourel, E. Deloule, L. Tissandier, C. Rapin, R. Podor, Na2O
solu-bility in CaO–MgO–SiO2melts, Geochem. Cosmochim. Acta 75 (Issue 2) (2011)
608–628https://doi.org/10.1016/j.gca.2010.11.001.
[5] P. Tripathia, P. Kumaria, V.K. Mishrad, R. Singhc, S.P. Singhb, D. Kumara, Effect of PbO–B2O3–BaO–SiO2glass additive on dielectric properties of Ba0.5Sr0.5TiO3
cera-mics for radio-frequency applications, J. Phys. Chem. Solids 127 (2019) 60–67
https://doi.org/10.1016/j.jpcs.2018.12.006.
[6] L. Savio, C. Giallombardo, L. Vattuone, A. Kokalj, M. Rocca, Tuning the stoichio-metry of surface oxide phases by step morphology: Ag (511) versus Ag (210), Phys. Rev. Lett. 101 (2008) 266103https://doi.org/10.1103/PhysRevLett.101.266103. [7] R. Hopper, The Ceramic Spectrum: A Simplified Approach to Glaze and Color
Development, second ed., American Ceramic Society, 2008.
[8] W. Wong-Ng, R.S. Roth, T.A. Vanderah, H.F. McMurdie, Phase equilibria and crystallography of ceramic oxides, J Res Natl Inst Stand Technol 106 (6) (2001) 1097–1134,https://doi.org/10.6028/jres.106.0592001 Nov-Dec.
[9] Y. Wang, S. Yu, J. Chu, D. Chen, J. Chen, Study on the copper and iron coexisted coloring glaze and the mechanism of the fambe, J. Eur. Ceram. Soc. 38 (Issue 10) (2018) 3681–3688https://doi.org/10.1016/j.jeurceramsoc.2018.04.004. [10] F. Singer, S.S. Singer, Industrial Ceramics, Chapman and Hall, London, UK, 1963. [11] Z. Jiang, Q. Zhang, The structure of glass: a phase equilibrium diagram approach, Prog. Mater. Sci. 61 (2014) 144–215https://doi.org/10.1016/j.pmatsci.2013.12. 001.
[12] T. Pradell, J. Molera, A.D. Smith, M.S. Tite, The invention of lustre: Iraq 9th and 10th centuries AD, J. Archaeol. Sci. 35 (2008) 1201–1215https://doi.org/10.1016/ j.jas.2007.08.016.
[13] A. Caiger-Smith, Luster Pottery. Technique, Tradition and Innovation in Islam and the Western World, Faber & Faber, London, UK, 1985.
[14] I. Borgia, B. Brunetti, A. Giulivi, A. Sgamellotti, F. Shokouhi, P. Oliaiy, J. Rahighi, M. Lamehi-Rachti, M. Mellini, C. Viti, Characterisation of decorations on Iranian (10th–13th century) lusterware, Appl. Phys. A 79 (Issue 2) (2004) 257–261https:// doi.org/10.1007/s00339-004-2519-z.
[15] S. Padovani, C. Sada, P. Mazzoldi, B. Brunetti, I. Borgia, A. Sgamellotti, A. Giulivi, F. D'Acapito, G. Battaglin, Copper in glazes of Renaissance luster pottery: nano-particles, ions, and local environment, J. Appl. Phys. 93 (2003) 10058https://doi. org/10.1063/1.1571965.
[16] T. Pradell, J. Molera, J. Roque, M. Vendrell-Saz, A.D. Smith, E. Pantos, D. Crespo, Ionic-exchange mechanism in the formation of medieval luster decorations, J. Am. Ceram. Soc. 88 (5) (2005) 1281–1289,https://doi.org/10.1111/j.1551-2916.2005. 00223.x.
[17] I. Borgia, B. Brunetti, I. Mariani, A. Sgamellotti, F. Cariati, P. Fermo, M. Mellini, C. Viti, G. Padeletti, Heterogeneous distribution of metal nanocrystals in glazes of historical pottery, Appl. Surf. Sci. 185 (3) (2002) 206–216https://doi.org/10. 1016/S0169-4332(01)00659-6.
[18] C.C. Ban, M. Ramli, Properties of high calcium wood ash and densified silica fume blended cement, Int. J. Phys. Sci. 6 (28) (2011) 6596–6606,https://doi.org/10. 5897/IJPS11.1485.