Contents lists available atScienceDirect
Ecotoxicology and Environmental Safety
journal homepage:www.elsevier.com/locate/ecoenvAssessment of the cytotoxic and genotoxic potential of pillar[5]arene
derivatives by Allium cepa roots and Drosophila melanogaster haemocytes
Recep Liman
a,∗, Ahmed Nuri Kursunlu
b,
İbrahim Hakkı Ciğerci
c, Mustafa Ozmen
b, Yaser Acikbas
daUsak University, Faculty of Arts and Sciences, Molecular Biology and Genetics Department, 64300, Uşak, Turkey bSelcuk University, Faculty of Science, Chemistry Department, 42250, Konya, Turkey
cAfyon Kocatepe University, Faculty of Arts and Sciences, Molecular Biology and Genetics Department, 03200, Afyonkarahisar, Turkey dUsak University, Faculty of Engineering, Materials Science and Nanotechnology Department, 64200, Usak, Turkey
A R T I C L E I N F O Keywords: Pillar[5]arene Allium cepa Comet DNA damage Drosophila melanogaster A B S T R A C T
In this study pillar[5]arene (P5) and a quinoline-functionalized pillar[5]arene (P5-6Q) which is used for de-tecting radioactive element, gas adsorption and toxic ions were synthesized. These materials were characterized by Nuclear Magnetic Resonance (NMR), Fourier Transform Infrared (FTIR), elemental analysis, melting point, Mass Spectroscopy, Scanning Electron Microscopy (SEM) and Zeta Potential. The cytotoxic and genotoxic po-tential ofP5 and P5-6Q at distinct concentrations of 12.5, 25, 50, and 100μg/mL were also investigated by Allium ana-telophase and comet assays on Allium cepa roots and Drosophila melanogaster haemocytes.P5 and P5-6Q showed dose dependent cytotoxic effect by decreasing mitotic index (MI) and genotoxic effect by increasing chromosomal aberrations (CAs such as disturbed anaphase-telophase, polyploidy, stickiness, chromosome lag-gards and bridges) and DNA damage at the exposed concentrations. These changes inP5-6Q were lower than P5. Further research is necessary to clarify the cytotoxic and genotoxic action mechanisms ofP5 and P5-6Q at molecular levels.
1. Introduction
Pillararene macrocycles are new headliners of supramolecular chemistry following to cucurbiturils, cyclodextrins, calixarenes, and crown ethers. The excellent symmetrical nature and practical functio-nalization of these macrocycles have afforded them unique properties such as excellent host-guest, high stability, multi-functionality, etc. In the construction of interesting supramolecular interactions (Nierengarten et al., 2016;Ogoshi et al., 2013). Day by day, the new studies on pillararene gain importance in many applications such as material chemistry, biochemistry and pharmacology due to their fas-cinating inter and intra molecules interactions. Most of these studies focused on host-guest principle while it is limited in their interaction with DNA (Nierengarten et al., 2013;Yakimova et al., 2018). Quinoline (1-Azanaphthalene, benzo[b]pyridine) and quinolone derivatives which are found many synthetic and natural products showed many biologic activities such as antimalarial, analgesic, anti-inflammatory, antineoplastic, antibacterial, antifungal, anticancer and anthelmintic activity etc. (Chu et al., 2019;Marella et al., 2013).P5 having quinoline fragments has been synthesized for as radioactive element (Fang et al., 2015), gas adsorption (Kursunlu et al., 2017) and toxic ions (Ma et al.,
2019; Yang et al., 2019) detection. Limited information is available about toxicity and harmful effects of pillararene derivatives.
Allium ana-telophase test is widely used to determine of cytotoxic and genotoxic effects of chemicals. It is a quick, easy, highly accurate and reproducible. It shows good correlation with other test systems. They have large size cells containing large sized chromosomes (2n = 16). So they are suitable for checking mitotic phases and CAs during mitosis. It is also accepted as a standard assay by the United Nations Environmental Programme, the United States Environmental Protection Agency and the World Health Organization. (Firbas and Amon, 2014;Leme and Marin-Morales, 2009;Liman et al., 2018;Ma et al., 2005;Palmieri et al., 2016;Rank and Nielsen, 1994).
A. cepa roots and D. melanogaster haemocytes have been extensively used for the measurement of the DNA damage by comet assay which is also known single cell gel electrophoresis. Because this technique is relatively simple, sensitive, versatile and low cost (Ávalos et al., 2018; Carmona et al., 2016;Kaygisiz and Cigerci, 2017;Liman et al., 2019; Mangalampalli et al., 2018;Pakrashi et al., 2014).
Despite the mentioned superior properties of the arenes, the cyto-toxic and genocyto-toxic effects of these compounds are not yet known sufficiently. Since cytotoxic and genotoxic effects are generally studied
https://doi.org/10.1016/j.ecoenv.2020.110328
Received 12 October 2019; Received in revised form 6 February 2020; Accepted 12 February 2020
∗Corresponding author. Uşak University, Faculty of Arts and Sciences, Molecular Biology and Genetics Department, 1 Eylül Campus, 64300, Usak. Turkey..
E-mail addresses:rliman@hotmail.com,recep.liman@usak.edu.tr(R. Liman).
Ecotoxicology and Environmental Safety 192 (2020) 110328
Available online 17 February 2020
0147-6513/ © 2020 Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
T
with commercial substances, a new generation macrocycle was used in this study. We preferred to bind quinoline on the deca-arms of the pillar [5]arene, thus provides dynamic inter&intra molecular interactions or hydrogen bonds that easily capable of participating in inclusion com-plexation form. The objective of this study is to evaluate the cytotoxic and genotoxic effects of pillar[5]arene macrocycles (P5 and P5-6Q) on A. cepa roots by Allium ana-telophase and comet assay and on D. mel-anogaster haemocytes by comet assay. Important objectives for future CAs and DNA damage applications of pillar[n]arenes is to scrutinize the cytotoxic and genotoxic effect of the pillar[n]arene derivatives and to extend this strategy to different useful macrocycles.
