ORIGINAL PAPER
Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 13197
Efficacy of Light Emitting diodes (LEDs) Lighting System for In vitro Shoot
Regeneration of Medicinal Water hyssop (Bacopa monnieri L. PENNEL)
Received for publication,February, 23, 2015 Accepted, July, 6, 2017 MEHMET KARATAŞ1*, MUHAMMAD AASIM1, MURAT DAZKIRILI2
1Department of Biotechnology, Faculty of Science, Necmettin Erbakan University, Konya, Turkey 2Department of Biology, Kamil Ozdag Faculty of Science, Karamanoglu Mehmetbey University, Karaman, Turkey
*Address for correspondence to: mkaratas@kmu.edu.tr; mshazim@gmail.com
Abstract
Efficacy of different Light Emitting Diodes (LEDs) on in vitro whole plant regeneration of water hyssop (Bacopa monnieri L. Pennel) was investigated in this study. Shoot tip explants were cultured on MS medium with BA (0.25, 0.50 and 1.0 mg l-1) and incubated under Red:Blue (R:B) LEDs in different combinations (4:1, 3:1, 2:1 and 1:1 R:B and white LEDs) for 10 weeks. Callus induction and shoot regeneration was noted on all explants irrespective of LEDs type or BA concentration. Shoots per explant and shoot length ranged 6.56-10.42 and 0.96-1.71cm respectively. Maximum number of 10.42 shoots with highest shoot length of 1.71 cm was achieved under 1:1 R:B LEDs. Whereas, different BA concentrations generated 4.97-11.98 shoots per explant with shoot length in the range of 0.93-1.35 cm. Maximum shoots per explant (10.35) with more longer shoots (1.31 cm) was achieved on MS medium containing 1.0 mg l-1 BA. Comparing of BA × LEDs, maximum number of 15.17 shoots per explant was induced under 1.0 mg l-1 BA × 2:1 R:B LEDs. Whereas, longer shoots (2.68 cm) were scored under 0.5 mg l-1 BA × 2:1 R:B LEDs. Regenerated shoots were rooted using 1.0 mg l-1 IBA in four weeks followed by acclimatization in the aquariums provided with tap water. Results revealed that LEDs in combination with BA can be successfully employed for multiple shoot regeneration of water hyssop.
Keywords: In vitro, Light emitting diodes, Shoot regeneration, Water hyssop
1. Introduction
Light is an important physical factor for all living organisms and plants that are sensitive to different light spectrum like blue, red or far red lights (QUAIL, [1]). These light spectrum photoreceptors control plant growth and development like germination, seedling establishment, shade-avoidance responses, circadian clock and vegetative to reproductive growth transition (CHEN et al., [2]). Conventional light sources for plant tissue cultures are fluorescent lamps but they have many limitations like more energy consumption, early replacement and heating etc.
Light Emitting Diodes (LEDs) are semiconductor diodes available in infrared (IR) to near ultraviolet (UV) spectral range (TAMULAITIS et al., [3]), which enable the wavelengths to coordinate with plant photoreceptors and affects the plant morphology and composition. These LEDs can be used as monochromatic spectral source or in different combinations of red and blue (HEO et al., [4]) in agricultural and biological studies. LEDs offer alternatives for traditional fluorescence lamps for in vitro studies (LIAN et al., [5], LI et al., [6]) and molecular biology techniques (WHELAN et al., [7]). Successful application of LEDs for in vitro studies like plant growth and development (WU et al., [8]), plant regeneration (NAHAR et al., [9]) and production of secondary metabolites (PARK et al., [10]) has been confirmed.
13198 Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 Besides that, more energy-efficiency with less heat emission (MORROW, [11]) and reduced electric energy consumption (FLAIG et al., [12]) are other advantages of adapting LEDs lighting system.
