Original Report: Patient-Oriented, Translational Research
Am J Nephrol 2011;33:491–498 DOI: 10.1159/000327829
Attenuated Cardiovascular Response to
Sympathetic System Activation during Exercise in
Patients with Dialysis-Induced Hypotension
Hakan Fotbolcu
a
Dursun Duman
c
Sebahat Alısır Ecder
b
Vecih Oduncu
d
Cihan Cevik
e
Kursat Tigen
d
Gokce Şirin
a
Emre Özker
a
Burak Kıran
d
Yelda Basaran
d
a
Division of Cardiology, Goztepe Medical Park Hospital, b Nephrology and Dialysis Unit, Goztepe Training and Research Hospital, c
Department of Cardiology, Medipol University, and d Division of Cardiology, Kartal Kosuyolu Heart Education and Research Hospital, Istanbul , Turkey; e
Cardiology Division, Texas Heart Institute St. Luke’s Episcopal Hospital, Baylor College of Medicine, Houston, Tex. , USA
SBP response values compared to the Non-DIH (34.88 8 15.01 vs. 55.67 8 25.42, p = 0.002) and controls (34.88 8 15.01 vs. 59.70 8 23.04, p ! 0.001). Conclusion: Patients with DIH have inadequate sympathetic activity of the cardiovas-cular system during exercise and impaired left ventricardiovas-cular systolic function. Both factors could contribute to the devel-opment of hypotension during hemodialysis.
Copyright © 2011 S. Karger AG, Basel
Introduction
Dialysis-induced hypotension (DIH) is a common complication in hemodialysis (HD) patients, occurring in one third [1] . This condition is also associated with increased morbidity and decreased overall quality of life [1, 2] . Previous studies have supported the imbalance between decreased plasma volume during HD and the counter-regulatory cardiovascular/neurohormonal mech anisms [3, 4] . Furthermore, cardiac arrhythmias, decreased cardiac output, pericardial disease, intravas-cular volume depletion, and the use of certain dialysates (such as acetate) may be responsible for this condition, though the exact mechanism of DIH is still unclear [3–6] . Key Words
Autonomic nervous system ⴢ Chronotropic incompetence ⴢ Dialysis ⴢ Heart rate recovery ⴢ Hypotension ⴢ Systolic blood pressure
Abstract
Background: We wished to investigate potential causes of dialysis-induced hypotension (DIH), including the attenuat-ed cardiovascular response to sympathetic system activa-tion during exercise and myocardial dysfuncactiva-tion. Methods: This study included 26 end-stage renal disease (ESRD) pa-tients with DIH, 30 ESRD papa-tients without DIH (Non-DIH), and 30 control subjects. Each patient was evaluated with echo-cardiography and a symptom-limited treadmill stress test. The chronotropic index (CRI), heart rate recovery (HRR), sys-tolic blood pressure response to exercise (SBP response), and tissue Doppler systolic myocardial velocities were calculat-ed. Results: The HRR and velocities were reduced in dialysis patients compared to controls; however, they were similar in patients with and without DIH. Patients with DIH had the lowest CRI compared to the Non-DIH group (0.62 8 0.15 vs. 0.73 8 0.17, p = 0.020) and controls (0.62 8 0.15 vs. 0.86 8 0.11, p ! 0.001). Similarly, patients with DIH had the lowest
Received: March 6, 2011 Accepted: March 22, 2011 Published online: May 5, 2011
Patients with end-stage renal disease (ESRD) on HD commonly have autonomic nervous system (ANS) dys-function [7] . ANS dysdys-function is even more common in uremic patients with DIH [7–9] . Several components of the ANS can be altered in patients with DIH, such as de-creased baroreceptor sensitivity, or the parasympathetic or sympathetic pathways; however, it is difficult to eluci-date which segment or segments of the ANS are impaired in uremic patients [7–10] . In this study, we tested a hy-pothesis suggesting that an attenuated cardiovascular re-sponse to sympathetic system activation during exercise together with tissue-Doppler-derived myocardial dys-function may play a role in the pathophysiology of DIH in patients with ESRD.
Methods
This study included 26 ESRD patients with DIH, 30 ESRD pa-tients without DIH (Non-DIH), and 30 control subjects. The fol-lowing patients were excluded from this study: hypertensive pa-tients who were on a medication which may affect ANS (verap-amil, diltiazem,  -blockers, ␣ -blockers), severe morbid obesity (BMI 1 35), moderate or severe heart valve insufficiency and/or stenosis, congenital heart disease, atrial fibrillation, left bundle branch block, established coronary artery disease [i.e. patients who had a history of myocardial infarction, unstable angina pectoris, angiographically proven significant coronary stenosis (more than 50% lumen stenosis in at least 1 coronary artery in coronary angiography) or had undergone revascularization, or an abnormal exercise test], left ventricular (LV) ejection fraction ! 50%, symptomatic heart failure, poor echocardiographic image quality, stroke, chronic obstructive pulmonary disease, or an or-thopedic or musculoskeletal disorder.