2. Materials and methods 2.1. Chemicals
All chemical materials were used as supplied from chemical fac-tories (Sigma-Aldrich, Acros, Alfa-Aesar) without further purification. Thin Layer Chromatography (TLC) was performed on aluminum sheets coated with silica gel 60 F254(Merck). Silica gel (0.040–0.200 mm) for
column chromatography was supplied from Sigma-Aldrich. The fol-lowing abbreviations were used to define the multiplicities: s = singlet, d = doublet, t = triplet, m = multiplet, and their combinations. 2.2. Instruments
The main macrocycle has been prepared by known steps (Atacan et al., 2019). Starting from commercially available material 1,4-bis(2-iodoethoxy)benzene as given inScheme 1. Iodo-alkylation of 1,4-bis(2-iodoethoxy)benzene was carried out with carbon tetraiodide using tri-phenylphosphine in acetonitrile to afford di-iodo derivative, 1,4-bis(2-iodoethoxy)benzene. The cyclized derivative of pillar[5]arene in-cluding deca-iodine was prepared by the reaction of 1,4-bis(2-io-doethoxy)benzene with paraformaldehyde and borontrifluoride die-thyletherate in dichloroethane. The final product, P5-6Q, and its
precursor molecules were well characterized by 1H and 13C NMR (Varian 400 MHz), FT-IR (Bruker), mass spectroscopy (Bruker) and elemental analysis (Truspec) (in Supp. Data).
2.3. The synthesis of pillar[5]arene including deca-iodine
As a known procedure (Atacan et al., 2019), paraformaldehyde (1.02 g, 36 mmol) and 1,4-bis(2-iodoethoxy)benzene (5.64 g, 13.5 mmol) were dissolved in dichloroethane (100 mL) and then BF3.O
(C2H5)2(1.6 g, 13.5 mmol) was added to this mixture under argon
at-mosphere and the reaction mixture was stirred at room temperature for 3 h. Water (300 mL) was then added following the completing of re-action for the extrre-action and the organic phase was separated. Organic phase was dried with Na2SO4and the solvents evaporated. The raw
product was purified by column chromatography (silica gel; cyclo-hexane/dichloromethane) to afford pillar[5]arene including di-iodine (2 g, 37%) as a white solid. M.P.: 276 °C.1H NMR (400 MHz, CDCl
3, r.
t.)δ (ppm): 6.92 (s, 10H), 4.33 (t, 20H, J = 5.4 Hz), 3.87 (s, 10H), 3.52 (t, 20H, J = 5.4 Hz).13C NMR (100 MHz) δ (ppm): 149.91, 126.02,
116.35, 67.45, 30.01, 3.90. Elemental Analysis calcd.: C55H60I10O10: C,
30.71; H, 2.82; found: C, 30.60; H, 3.02. 2.4. The synthesis of P5-6Q
A mixture of potassium carbonate (1.66 g, 12 mmol), 6-hydro-xyquinoline (0.6 g, 4.2 mmol), and pillar[5]arene including deca-iodine (0.86 g, 0.4 mmol) in acetonitrile (120 mL) were refluxed for 72 h under argon atmosphere. The reaction mixture was cooled at room temperature, residue wasfiltered, and the organic phase was evapo-rated under vacuum. The product was purified by chromatography (silica gel; cyclohexane/dichloromethane) followed by recrystallization from ethyl acetate to afford pillar[5]arene-quinoline (0.73 g, 79%) as a white solid. M.P.: 103 °C. 1H-NMR (400 MHz, DMSO‑d
6, room
tem-perature)δ (ppm): 8.67 (d, 10H), 7.75 (d, 10H) 7.65 (d, 10H), 7.37 (d, 10H), 7.35–7.10 (m, 20H), 7.05 (s, 10H), 3.82 (m, 30H), 3.51 (t, 20H).
13
C-NMR (100 MHz)δ (ppm): 157.44, 150.51, 146.42, 144.61, 139.22, 135.78, 132.89, 129.48, 129.15, 124.47, 123.99, 112.24, 105.57, 67.24, 65.69, 30.11. Elemental analysis calcd. C145H120N10O20: C,
74.98; H, 5.21; N, 6.03; found: C, 75.12; H, 5.78; N, 6.02. [M+H]+:
2324.2.
2.5. Characterization of P5 and P5-6Q
Scanning electron microscope (SEM) images were taken with LEO 1430 VP SEM. 20 kV wasfixed as the value of acceleration voltage in SEM instrument and all samples were gold sputtered before analyses. The zeta potential values were obtained at 25 °C using Zetasizer Malvern Nano ZS. Zeta-potential was used to investigate surface elec-trical characteristic properties of P5 and P5-6Q in ultrahigh-purity water. Zetasizer (Version 7.11) were also used to investigate the size distribution of the P5 and P5-6Q particles in the distilled water.