Water hyssop (Bacopa monnieri L. Pennel) also known as “Brahmi” is an important medicinal creeping herb that grows in damp marshy places. Several alkaloids, flavonoids, stigmastrol, beta-sitosterol, saponins and bacopasaponins have been reported in water hyssop (ALI et al., [13]) and that are used as cardiac or brain tonic, memory enhancement. They are also used for treatment of anxiety or epileptic disorders (MUKHERJEE et al., [14], VIJAYAKUMAR et al., [15]). Treating anti-inflammatory, antipyretic, diuretic and analgesic problems (VOHORA et al., [16], STOUGH et al., [17]), curing of insanity, hoarseness, epilepsy, snake bite, enlargement of spleen, leprosy, rheumatism, ring worm and eczema (BASU & WALIA, [18]) has been reported using these plant extracts. Similarly, compounds are in use for treating bronchitis, irritable bowel syndrome, asthma, and gastric ulcers (SHAKOOR et al., [19]). Besides that, it is very popular ornamental aquarium plant all over the world and attracts attention of people due to its lush green appearance and white colored flowers (KARATAS et al., [20]).
Water hyssop is collected from wild due to extensive use in pharmaceutical studies. High demand and collection without management has threatened the plant and has been reported as endangered species (TANVIR et al., [21], TIWARI et al., [22]). There is dire need to develop strategies for conserving plant by using traditional propagation techniques or by employing modern biotechnological techniques. Although, several reports on in vitro propagation of water hyssop has been reported (VIJAYAKUMAR et al., [15], KARATAS et al., [20], TIWARI et al., [22]). But still, there is need to develop new protocols on in vitro plant regeneration and strategies for secondary metabolites isolation/production. Application of LEDs lighting system can be employed for in vitro shoot regeneration along with altering the metabolites (PARK et al., [10], SHOHAEL et al., [23], GANGADHAR et al., [24], JEONG et al., [25], KIM et al., [26]). Therefore, the main focus of this study was to establish a reliable in vitro plant regeneration system using different LED lights that may provide plant materials for future research.
2. Materials and Methods
The plants were collected from local aquatic plants traders of Karaman province of Turkey. Twigs were separated and cut into pieces having 2-3 nodes. Thereafter, they were cleaned with tap water for 5 minutes. After cleaning process, surface sterilization of twigs was performed by using 16% H2O2 (v/v) for 10 min by continuous stirring using magnetic stirrer.
It was followed by rinsing 5 × 3 min with sterilized distilled water. Thereafter, twigs were inoculated on MS (MURASHIGE & SKOONG, [27]) medium for 15 days to get explants without any bacterial or fungal contamination.
Shoot tip explants were carefully separated from twigs and inoculated on MS medium containing 0.25, 0.50 and 1.0 mg l-1 6-benzylaminopurine (BA) in Magenta GA7 vessels
(Table 1). After that, they were incubated under 16 h light photoperiod using R:B and white light. There were 2 factors in this experiment; i.e. 5 light sources (4:1 R:B, 3:1 R:B, 2:1 R:B, 1:1 R:B and white light; Figure 1) and three concentrations of BA (0.25, 5 and 1.0 mgL-1) and
three replicates.
The regenerated shoots were transferred onto agar-solidified MS rooting medium containing 1.0 mg l-1 Indole-3-butyric acid (IBA) in Magenta GA7 vessels for inducing root
growth. Regeneration and rooting medium used in the whole study were supplemented with 30 g/l (w/v), solidified with 0.65% (w/v) agar at pH 5.8. The culture mediums were autoclaved at
Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 13199 104 kPa atmospheric pressure and 120°C for 21 min. Well developed, healthy plantlets were acclimatized after 2 weeks by removing gel adhering to roots of the micropropagated plantlets and acclimatized in aquariums containing tap water under R:B (1:1) LED lights with temperature range of 26 ± 2°C.