A total of 522 patients with ESRD were evaluated to determine DIH during a 6-month run-in period at 5 hemodialysis centers. After the rin phase, 26 patients with DIH who had been un-dergoing 4-hour sessions of HD 3 times a week for 6 2 years were enrolled. We also included 30 age- and sex-matched patients un-dergoing HD without DIH from this cohort with a similar diabe-tes mellitus and hypertension incidence. The control group in-cluded 30 patients without renal failure (serum creatinine ! 1.2 mg/dl). Diabetes was diagnosed according to World Health Orga-nization criteria [11] . Participants were classified as hypertensive if resting systolic blood pressure (SBP) was 6 140 mm Hg and/or diastolic blood pressure (DBP) was 6 90 mm Hg, or if they took antihypertensive medications. All study patients underwent a thorough clinical anthropometric laboratory investigation. Blood samples were obtained on the day following HD, before the car-diac stress test and echocardiographic examination. Heights and weights of all subjects were measured, and the BMI was calcu-lated as weight (kg) divided by height 2 (m).
As previously reported [12] , DIH was defined as the presence of either or both of the following conditions on more than 30 oc-casions from among 50 previous sessions of dialysis: (1) a fall in SBP to ! 90 mm Hg during dialysis; (2) a fall in SBP of 1 25% from
the start of dialysis associated with symptoms related to hypoten-sion, including dizziness, vomiting, nausea, and muscle cramps. Blood pressure was measured on the same arm by trained nursing staff intermittently at 30-min intervals using a mercury sphygmomanometer with the patients seated in multi-adjustable chairs. The start and end positions were semi-recumbent. When sudden symptomatic hypotension occurred, blood pressure mea-surements were repeated in the reverse Trendelenburg position, and hypertonic saline boluses were given as needed. The ultrafil-tration rate (% body weight reduction) during dialysis sessions was adjusted according to the presumed dry weight (assessed as the postdialysis patient’s weight when normotensive and free of edema). During the follow-up period, patients received standard HD using a bicarbonate bath and substituted with cellulose and synthetic membranes. Blood flow rates ranged from 350 to 400/ min, the dialysate flow rate was 500 ml/min, and the dialysate temperature was 36 ° C. Ultrafiltration was linear and the ultrafil-tration rate was adapted to reach the dry weight in the preset di-alysis time. No sodium or blood volume profiling was carried out. Dialysate contained 140 mmol/l Na + , 1 mmol/l K + , 33 mmol/l HCO3– , 105 mmol/l Cl – , 1.25 mmol/l Ca2+ , 0.25 mmol/l Mg2+ , and 11 mmol/l glucose. Kt/V was calculated from total loss of urea nitrogen in the spent dialysate using the Watson equation [13] . Mean values of Kt/V were calculated from monthly predialysis measurements.
Echocardiographic Measurements
Each patient underwent standard transthoracic 2D echocar-diography and exercise testing between 10: 00 and 16: 00 h on the next day of HD. All echocardiographic assessments were per-formed by 2 operators, who were blinded to the clinical and labo-ratory results of the study group. Vivid 3D echocardiography equipment (GE Vingmed, Horten, Norway) with a 2.5-MHz phased-array transducer was used for each study subject. LV di-mension and wall thickness were measured from 2D guided M-mode echocardiographic tracings at mid-chordal level on the parasternal long-axis view. The M-mode traces were recorded at a speed of 50 mm/s. Ejection fraction was calculated using the Teicholz formula. The LV mass was estimated by using the ana-tomically validated formula of Devereux et al. [14] . It was indexed for body surface area to estimate the LV mass index. The tissue Doppler imaging program was set to pulse wave Doppler mode. Filters were set to exclude high-frequency signals. Gains were minimized to allow a clear tissue signal with minimal back-ground noise. The tissue Doppler imaging of the systolic velocities was obtained from the apical 4-chamber view. A 1.5-mm sample volume was placed at the lateral corner of the mitral annulus.