2.6. A. cepa anaphase-telophase test
The A. cepa anaphase-telophase test was carried out following the modified protocol ofRank (2003)in the root meristem cells of A. cepa as described inLiman et al. (2019). Healthy and equal-sized A. cepa bulbs (25–30 mm in diameter) were purchased from local market. The outer shells of the bulbs and dried roots were removed. When the onion roots reached 2–3 cm after 2 days, they were exposed to 4 randomly selected doses ofP5 and P5-6Q (12.5, 25, 50 and 100μg/mL) that were directly suspended in distilled water and dispersed by ultrasonic vi-bration (Bandelin Sonorex Digitec DT100, Germany, 320 W, 35 kHz) for 30 min before treatment, distilled water (negative control) and 10μg/ mL of MMS (positive control) for 4 h at the room temperature (21 ± 4 °C). Application time was based on previously published data (Rajeshwari et al., 2016;Saha and Gupta, 2017). The root tips (~1 cm, 3 roots from 5 onions per application) were fixed in ethanol:glacial acetic acid (3:1, v/v) overnight at 4 °C then stored in alcohol (70%) at 4 °C. Fixed root tips were hydrolyzed in 2 mL of 1 N HCl for 8–10 min at 60 °C. Root tips were washed with distilled water and then stained with Feulgen stain for 20–25 min at room temperature. 5000–5200 cells (1000–1040 cells one slide per bulb) and 500 ana-telophase cells (100 ana-telophase cells one slide per bulb) from the dark stained root tips (1–2 mm length) were counted for MI and CAs frequencies by a trino-cular light microscope (Nikon Eclipse Ci-L, Japan)fitted with a CMOS camera (Argenit, Kameram5, Turkey) according toSaxena et al. (2005). The following formulas were used in the calculation of MI, phase index and CA.
= + + +
MI (%) Prophase Metaphase Anaphase Telophase Total number of cells x100
=
+ + +
Phase index (%) Particular phase
Prophase Metaphase Anaphase Telophasex100
=
−
CA (%) Total aberrant cells 100 ana telophase cellsx100
2.7. Drosophila strain and treatments
The wild type strain, Oregon R+, kindly supplied by Prof. Dr. Bülent Kaya of Akdeniz University-Turkey, was used for determining DNA damage by Comet assay. Third instar larvae (72 ± 4 h old) were ex-posed to four concentrations ofP5 and P5-6Q (12.5, 25, 50 and 100μg/ mL) in plastic vials containing of Drosophila instant medium for 24 h at 25 ± 1 °C and ~60% relative humidity. Distilled water and 8 mM CoCl2were used as negative and positive controls, respectively.
2.8. Comet assay
The alkaline comet assay was performed for determining of DNA damage in root meristem cells of A. cepa which were exposed to same concentrations ofP5 and P5-6Q as used for Allium test by following the protocol proposed by Tice et al. (2000)with slight modifications as described inLiman et al. (2019). The treated root tips were gently sliced using razor blade to isolate the nuclei in 600μL ice-cold Tris-MgCl2
buffer (4 mM MgCl2–6H2O, 0.2 M Tris, 0.5% w/v Triton X-100, pH 7.5)
and thenfiltered through 60 μm meshes Nylon filter. They were cen-trifuged at 1200 rpm for 7 min at 4 °C. Pellet was suspended with PBS. Nuclei suspension (50 μL) was mixed with 1.5% low melting point agarose in PBS (50μL) at 37 °C and pipetted over 1% normal melting point agarose coated slides. Slides were covered with coverslips and kept on ice for 5 min.
D. melanogaster larvae hematocytes were obtained according to Irving et al. (2005). Larvae (96 ± 4 h old) were collected from food media, washed in water, sterilized in 5% sodium hypochlorite solution for quickly. 60–80 larvae were used for hematocytes isolation. Larvae were disrupted with two forceps and the haemolymph and circulating haemocytes were directly collected in cold PBS solution containing 0.07% PTU. Haemolymph was centrifuged at 300×g for 10 min at 4 °C; the supernatant was discarded and the haemocyte pellet was suspended in 20μL of cold PBS. The comet assay was performed as previously described (Singh et al., 1988) with minor modifications as described in Carmona et al. (2011). Cells were carefully resuspended in 80μL of 0.75% low melting point agarose, layered onto microscope slides coated one day before with 100μL of 1% normal melting point agarose. Two gels were mounted on each slide and covered with a coverslip. Immediately after agarose solidification (10 min, 4 °C), the coverslips were removed and the slides were immersed in cold, freshly made lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, 1% Triton X-100
and 1% N-lauroylsarcosinate, pH 10) for 2 h at 4 °C in a dark chamber. Other steps of the experiment were performed both the Allium and Drosophila comet assays as follow procedure. The slides were placed in alkaline buffer (1 mM EDTA and 300 mM NaOH, pH > 13) at 4 °C for 20 min and electrophoresed at 25 V and 300 mA for 20 min at 4 °C. Slides were neutralized with 0.4 M Tris (pH 7.5) thrice and stained with 70μL EtBr solution (20 μg/mL) for 5 min. DNA damage expressed as arbitrary unit (AU) [classified to 5 class; 0: undamaged, 1: mild da-mage, 2: moderate dada-mage, 3: severe dada-mage, 4: complete damage] was analyzed on randomly selected 50 nuclei per each slide by using BAB fluorescence microscope (TAM-F, Turkey) according toKocyigit et al. (2015)as shown in Supp. Data. Each treatment was repeated thrice.
2.9. Statistical analysis
The values are expressed as mean ± standard deviation. MI, mi-totic phases, CAs and DNA damage results were analyzed using One-way ANOVA with Duncan's multiple comparison test at p < 0.05. Pearson correlation test was also applied to between dose-response and time-response relationships. All the statistical analyses were performed using SPSS ver. 23.