Data about frequency of callus induction (%), shoot induction (%), shoots per explant and shoot length were taken after 10 weeks of culture and subjected to statistical analysis by drawing univariate analysis. IBM SPSS17 for Windows was used for statistical analysis and Duncan’s Multiple Range Test (DMRT) was employed for post hoc tests. Data presented in percentages pertained to arcsine transformation (SNEDECOR & COCHRAN, [28]).
3. Results
The study was designed to investigate the response of different LEDs light combinations (4:1, 3:1, 2:1 and 1:1 R:B and white LED) and BA concentrations on axillary shoot regeneration. Results revealed that single shoot initiation started from shoot tip explant within 7-10 days irrespective of BA concentration, LED light types or their combinations. Callus induction started at the basal end within 2 weeks that later on resulted in induction of shoot buds that turned into multiple shoot induction after 4 weeks. Callus and shoot regeneration frequency was recorded 100 % in response to BA concentration, therefore, were not subjected to statistical analysis. Whereas, Analysis of variance for rest of the parameters showed statistically significant (p≤0.01) effects of BA concentration for LED lights and their interactions (BA × LEDs) on both shoots per explant and shoot length.
Different BA concentrations exerted statistically significant effects on shoots per explant and shoot length of water hyssop. Shoots per explant and shoot length ranged 4.97-11.98 and 0.93-1.35 cm respectively on different BA concentrations used in the study. Both minimum number of shoots (4.97) and shorter shoots (0.93 cm) were recorded on 0.25 mg l-1
BA containing medium. Further increase of BA concentration led to increase in shoots per explant and shoot length. Maximum number of shoots (11.98) was scored on medium containing 1.0 mg l-1 BA. Whereas, the longest shoots (1.35 cm) were obtained on medium
containing 0.50 mg l-1 BA followed by 1.31 cm on medium containing 1.0 mg l-1 BA.
Shoots per explant and shoot length showed statistically significant response to different LEDs lights. Shoot tip explants induced 6.56-10.42 shoots per explant when exposed to LEDs lights. Maximum number of 10.42 shoots were scored under 2:1 and 1:1 R:B LEDs lights. Whereas, white and 3:1 R:B LEDs induced 6.78 and 6.56 shoots respectively. Likewise, response of shoot length was similar to that of shoots per explant and equal concentration of R:B LEDs was promotory and ended up with comparatively longer shoots (1.71 cm) compared to all other LEDs types used in the study. The results further showed that increased Red LED light intensity in combination with Blue LEDs was inhibitory and caused reduction in shoot length. Relatively shorter shoots (0.96 cm) were scored under 3:1 R:B LEDs. Overall, shoot length ranged 0.96-1.71
under different LEDs lighting system. Figure 1. An overview of Light Emitting Diodes (LEDs); (a) 4:1 R:B, (b) 3:1 R:B,
(c) 2:1 R:B, (d) 1:1 R:B and (e) white LEDs.
13200 Romanian Biotechnological Letters, Vol. 23, No. 1, 2018
Figure 2. Multiple shoot induction from shoot tip explant under different LEDs lights of water hyssop (a) shoot tip explant (b) single shoot induction after two weeks, (c) multiple shoot induction after four weeks and (d) 10 weeks (e) rooted plantlets
Interactive effects of BA × LEDs had significant effects on number of shoots per explant and mean shoot length. Shoots per explant responded variably to BA × LEDs that ranged 3.33-15.17. Under White LEDs, all BA concentrations generated almost equal number of shoots per explant. 0.25 mg l-1 BA in combination with all LEDs was least responsive and
resulted in minimum number of shoots per explant. Results also revealed gradual increase in shoots per explant with increased BA concentration under all R:B LEDs and resulted in 3 folds shoots per explants under R:B LEDs with 1.0 mg l-1 BA. Maximum number of 15.17
shoots per explants were registered on MS medium containing 1.0 mg l-1 BA × 2:1 R:B
LEDS. Contrarily, minimum number of 3.33 shoots per explants were registered on MS medium containing 0.25 mg l-1 BA × 2:1 R:B LEDs.