Exercise Testing
The modified Bruce standard protocols were used for symp-tom-limited treadmill testing, during which leaning on handrails was explicitly not allowed [15, 16] . With each stage of exercise and recovery (first 3 min), data on symptoms, rhythm, heart rate, blood pressure, workload (in metabolic equivalents; METs), and ST segment changes were collected. Exercise capacity in METs was estimated from standard published tables [16] . Patients were credited with an appropriate time-based proportion of the esti-mated METs if the final stage of exercise was not completed. Per laboratory protocol, exercise could be stopped for marked ( 1 2.5 mm) ST segment depression, exercise SBP 1 250 mm Hg, and
ven-tricular tachycardia. The exercise ECG was considered positive if there was 0.1 mV or more of J point depression and the ST segment was flat or downward sloping 0.08 s after the J point.
Heart rate recovery (HRR) was defined as: peak heart rate – recovery heart rate (at 1 min after exercise) [17] . The chronotrop-ic index (CRI) was defined as: (peak heart rate – resting heart rate)/(220 – age – resting heart rate), with an abnormal value (chronotropic incompetence) defined as ^ 0.80 [18] . Blood pres-sure recovery (SBP ratio) was defined as the ratio of the SBP at 3 min into recovery to the SBP at peak exercise [19] . Blood pres-sure response to exercise (the SBP response) was defined as: SBP at peak exercise – SBP at baseline.
Assessment of Blood Pressure
Pre-exercise and exercise blood pressures were measured manually by the same nurse with a random-zero sphygmoma-nometer (cuff size 14 ! 54 cm; Hawsksley). The blood pressure measurements were performed every 2 min during the treadmill exercise test. The highest SBP achieved during the exercise test was defined as the peak SBP. The blood pressures of the study population were measured during the recovery stage at regular intervals of 1 and 3 min while the subjects were seated.
Statistical Analysis
All statistical analyses were performed using SPSS 11.5 (SPSS Inc., Chicago, Ill., USA). Data are presented as means 8 SD for each group of measurements. The frequency distribution of the categorical variables was tested with a 2 ! 2 contingency table. The continuous variables of 2 groups were compared with Stu-dent’s t test, and the Mann-Whitney U test was used when a non-normal distribution was present. ANOVA and Kruskal-Wallis tests were used during the comparison of 3 groups with each oth-er, depending on the normal distribution of the data. For the post hoc test, we used the Tukey HSD test for the variables that were significant in ANOVA, and the Mann-Whitney U test for the
vari-ables that were significant in the Kruskal-Wallis test. Correlations between the variables were tested by Pearson correlation analysis. ANCOVA was used for the CRI and SBP response, and creatinine, phosphorus, hematocrit, and BMI values were used as covariates. Since the urea and creatinine levels had a significant intercorrela-tion with each other, we only included creatinine levels in the model. In all statistical analyses, two-tailed values of p ! 0.05 were considered to indicate statistical significance.
Results
Fifty-six HD patients (mean age 43.9 8 11.8 years) and 30 controls (mean age 43.4 8 13.6 years) were included in this study. Baseline clinical and biochemical parame-ters of the study population are presented in table 1 . All groups were similar in terms of age, gender, incidence of diabetes and hypertension, antihypertensive medication profile, serum potassium, serum calcium, and serum al-bumin levels. The control group had an increased BMI compared to the patients on HD (p = 0.011). Echocardio-graphic parameters of all patients are presented in table 2 . Dialysis patients had increased LV wall thickness, LV mass, and mass index compared to the control group. The LV ejection fraction was similar in the 3 groups (p = 0.703). DIH (8.03 8 1.90 cm/s) and Non-DIH (8.31 8 1.68 cm/s) groups had significantly reduced (p ! 0.001) tissue Doppler systolic velocities compared to the con-trols (10.78 8 3.21 cm/s). The tissue Doppler systolic myocardial velocities of DIH and Non-DIH subgroups
Table 1. B aseline clinical and biochemical parameters
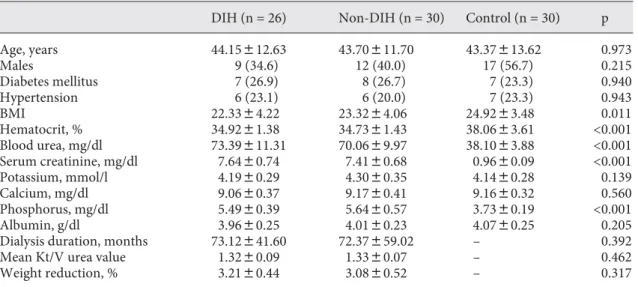
DIH (n = 26) Non-DIH (n = 30) Control (n = 30) p
Age, years 44.15812.63 43.70811.70 43.37813.62 0.973 Males 9 (34.6) 12 (40.0) 17 (56.7) 0.215 Diabetes mellitus 7 (26.9) 8 (26.7) 7 (23.3) 0.940 Hypertension 6 (23.1) 6 (20.0) 7 (23.3) 0.943 BMI 22.3384.22 23.3284.06 24.9283.48 0.011 Hematocrit, % 34.9281.38 34.7381.43 38.0683.61 <0.001 Blood urea, mg/dl 73.39811.31 70.0689.97 38.1083.88 <0.001 Serum creatinine, mg/dl 7.6480.74 7.4180.68 0.9680.09 <0.001 Potassium, mmol/l 4.1980.29 4.3080.35 4.1480.28 0.139 Calcium, mg/dl 9.0680.37 9.1780.41 9.1680.32 0.560 Phosphorus, mg/dl 5.4980.39 5.6480.57 3.7380.19 <0.001 Albumin, g/dl 3.9680.25 4.0180.23 4.0780.25 0.205
Dialysis duration, months 73.12841.60 72.37859.02 – 0.392
Mean Kt/V urea value 1.3280.09 1.3380.07 – 0.462
Weight reduction, % 3.2180.44 3.0880.52 – 0.317
were statistically similar (8.03 8 1.90 vs. 8.31 8 1.68 cm/s, respectively, p = 0.896).