3. Results and discussion
1H-NMR of 1,4-bis(2-iodoethoxy)benzene, the signals observed at
3.52 ppm (triplet) and 4.33 ppm (triplet) and 6.92 ppm (singlet) for aliphatic and aromatic protons, respectively. (in Supp. Data). The tri-plet peaks of–CH2 fragments attached to oxygen atoms at 4.33 and
3.52 ppm closed to each other in new macrocycle observed at 3.87 and 3.51 ppm. In 1H-NMR of pillar[5]arene including deca-iodine, the methylene bridge protons appeared at 3.87 ppm as triplet, so that same peak was observed at 3.83 ppm in spectrum ofP5-6Q. In P5-6Q com-pound, the aromatic hydrogen peaks on quinoline unit raised between 7
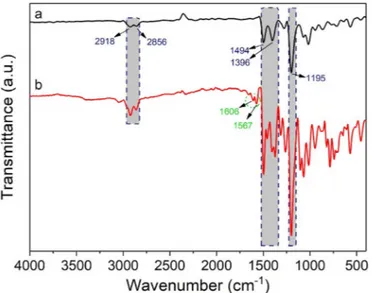
and 9 ppm in different splitting's (singlet, doublet or triplet). Moreover, the FT-IR spectra of including deca-iodine and pillar[5]arene-quinoline were recorded by FT-IR spectrometry as given inFig. 1. The small vi-brations between 2918 and 2856 cm−1in the FT-IR spectra of both pillar[5]arene including deca-iodine and pillar[5]arene-quinoline was assigned to the aliphatic and aromatic C–H stretching vibrations in –CH or–CH2moieties. Moreover, the sharp broad band at 1195 cm−1
cor-responds to the etheric C–O–C stretching in the FT-IR spectrum of pillar [5]arene including deca-iodine. This broad band was observed as more broadly in the spectrum of pillar[5]arene-quinoline. Similarly, the C]C stretching bands around 1500 cm−1 were observed in a wider and Fig. 1. FT-IR spectra of P5 including deca-iodine (a) and P5-6Q (b).
Fig. 2. SEM images of surface adhesion of P5 and P5-6Q on Allium root; (a) control root showing the absence of particle adhesion to the surface, (b) P5 and c) P5-6Q. Table 1
Characterization ofP5 and P5-6Q. Particles Average
diameter ± SD (μm)
PdI Zeta potential ± SD (mV) Electrophoretic mobility (μm cm s−1V−1) P5 0.901 ± 0.19 0.797 −12.73 ± 1.28 −9.96 P5-6Q 1.970 ± 0.26 0.460 −7.65 ± 0.18 −5.99 SD: Standard Deviation.
multiple form in the spectrum of pillar[5]arene-quinoline. The vibra-tion bands in lower frequency from 1600 cm−1 in the spectrum (Fig. 1b) were attributed to C]N stretching in quinoline units. All changes were approved that 6-hydroxyquinolines bound to main pillar [5]arene skeleton.
Fig. 2a refers to control sample forP5 and P5-6Q, while the re-vealed adsorption and treated roots ofP5 and P5-6Q to the root surface are given in Fig. 2b and c, respectively. Zetasizer was used to in-vestigate the physicochemical properties ofP5 and P5-6Q particles and the obtained values are given inTable 1. The average diameter results display that the mean diameter ofP5-6Q (1.97 ± 0.26μm) larger than P5 (0.901 ± 0.19μm) measured in purity water suspension. This re-sult can be interpreted that the effect of particle agglomeration on the formation of P5 in purity water is lower when compared with the for-mation ofP5-6Q. The Zeta potential (ζ), PdI and electrophoretic mo-bility values ofP5 and P5-6Q in water werefixed or calculated and all results displayed inTable 1. FromTable 1,P5 and P5-6Q gives negative zeta potential value and these results can be explained with electrical properties ofP5 particle. This particle stored negative charges due to functionalization with 1,4-bis(2-iodoethoxy)benzene of P5. The inside surface of P5 cavity is negative due to the ethoxy functional groups on the skeletons contains electron-donating group. All results are reported as an average value of 3 measurements.
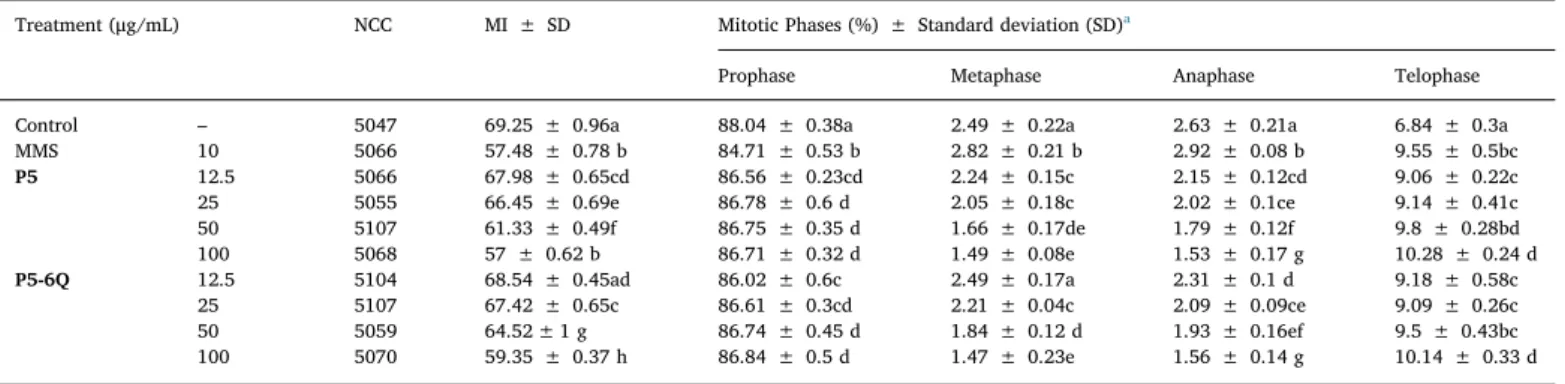