The treadmill cardiac stress test parameters of the di-alysis patients and the control group are presented in ta-ble 3 . The baseline heart rates of DIH (93.08 8 14.06 beats/min) and Non-DIH groups (92.20 8 15.20 beats/ min) were significantly increased (p ! 0.001) compared to the control group (77.80 8 14.65 beats/min). Baseline heart rates were similar between DIH and Non-DIH groups (93.08 8 14.06 vs. 92.20 8 15.20 beats/min, p = 0.974). The peak heart rate of patients with DIH was less than the controls’ (146.15 8 13.70 vs. 163.13 8 20.99 beats/min, p = 0.002). However, the peak heart rates in patients with DIH versus Non-DIH (146.15 8 13.70 vs.
153.07 8 18.17 beats/min, p = 0.331) and Non-DIH versus controls (153.07 8 18.17 vs. 163.13 8 20.99 beats/min, p = 0.084) were similar. DIH groups had lower baseline SBP compared to Non-DIH and controls (116.15 8 30.89 vs. 142.53 8 21.56 mm Hg, p ! 0.001, and 116.15 8 30.89 vs. 131.67 8 17.30 mm Hg, p = 0.042). Baseline SBP of Non-DIH and controls were similar (142.53 8 21.56 vs. 131.67 8 17.30 mm Hg, p = 0.180). The DIH group had lower peak SBP compared to Non-DIH and control groups (151.04 8 30.34 vs. 198.20 8 26.22 mm Hg, p ! 0.001, and 151.04 8 30.34 vs. 191.37 8 27.32 mm Hg, p ! 0.001). Peak SBP were similar between Non-DIH and controls. (198.20 8 26.22 vs. 191.37 8 27.32 mm Hg, p = 0.611). The DIH group had lower baseline DBP compared to
Table 2. E chocardiographic parameters of all patients
DIH Non-DIH Control p
IVS-D, cm 1.0580.15 1.0680.12 0.9780.17 0.082 PW-D, cm 0.9680.14 0.9580.10 0.8680.16 0.012 LVEDD, cm 4.7480.41 4.7280.27 4.5980.36 0.240 LVESD, cm 3.1080.44 3.0480.32 2.9080.39 0.135 LVEF, % 63.984.9 64.682.5 63.983.4 0.703 LV mass, g 169.2841.1 169.3838.2 138.6847.8 0.009 LV mass index g/m³ 108.7827.6 104.0827.6 71.6821.2 <0.001 TDVS, cm/s 8.0381.90 8.3181.68 10.7883.21 <0.001
I VS-D = Interventricular septum diastolic thickness; PW-D = posterior wall diastolic thickness; LVEDD = LV end-diastolic diameter; LVESD = LV end-systolic diameter; LVEF = LV ejection fraction; TDVS = tissue Doppler systolic velocity.