The effect of P5 and P5-6Q on mitotic and phase indices in A. cepa root tip cells is shownTable 2.P5 and P5-6Q reduced the MI in a dose dependent manner (r = −0.973 p = 0.01 for P5 and r = −0.945 p = 0.01 forP5-6Q). P5-6Q decreased the MI values less than P5 and these decreases were found to be statistically significant except 12.5 μg/ mL. P5 and P5-6Q increased anaphase index and decreased other
indices statistically (except 12.5μg/mL of P5-6Q at metaphase). The changes of cell cycle such as inhibition of DNA synthesis (Sudhakar et al., 2001) or blocking of G2 phase (El-Ghamery et al., 2000), che-mical(s) mitodepressive activity (Sharma and Vig, 2012), or inhibition of DNA polymerase by the inhibition of specific protein (Hidalgo et al., 1989), or mitotic stage duration changes (Chauhan and Gupta, 2005) and ROS disturbance homeostasis (Livanos et al., 2012) can be caused to a significant decrease in MI. Amphiphilic pillar[5]arene containing nanotubes showed also low toxicity in lung cancer cells and Chinese hamster lungfibroblast cell (Yu et al., 2013). Water-soluble pillar[5] arenes containing amide and ammonium fragments 2 and 3 showed low cytotoxicity only at the highest concentrations studied (500 μg/mL) against A549 cells reducing cell viability to 70 and 73%, respectively (Skvortsova et al., 2018). Unlike our result, some of the polycationic dendritic pillar[5]arene derivatives (Nierengarten et al., 2013) and hydrazino-pillar[5]arene functionalized graphene (Mao et al., 2016), which are soluble in the water showed very low toxicity. Gibberellin acid loaded water soluble carboxylated pillar[5]arenes induced the growth of Arabidopsis thaliana and cabbage (Li et al., 2019). Recently, three water-soluble pillar[5]arenes were also synthesized as plant growth regulators for wheat to induce the growth rate of buds and roots in the seedling cultivation stage (Shangguan et al., 2019).
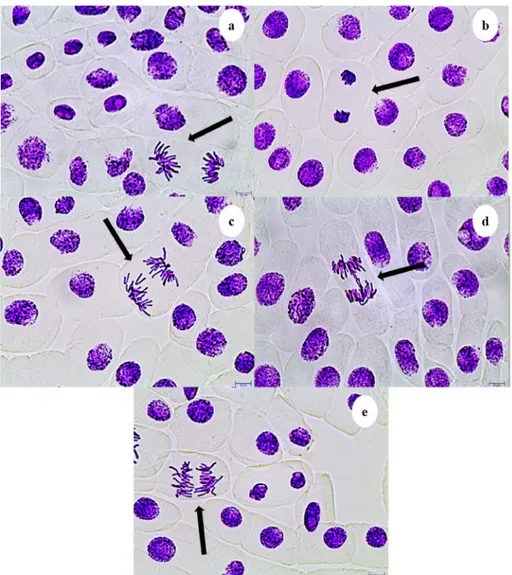
A dose dependent increase of CAs in A. cepa root anaphase-telo-phase cells with P5 (r = 0.931 p = 0.01) and P5-6Q (r = 0.902 p = 0.01) treatment was observed (Table 3andFig. 3). Total CAs forP5 was found higher thanP5-6Q. The most common CA was a disturbed ana-telophase (3.2% at 100 μg/mL of P5, Fig. 3a), and the least common CA was the chromosome laggards (0.8% at 12.5μg/mL of P5-6Q,Fig. 3c), which may be caused by degraded microtubules or failure of chromosome to move toward the poles (Evseeva et al., 2005;Kumari et al., 2009;Rajeshwari et al., 2016;Singh and Roy, 2017). Stickiness (Fig. 3b), an indicator of toxicity; may be caused by formation cross-linking of DNA-DNA or DNA-protein (Amin, 2002; Barbério et al., 2011). Anaphase bridges (Fig. 3d) may cause chromosome laggards by showing clastogenic effect due to formation of dicentric chromosomes, stickiness, changes in replication enzyme activity, breakage or fusion of chromosomes and unequal chromatid exchange (Dutta et al., 2016; El-Ghamery et al., 2000;Luo et al., 2004). Polyploidy (Fig. 3e) may result from abnormal segregation of chromosomes during the cell division (Nefic et al., 2013;Palsikowski et al., 2018).
P5 and P5-6Q (except 12.5μg/mL for P5-6Q in D. melanogaster) significantly induced DNA damage in A. cepa roots and D. melanogaster haemocytes (Table 4). DNA damage ranged from 124.33 ± 2.52 to 133.33 ± 2.52 forP5 and 82.67 ± 0.58 to 126 ± 3 for P5-6Q in A. cepa roots. These values were found 7.67 ± 0.58 to 21 ± 2 forP5 and 4.67 ± 0.58 to 17.67 ± 1.15 forP5-6Q in D. melanogaster. Pillar[5] arenes containing amide and ammonium fragments did not show mu-tagenic activity in Salmonella typhimurium TA100 in the concentration range of 100–500 μg/plate (Skvortsova et al., 2018).
Table 2
Mitotic index and mitotic phases changes in A. cepa meristematic cells treated withP5 and P5-6Q.
Treatment (μg/mL) NCC MI ± SD Mitotic Phases (%) ± Standard deviation (SD)a
Prophase Metaphase Anaphase Telophase
Control – 5047 69.25 ± 0.96a 88.04 ± 0.38a 2.49 ± 0.22a 2.63 ± 0.21a 6.84 ± 0.3a
MMS 10 5066 57.48 ± 0.78 b 84.71 ± 0.53 b 2.82 ± 0.21 b 2.92 ± 0.08 b 9.55 ± 0.5bc P5 12.5 5066 67.98 ± 0.65cd 86.56 ± 0.23cd 2.24 ± 0.15c 2.15 ± 0.12cd 9.06 ± 0.22c 25 5055 66.45 ± 0.69e 86.78 ± 0.6 d 2.05 ± 0.18c 2.02 ± 0.1ce 9.14 ± 0.41c 50 5107 61.33 ± 0.49f 86.75 ± 0.35 d 1.66 ± 0.17de 1.79 ± 0.12f 9.8 ± 0.28bd 100 5068 57 ± 0.62 b 86.71 ± 0.32 d 1.49 ± 0.08e 1.53 ± 0.17 g 10.28 ± 0.24 d P5-6Q 12.5 5104 68.54 ± 0.45ad 86.02 ± 0.6c 2.49 ± 0.17a 2.31 ± 0.1 d 9.18 ± 0.58c 25 5107 67.42 ± 0.65c 86.61 ± 0.3cd 2.21 ± 0.04c 2.09 ± 0.09ce 9.09 ± 0.26c 50 5059 64.52 ± 1 g 86.74 ± 0.45 d 1.84 ± 0.12 d 1.93 ± 0.16ef 9.5 ± 0.43bc 100 5070 59.35 ± 0.37 h 86.84 ± 0.5 d 1.47 ± 0.23e 1.56 ± 0.14 g 10.14 ± 0.33 d
a Different letters in the same columns are statistically significant (P ≤ 0.05). NCC: Number of Cells Counted.
Table 3
Ana-telophase anomalies in A. cepa root meristematic cells treated withP5 and P5-6Q.
Treatment (μg/mL) NCC Anaphase-Telophase Anomalies %
DAT CL S AB P TA ± SDa Control – 500 2 0.8 1 1 0.8 5.6 ± 0.55a MMS 10 500 2.8 2.4 2.4 1.8 2.4 11.8 ± 0.45b P5 12.5 500 2.4 1 1.4 1.2 1.6 7.6 ± 0.55cd 25 500 2.8 1.2 1.6 1.6 1.8 9 ± 0.71e 50 500 2.8 1.6 1.8 1.6 2 9.8 ± 0.45 fg 100 500 3.2 1.6 2.2 1.8 2.6 11.4 ± 0.55b P5-6Q 12.5 500 2.4 0.8 1.2 1.2 1.4 7 ± 0.71c 25 500 2.6 1.2 1.4 1.2 1.8 8.2 ± 0.84d 50 500 2.8 1.2 1.6 1.6 2 9.2 ± 0.45ef 100 500 3 1.6 1.8 1.6 2.2 10.2 ± 0.45g SD: Standard Deviation NCC: Number of Cells Counted DAT: Disturbed Anaphase-Telophase CL: Chromosome Laggards S: Stickiness AB: Anaphase Bridge P: Polyploidy TA: Total Anomalies.
4. Conclusion
We have prepared two pillar[5]arene based macrocycle derivatives in this study to examine their cytotoxic and genotoxic potential. The results of the present study showed thatP5 and P5-6Q induced toxicity by reducing MI and genotoxicity by increasing CAs and DNA damage in
A. cepa roots and D. melanogaster haemocytes. Further research is ne-cessary to clarify the cytotoxic and genotoxic action mechanisms ofP5 andP5-6Q at molecular levels. Since they have potential harmful ef-fects as well as beneficial effects, their use should be taken into con-sideration.
Author contributions
This work was carried out in collaboration between all authors. “Authors ANK and MO” synthesized and characterized P5 and P5-6Q. “Author YA” investigated the physicochemical properties of the P5 and P5-6Q in the distilled water.“Authors RL and İHC” performed A. cepa ana-telophase and Comet assays. All authors discussed the results, commented on the manuscript and approved thefinal manuscript. Acknowledgements
We are thankful to Research Foundation of Selcuk University (BAP-192019) for partially supporting. The authors express their apprecia-tion to Prof. J.-F. Nierengarten and Dr. I. Nierengarten for helpful dis-cussions.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// Fig. 3. CAs induced by P5 and P5-6Q observed in Allium ana-telophase cells a: Disturbed ana-telophase, b: Stickiness, c: Chromosome laggards, d: Anaphase bridge, e: Polyploidy. Scale bars 10μm.
Table 4
P5 and P5-6Q induced DNA damage in A. cepa roots and D. melanogaster hae-mocytes.
Treatment Concentration (μg/ mL)
DNA Damage (Arbitrary Unit ± SD)a
A. cepa D. melanogaster Negative control – 12.67 ± 1.53a 3.67 ± 1.15a MMS CoCl2 10 8 mM 148 ± 2 b - - 28 ± 1,73 b P5 12.5 124.33 ± 2.52c 7,67 ± 0,58c 25 125.67 ± 1.53cd 10.33 ± 1,15de 50 129 ± 1 d 12 ± 1e 100 133.33 ± 2.52e 21 ± 2 g P5-6Q 12.5 82.67 ± 1.53f 4,67 ± 0,58a 25 90.33 ± 3.06 g 7,67 ± 1,15c 50 104 ± 4 h 9,33 ± 0,58cd 100 126 ± 3cd 17.67 ± 1,15f
a Different letters in the same columns are statistically significant (P ≤ 0.05).
doi.org/10.1016/j.ecoenv.2020.110328. References
Amin, A.W., 2002. Cytotoxicity testing of sewage water treatment using Allium cepa chromosome aberration assay. Pakistan J. Biol. Sci. 5, 184–188.
Atacan, K., Kursunlu, A.N., Ozmen, M., 2019. Preparation of pillar[5]arene immobilized trypsin and its application in microwave-assisted digestion of cytochrome c. Mater. Sci. Eng. 94, 886–893.
Ávalos, A., Haza, A.I., Mateo, D., Morales, P., 2018. In vitro and in vivo genotoxicity as-sessment of gold nanoparticles of different sizes by comet and SMART assays. Food Chem. Toxicol. 120, 81–88.