Table 3. Treadmill parameters of all patients
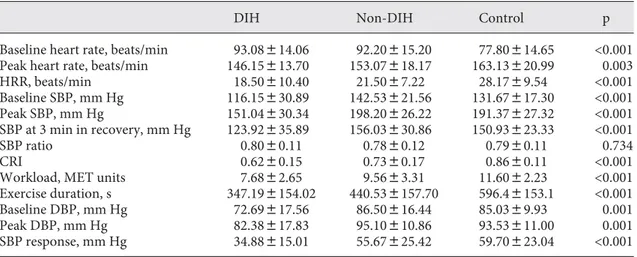
DIH Non-DIH Control p
Baseline heart rate, beats/min 93.08814.06 92.20815.20 77.80814.65 <0.001
Peak heart rate, beats/min 146.15813.70 153.07818.17 163.13820.99 0.003
HRR, beats/min 18.50810.40 21.5087.22 28.1789.54 <0.001 Baseline SBP, mm Hg 116.15830.89 142.53821.56 131.67817.30 <0.001 Peak SBP, mm Hg 151.04830.34 198.20826.22 191.37827.32 <0.001 SBP at 3 min in recovery, mm Hg 123.92835.89 156.03830.86 150.93823.33 <0.001 SBP ratio 0.8080.11 0.7880.12 0.7980.11 0.734 CRI 0.6280.15 0.7380.17 0.8680.11 <0.001
Workload, MET units 7.6882.65 9.5683.31 11.6082.23 <0.001
Exercise duration, s 347.198154.02 440.538157.70 596.48153.1 <0.001
Baseline DBP, mm Hg 72.69817.56 86.50816.44 85.0389.93 0.001
Peak DBP, mm Hg 82.38817.83 95.10810.86 93.53811.00 0.001
Non-DIH patients and controls (72.69 8 17.56 vs. 86.50 8 16.44 mm Hg, p = 0.002, and 72.69 8 17.56 vs. 85.03 8 9.93 mm Hg, respectively, p = 0.008). Baseline DBP was similar between Non-DIH patients and controls (86.50 8 16.44 vs. 85.03 8 9.93 mm Hg, p = 0.923). The DIH group had lower peak DBP levels compared to the Non-DIH and control groups (82.38 8 17.83 vs. 95.10 8 10.86 mm Hg, respectively, p = 0.002, and 82.38 8 17.83 vs. 93.53 8 11.00 mm Hg, respectively, p = 0.007). Peak DBP levels were similar between Non-DIH and control groups (95.10 8 10.86 vs. 93.53 8 11.00 mm Hg, p = 0.893).
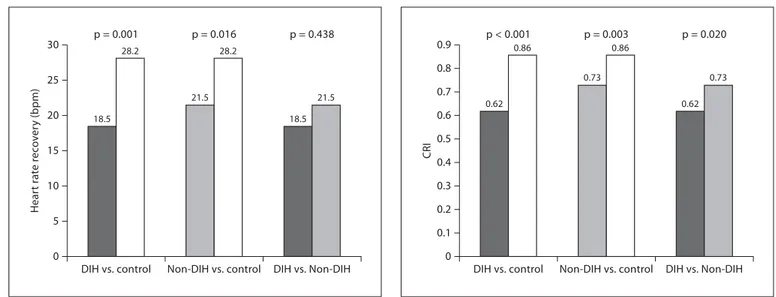
DIH and Non-DIH groups were similar in term of HRR (18.50 8 10.40 vs. 21.50 8 7.22 beats/min, p = 0.438). Both DIH and Non-DIH patients had lower HRR values compared to the control group ( fig. 1 ). Patients with DIH had the lowest CRI values (0.62 8 0.15) com-pared to the Non-DIH (0.73 8 0.17) and the controls (0.86 8 0.11) ( fig. 2 ). Twenty-four patients (92.3%) with DIH, 18 patients with Non-DIH (60%), and 9 controls (30%) had chronotropic incompetence. Patients with DIH had significantly lower SBP response compared to the Non-DIH and control groups ( fig. 3 ). The 3 groups were similar in terms of SBP ratio (p = 0.734). The statis-tically significant difference was persistent between DIH and Non-DIH groups for CRI and SBP response, even af-ter adjusting for baseline creatinine, phosphorus, hema-tocrit, and BMI levels with ANCOVA (0.69 8 0.09 vs. 0.79 8 0.08 mm Hg, p = 0.017, 50.15 8 11.86 vs. 66.74 8 11.32 mm Hg, p = 0.005, respectively).
Both DIH and Non-DIH patients had a significantly lower exercise duration than the controls (347.2 8 154.0 vs. 596.4 8 153.1 s, p ! 0.001, 440.5 8 157.7 vs. 596.4 8 153.1 s, p = 0.001, respectively). The exercise duration was similar between DIH and Non-DIH groups (347.2 8 154.0 vs. 440.5 8 157.7 s, p = 0.069). MET values of the DIH and Non-DIH groups were similarly lower than those of the control group (7.68 8 2.65 vs. 11.60 8 2.23 units, p ! 0.001; 9.56 8 3.31 vs. 11.60 8 2.23 units, p = 0.015, respectively). The DIH group had lower MET levels
0 5 10 15 20 25 30
DIH vs. control Non-DIH vs. control DIH vs. Non-DIH
p = 0.001 p = 0.016 p = 0.438 18.5 28.2 21.5 28.2 18.5 21.5 Hear t rat e r e c o v e ry (bpm) 0 0.1 0.2 0.3 0.5 0.4 0.6 0.8 0.7 0.9
DIH vs. control Non-DIH vs. control DIH vs. Non-DIH
p < 0.001 p = 0.003 p = 0.020 0.62 0.86 0.73 0.86 0.62 0.73 CRI 0 10 20 30 40 50 60
DIH vs. control Non-DIH vs. control DIH vs. Non-DIH
p < 0.001 p = 0.756 p = 0.002 34.8 59.7 55.6 59.7 34.8 55.6 S y st olic blood pr essur e r esponse (mm Hg)
Fig. 1. HRR of patients with DIH/Non-DIH and controls. Fig. 2. CRI of DIH, Non-DIH, and controls.
compared to the Non-DIH group (7.68 8 2.65 vs. 9.56 8 3.31 units, p = 0.036). There was no significant correlation between the dialysis time and treadmill test parameters.
Discussion
In our study, we found that the heart rate response to exercise was reduced in dialysis patients, and they devel-oped chronotropic incompetence. Furthermore, DIH pa-tients had more significant chronotropic incompetence compared to the Non-DIH group. This finding was also similar for the SBP response to exercise. The tissue Dop-pler study indicated a decreased LV systolic function in the dialysis patients in our study population, although the ejection fractions were within normal values. In our study, we assessed the LV contractile function simultane-ously with cardiac sympathetic activation; therefore, our results provided significant evidence indicating the pathophysiological mechanisms of DIH.
Two neurohormonal mechanisms influence the SBP response to exercise. Parasympathetic and sympathetic efferent stimulation influence a cardiovascular response central command. The autonomic efferent sympathetic response resulting from intramuscular afferent receptors sensitive to metabolic products produced by skeletal muscle is known as the muscle metaboreflex [20] . After the onset of exercise, there is an initial increase in heart rate that results from a decline in parasympathetic tone. Subsequently, there is an increase in sympathetic activity that results in an increase in SBP and contributes to the increase in heart rate. The sympathetic response results from the release of catecholamines from the central med-ullary and spinal neuronal circuits (central command) [21] . There is also sympathetic stimulation that results from the release of catecholamines from large muscle groups. These muscle groups contain afferent mechano-receptors that are stimulated by muscle contraction and release catecholamines [22, 23] . Similar to exercise, a sud-den reduction in the intravascular volume during HD stimulates the sympathetic system, which increases the heart rate and total peripheral vascular resistance. This mechanism prevents the imbalance between the vascular capacitance and plasma under-filling; therefore, it main-tains the systemic arterial pressure. In DIH patients, the vasoconstrictive response to the HD-induced changes in total plasma volume seems to be altered and could be re-sponsible for a sudden drop in arterial pressure [24, 25] . The rise in blood pressure with sympathetic activation is predominantly caused by an increase in cardiac output,
which is related to LV systolic function and heart rate. In addition, the increase in peripheral vascular resistance elevates the systemic blood pressure (mean arterial pres-sure = stroke volume ! heart rate ! peripheral vascular resistance). Our results indicated that the reduced activa-tion of the sympathetic system on the cardiovascular sys-tem caused a blunted heart rate and LV inotropic effect. The tissue Doppler values of DIH and Non-DIH patients were similar, but lower compared to the controls. There-fore, we hypothesized that impaired LV systolic function could be a contributor to the development of DIH.
There are significant publications on peripheral vas-cular resistance in patients with ESRD in the literature: the autonomic neuropathy often present in uremia [8] , an impaired vascular adrenoceptor function [9] , or a de-creased vascular response to angiotensin II infusion sec-ondary to a reduced angiotensin II receptor number [26] , and others. Hypotensive HD patients display even higher plasma catecholamine levels than uremic patients [7, 9, 26] , while the pressor response to noradrenaline infusion is markedly blunted when compared to normotensive HD patients [7, 9, 26] , suggesting the presence of postsyn-aptic vascular resistance to the sympathetic stimuli and a compensatory sympathetic activation. Previous authors have ascribed this peripheral resistance to catechol-amines to a reduced vascular adrenoceptor number and/ or function [9] . The study of Coll et al. [27] demonstrated the reduced peripheral vascular resistance and increased vascular compliance in patients with DIH. In our study, the baseline blood pressure levels of the DIH group were lower than in the Non-DIH group. This finding may in-dicate the reduced baseline peripheral vascular resistance in patients with DIH. Our results do not indicate a change in peripheral arterial resistance during exercise in gen-eral. Studies investigating the change in peripheral vas-cular resistance during sympathetic activation are need-ed to demonstrate the role of peripheral vascular resis-tance in the blunted blood pressure response in patients with DIH.