Barbério, A., Voltolini, J.C., Mello, M.L.S., 2011. Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicology 20, 927–935.
Carmona, E.R., Creus, A., Marcos, R., 2011. Genotoxicity testing of two lead-compounds in somatic cells of Drosophila melanogaster. Mutat. Res-Gen. Tox. En. 724, 35–40.
Carmona, E.R., Inostroza-Blancheteau, C., Rubio, L., Marcos, R., 2016. Genotoxic and oxidative stress potential of nanosized and bulk zinc oxide particles in Drosophila melanogaster. Toxicol. Ind. Health 32, 1987–2001.
Chauhan, L.K.S., Gupta, S.K., 2005. Combined cytogenetic and ultrastructural effects of substituted urea herbicides and synthetic pyrethroid insecticide on the root meristem cells of Allium cepa. Pestic. Biochem. Physiol. 82, 27–35.
Chu, X.M., Wang, C., Liu, W., Liang, L.L., Gong, K.K., Zhao, C.Y., Sun, K.L., 2019. Quinoline and quinolone dimers and their biological activities: an overview. Eur. J. Med. Chem. 161, 101–117.
Dutta, J., Ahmad, A., Singh, J., 2016. Study of industrial effluents induced genotoxicity on Allium cepa L. Caryologia 71, 39–145.
El-Ghamery, A.A., El-Nahas, A.I., Mansour, M.M., 2000. The action of atrazine herbicide as an indicator of cell division on chromosomes and nucleic acid content in root meristems of Allium cepa and Vicia faba. Cytologia 65, 277–287.
Evseeva, T.I., Geras’kin, S.A., Shuktomova, I.I., Taskaev, A.I., 2005. Genotoxicity and cytotoxicity assay of water sampled from the underground nuclear explosion site in the north of the perm region (Russia). J. Environ. Radioact. 80, 59–74.
Fang, Y., Li, C., Wu, L., Bai, B., Li, X., Jia, Y., Yuan, L., 2015. A non-symmetric pillar [5] arene based on triazole-linked 8-oxyquinolines as a sequential sensor for thorium (IV) followed byfluoride ions. Dalton Trans. 44, 14584–14588.
Firbas, P., Amon, T., 2014. Chromosome damage studies in the onion plant Allium cepa L. Caryologia 67, 25–35.
Hidalgo, A., Gonzales-Reyes, J.A., Navas, P., Garcia-Herdugo, G., 1989. Abnormal mitosis and growth inhibition in Allium cepa roots induced by propham and chlorpropham. Cytobios 57, 7–14.
Irving, P., Ubeda, J.M., Doucet, D., Troxler, L., Lagueux, M., Zachary, D., Hoffmann, J.A., Hetru, C., Meister, M., 2005. New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol. 7, 335–350.
Kaygisiz, S.H., Cigerci, I.H., 2017. Genotoxic evaluation of different sizes of iron oxide nanoparticles and ionic form by SMART, Allium and comet assay. Toxicol. Ind. Health 33, 802–809.
Kocyigit, A., Keles, H., Selek, S., Guzel, S., Celik, H., Erel, O., 2015. Increased DNA da-mage and oxidative stress in patients with cutaneous leishmaniasis. Mutat. Res-Gen. Tox. En. 585, 71–78.
Kumari, M., Mukherjee, A., Chandrasekaran, N., 2009. Genotoxicity of silver nano-particles in Allium cepa. Sci. Total Environ. 407, 5243–5246.
Kursunlu, A.N., Acikbas, Y., Ozmen, M., Erdogan, M., Capan, R., 2017. Preparation of pillar [5] arene-quinoline Langmuir–blodgett thin films for detection of volatile or-ganic compounds with host–guest principles. Analyst 142, 3689–3698.
Leme, D.M., Marin-Morales, M.A., 2009. Allium cepa test in environmental monitoring: a review on its application. Mutat. Res. Rev. Mutat. 682, 71–81.
Li, X., Han, J., Wang, X., Zhang, Y., Jia, C., Qin, J., Wang, C., Wu, J.-R., Fang, W., Yang, Y.-W., 2019. A triple-stimuli responsive hormone delivery system equipped with pillarene magnetic nanovalves. Mater. Chem. Front. 3, 103–110.
Liman, R., Acikbas, Y., Ciğerci, İ.H., 2019. Cytotoxicity and genotoxicity of cerium oxide micro and nanoparticles by allium and comet tests. Ecotoxicol. Environ. Saf. 168, 408–414.
Liman, R., Ciğerci, İ.H., Gökçe, S., 2018. Cytogenetic and genotoxic effects of rosmaniric acid on Allium cepa L. root meristem cells. Food Chem. Toxicol. 121, 444–449.
Livanos, P., Galatis, B., Quader, H., Apostolakos, P., 2012. Disturbance of reactive oxygen species homeostasis induces a typical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cyto. Hoboken. 69, 1–21.
Luo, L.Z., Werner, K.M., Gollin, S.M., Saunders, W.S., 2004. Cigarette smoke induces anaphase bridges and genomic imbalances in normal cells. Mutat. Res-Fund. Mol. M. 554, 375–385.
Ma, T.H., Cabrera, G.L., Owens, E., 2005. Genotoxic agents detected by plant bioassays. Rev. Environ. Health 20, 1–14.
Ma, X.Q., Wang, Y., Wei, T.B., Qi, L.H., Jiang, X.M., Ding, J.D., Lin, Q., 2019. A novel AIE chemosensor based on quinoline functionalized Pillar[5]arene for highly selective
and sensitive sequential detection of toxic Hg2+and CN−. Dyes Pigments 164, 279–286.
Mangalampalli, B., Dumala, N., Grover, P., 2018. Allium cepa root tip assay in assessment of toxicity of magnesium oxide nanoparticles and microparticles. J. Environ. Sci. 66, 125–137.