The baseline heart rate levels of the dialysis patients were higher compared to the controls in our study. This could be explained by the increased incidence of anemia among the dialysis patients. Recent studies have high-lighted the prognostic value of an exercise treadmill test featuring HRR after exercise [28] . A rapid HRR response to exercise is considered to be a marker of physical fitness. HRR is mediated by vagal reactivation, and the rate at which HR declines appears to be a reflection of faster re-covery from the sympathetic drive necessary during ex-ercise [29] . Increased vagal activity associated with a
fast-er HRR has been showed to be associated with a decreased risk of death [30] . The HRR which indicated post-exercise parasymphatetic reactivation was lower in dialysis pa-tients compared to controls; however, DIH and Non-DIH groups were similar in terms of HRR ( fig. 1 ).
Consistent with a previous study showing the role of delayed slowing of HR [28] , elevated SBP immediately af-ter exercise may also reflect the overactivity of the sym-pathetic nervous system and attenuated vagal reactiva-tion. Autonomic dysfunction and vasoreactivity abnor-malities may account for the gradual decrease in SBP after exercise [31, 32] . An attenuated decrease in exercise blood pressure also may be attributable to poor arterial compliance in individuals with underlying vascular smooth muscle hypertrophy and subclinical arterioscle-rotic changes [33] . In our study, SBP ratio values were similar among all groups. We believe that the preserved vascular compliance caused the unchanged SBP ratio val-ues among these patients. The similar SBP ratio between the subgroups excludes the over-reactivation of periph-eral vascular resistance during the relaxation period in each subgroup.
Epidemiological studies have demonstrated an inverse association between physical fitness and the incidence of coronary heart disease or all-cause mortality in healthy or asymptomatic participants. Recently, Kodama et al. [34] reported that a 1-MET higher level of maximal aero-bic capacity was associated with 13 and 15% decrements in the risk of all-cause mortality and coronary heart dis-ease/cardiovascular disease in their meta-analysis, re-spectively. Similar to their findings, we demonstrated that MET values of the dialysis patients were lower than the controls’. This result was even more significant in DIH patients compared to Non-DIH patients.
Limitations of the Study
We demonstrated the inadequate sympathetic system response to exercise in patients with DIH; however, the etiology of this result remained unclear. This could either be from the sympathetic system itself or secondary to a tissue level resistance to the sympathetic stimulation. Molecular studies investigating the adrenoceptor re-sponse at the peripheral or cardiac tissue level are needed to answer this question. Furthermore, genetically based studies are necessary to demonstrate why the DIH group was more significantly affected by the impaired ANS ef-fects of uremia than the Non-DIH group.
In conclusion, we found that the CRI and SBP response were reduced, and SBP ratio values were preserved in tients with DIH compared to controls and Non-DIH pa-tients. These findings indicate that the cardiovascular re-sponse to sympathetic activation is diminished and pe-ripheral vascular compliance is preserved in this patient population.
Acknowledgments
The authors are grateful for the excellent nursing support from Yasemin Bozalp and Yeliz Ozkan Cetin during the treadmill car-diac stress tests, and the nursing care from Serpil Gunduz during dialysis for the study patients. We appreciate the excellent secre-tarial assistance of Ayten Bolut during the preparation of the manuscript. We also thank Ferit Ün and Bülent Sakaoğlu for their help in the statistical analysis of the revised manuscript.
Disclosure Statement
The authors report no conflict of interest.
References
1 Henrich WL: Hemodynamic instability
dur-ing hemodialysis. Kidney Int 1986; 30: 605–
612.
2 Maher JF, Schreiner GE: Hazards and
com-plications of dialysis. N Engl J Med 1965; 273:
370–377.
3 Daugirdas JT: Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis
2001; 38(suppl 4):S11–S17.
4 Andrulli S, Colzani S, Mascia F, et al: The role of blood volume reduction in the genesis of intradialytic hypotension. Am J Kidney
Dis 2002; 40: 1244–1254.
5 Lazarus JM, Henderson LW, Kjellstrand CM, et al: Cardiovascular instability during di-alysis. Trans Am Soc Artif Intern Organs
1982; 28: 656–665.
6 Davis CL, Henrich WL: Cardiac perfor-mance in chronic renal failure. Int J Artif
Or-gans 1985; 8: 7–10.
7 Campese VM, Romoff MS, Levitan D, et al: Mechanisms of autonomic nervous system
dysfunction in uremia. Kidney Int 1981; 20:
246–253.
8 Stojeva-Taneva O, Masin G, Polenakovic M, et al: Autonomic nervous system dysfunc-tion and volume nonresponsive hypotension in hemodialysis patients. Am J Nephrol 1991;
11: 123–126.