Mao, X., Liu, T., Bi, J., Luo, L., Tian, D., Li, H., 2016. The synthesis of pillar [5] arene functionalized graphene as afluorescent probe for paraquat in living cells and mice. Chem. Commun. 52, 4385–4388.
Marella, A., Tanwar, O.P., Saha, R., Ali, M.R., Srivastava, S., Akhter, M., Alam, M.M., 2013. Quinoline: a versatile heterocyclic. Saudi Pharmaceut. J. 21, 1–12.
Nefic, H., Musanovic, J., Metovic, A., Kurteshi, K., 2013. Chromosomal and nuclear al-terations in root tip cells of Allium cepa L. induced by alprazolam. Med. Arh. 67, 388–392.
Nierengarten, I., Deschenaux, R., Nierengarten, J.F., 2016. From Pillar[n]arene scaffolds for the preparation of nanomaterials to Pillar[5]arene- containing rotaxanes. Chimia 70, 61–66.
Nierengarten, I., Nothisen, M., Sigwalt, D., Biellmann, T., Holler, M., Remy, J.S., Nierengarten, J.F., 2013. Polycationic Pillar[5]arene derivatives: interaction with DNA and biological applications. Chem. Eur J. 19, 17552–17558.
Ogoshi, T., Yamafuji, D., Akutsu, T., Naito, M., Yamagishi, T., 2013. Achiral guest-in-duced chiroptical changes of a planar-chiral pillar[5]arene containing one pi-con-jugated unit. Chem. Commun. (J. Chem. Soc. Sect. D) 49, 8782–8784.
Pakrashi, S., Jain, N., Dalai, S., Jayakumar, J., Chandrasekaran, P.T., Raichur, A.M., Mukherjee, A., 2014. In vivo genotoxicity assessment of titanium dioxide nano-particles by Allium cepa root tip assay at high exposure concentrations. PloS One 9, e87789.
Palmieri, M.J., Andrade-Vieira, L.F., Trento, M.V.C., de Faria Eleutério, M.W., Luber, J., Davide, L.C., Marcussi, S., 2016. Cytogenotoxic effects of spent pot liner (SPL) and its main components on human leukocytes and meristematic cells of Allium cepa. Water, Air, Soil Pollut. 227, 156.
Palsikowski, P.A., Roberto Sommaggio, M.M., Souza, L.R., Morales, P.M., Marin, A.R., Morales, M.A., 2018. Ecotoxicity evaluation of the biodegradable polymers PLA, PBAT and its blends using Allium cepa as test organism. J. Polym. Environ. 26, 938–945.
Rajeshwari, A., Suresh, S., Chandrasekaran, N., Mukherjee, A., 2016. Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay. Adv 6, 24000–24009.
Rank, J., 2003. The method of allium anaphase-telophase chromosome aberration assay. Ekologija 1, 38–42.
Rank, J., Nielsen, M.H., 1994. Evaluation of the allium anaphase-telophase test in relation to genotoxicity screening of industrial wastewater. Mutat. Res-Environ. Mut. 312, 17–24.
Saha, N., Gupta, S.D., 2017. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips andflower buds of Allium cepa. J. Hazard Mater. 330, 18–28.
Saxena, P.N., Chauhan, L.K.S., Gupta, S.K., 2005. Cytogenetic effects of commercial for-mulation of cypermethrin in root meristem cells of Allium sativum: spectroscopic basis of chromosome damage. Toxicology 216, 244–252.
Shangguan, L., Shi, B., Chen, Q., Li, Y., Zhu, H., Liu, Y., Huang, F., 2019. Water-soluble pillar [5] arenes: a new class of plant growth regulators. Tetrahedron Lett. 60, 150949.
Sharma, S., Vig, A.P., 2012. Genotoxicity of atrazine, avenoxan, diuron and quizalofop-P-ethyl herbicides using the Allium cepa root chromosomal aberration assay. Terr. Aquat. Environ. Toxicol. 6, 90–95.
Singh, D., Roy, B.K., 2017. Evaluation of malathion-induced cytogenetical effects and oxidative stress in plants using Allium test. Acta Physiol. Plant. 39, 92–102.
Singh, N.P., McCoy, M.T., Tice, R.R., Schneider, E.L., 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191.
Skvortsova, P.V., Gruzdeva, E.V., Faizullin, D.A., Shurpik, D.N., Evtugyn, V.G., Zelenikhin, P.V., Khairutdinov, B.I., 2018. The interaction of water-soluble Pillar [5] Arenes containing amide and ammonium fragments with lipid bilayer.
BioNanoScience 8, 888–894.
Sudhakar, R., Ninge Gowda, K.N., Venu, G., 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia 66, 235–239.
Tice, R.R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., Sasaki, Y.F., 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206–221.
Yakimova, L.S., Shurpik, D.N., Guralnik, E.G., Evtugyn, V.G., Osin, Y.N., Stoikov, I.I., 2018. Fluorescein-Loaded solid lipid nanoparticles based on monoamine Pillar[5] arene: synthesis and interaction with DNA. Chem. Nano. Mat. 4, 919–923.
Yang, H.L., Dang, Z.J., Zhang, Y.M., Wei, T.B., Yao, H., Zhu, W., Lin, Q., 2019. Novel cyanide supramolecularfluorescent chemosensor constructed from a quinoline hy-drazone functionalized-pillar [5] arene spectrochim. Acta 220, 117136.
Yu, G., Ma, Y., Han, C., Yao, Y., Tang, G., Mao, Z., Huang, F., 2013. A sugar-functionalized amphiphilic pillar [5] arene: synthesis, self-assembly in water, and application in bacterial cell agglutination. J. Am. Chem. Soc. 135, 10310–10313.