9 Daul AE, Wang XL, Brodde OE: Arterial hy-potension in chronic hemodialyzed patients.
Kidney Int 1987; 32: 728–735.
10 Zoccali C, Ciccarelli M, Mallamaci F, et al: Parasympathetic function in haemodialysis
11 World Health Organization: Diabetes Mel-litus: A Report of a WHO Study Group (WHO publication No. 727). Geneva, World Health Organization, 1985.
12 Sato M, Horigome I, Chiba S, et al: Autonom-ic insuffAutonom-iciency as a factor contributing to dialysis-induced hypotension. Nephrol Dial
Transplant 2001; 16: 1657–1662.
13 Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric
mea-surements. Am J Clin Nutr 1980; 33: 27–39.
14 Devereux RB, Alonso DR, Lutas EM, et al: Echocardiographic assessment of the left ventricular hypertrophy: comparison
nec-ropsy findings. Am J Cardiol 1986; 57: 450–
458.
15 Doan AE, Peterson DR, Blackmon JR, et al: Myocardial ischemia after maximal exercise
in healthy men. Am Heart J 1965; 69: 11–21.
16 Fletcher GF, Froelicher VF, Hartley LH, et al: Exercise standards: a statement for health professionals from the American Heart
As-sociation. Circulation 1990; 82: 2286–2322.
17 Nishime EO, Cole CR, Blackstone EH, et al: Heart rate recovery and treadmill exercise score as predictors of mortality in patients
referred for exercise ECG. JAMA 2000; 284:
1392–1398.
18 Lauer MS, Francis GS, Okin PM, et al: Im-paired chronotropic response to exercise stress testing as a predictor of mortality.
JAMA 1999; 281: 524–529.
19 Amon KW, Richards KL, Crawford MH: Usefullness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984;
70: 951–956.
20 Ponikowski PP, Chua TP, Francis DP, et al: Muscle ergoreceptor overactivity reflects de-terioration in clinical status and cardiorespi-ratory reflex control in chronic heart failure.
Circulation 2001; 104: 2324–2330.
21 Christensen NJ, Galbo H: Sympathetic ner-vous activity during exercise. Ann Rev
Physiol 1983; 45: 139–153.
22 Miyahara T, Yokota M, Iwase M, et al: Mech-anism of abnormal postexercise systolic blood pressure response and its diagnostic value in patients with coronary artery
dis-ease. Am Heart J 1990; 120: 40–49.
23 Iellamo F, Pizzinelli P, Massaro M, et al: Muscle metaboreflex contribution to sinus node regulation during static exercise: in-sights from spectral analysis of heart rate
variability. Circulation 1999; 100: 27–32.
24 Converse RL Jr, Jacobsen TN, Jost CM, et al: Paradoxical withdrawal of reflex vasocon-striction as a cause of hemodialysis-induced
hypotension. J Clin Invest 1992; 90: 1657–
1665.
25 Landry DW, Oliver JA: Blood pressure insta-bility during hemodialysis. Kidney Int 2006;
69: 1710–1711.
26 Moore TJ, Lazarus JM, Hakim RM: Reduced angiotensin receptors and pressor responses in hypotensive hemodialysis patients.
Kid-ney Int 1989; 36: 696–701.
27 Coll E, Larrousse M, de la Sierra A, et al: Chronic hypotension in hemodialysis pa-tients: role of functional vascular changes and vasodilator agents. Clin Nephrol 2008;
69: 114–120.
28 Cole CR, Blackstone EH, Pashkow FJ, et al: Heart-rate recovery immediately after exer-cise as a predictor of mortality. N Engl J Med
1999; 341: 1351–1357.
29 Imai K, Sato H, Hori M, et al: Vagally medi-ated heart rate recovery after exercise is ac-celerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol
1994; 24: 1529–1535.
30 Hull SS Jr, Vanoli E, Adamson PB, et al: Do increases in markers of vagal activity imply protection from sudden death? The case of
scopolamine. Circulation 1995; 91: 2516–
2519.
31 Singh JP, Larson MG, Manolio TA, et al: Blood pressure response during treadmill testing as a risk factor for new-onset hyper-tension. The Framingham Heart Study.
Cir-culation 1999; 99: 1831–1836.
32 Lim PO, MacFadyen RJ, Clarkson PB, et al: Impaired exercise tolerance in hypertensive
patients. Ann Intern Med 1996; 124: 41–55.
33 Morrow K, Morris CK, Froelicher VF, et al: Prediction of cardiovascular death in men undergoing noninvasive evaluation for coro-nary artery disease. Ann Intern Med 1993;
118: 689–695.
34 Kodama S, Saito K, Tanaka S, et al: Cardio-respiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a