Interactive effects of BA × LEDs had significant effects on number of shoots per explant and mean shoot length. Shoots per explant responded variably to BA × LEDs that ranged 0.63-2.68 cm. Under White LEDs, shoot length decreased with increased BA concentrations. Similarly, shorter shoots were scored on medium containing 0.25 mg l-1 BA
under all LEDs. Results also revealed the gradually increase in shoot length with increased BA concentration under all R:B LEDs. The longest shoots (2.68 cm) were achieved on MS medium containing 0.50 mg l-1 BA × 1:1 R:B LEDS. Contrarily, shorter shoots (0.63 cm)
Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 13201
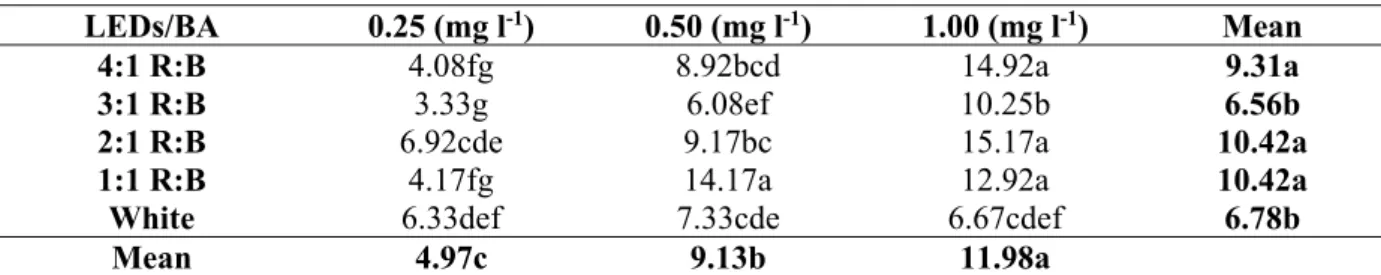
Table 1. Effects of different BA and LEDs concentrations on number of shoots per explant of water hyssop (Bacopa monnieri L. Pennel)
LEDs/BA 0.25 (mg l-1) 0.50 (mg l-1) 1.00 (mg l-1) Mean
4:1 R:B 4.08fg 8.92bcd 14.92a 9.31a
3:1 R:B 3.33g 6.08ef 10.25b 6.56b
2:1 R:B 6.92cde 9.17bc 15.17a 10.42a
1:1 R:B 4.17fg 14.17a 12.92a 10.42a
White 6.33def 7.33cde 6.67cdef 6.78b
Mean 4.97c 9.13b 11.98a
Values within a column followed by different letters are significantly different at 0.01 level of significance using DMRT
Table 2. Effects of different BA and LEDs concentrations on shoot length (cm) of water hyssop (Bacopa monnieri L. Pennel)
LEDs/BA 0.25 (mg l-1) 0.50 (mg l-1) 1.00 (mg l-1) Mean
4:1 R:B 0.63g 1.05def 1.45bc 1.04bc
3:1 R:B 0.83efg 0.92defg 1.12cdef 0.96c
2:1 R:B 1.17cde 1.20cde 1.27cd 1.21b
1:1 R:B 0.73fg 2.68a 1.70b 1.71a
White 1.27cd 0.92defg 1.03def 1.07bc
Mean 0.93b 1.35a 1.31a
Values within a column followed by different letters are significantly different at 0.01 level of significance using DMRT
For rooting of in vitro regenerated axillary shoots, ~1.0 cm long shoots were separated from explants under in vitro conditions, followed by inoculation of shoots on MS medium with 1.0 mg l-1 IBA for 4 weeks. Thereafter, the rooted plantlets were thoroughly cleaned
under tap water for removing adherent agar in the root zone and in vitro rooted plantlets were directly transferred to water filled aquariums fitted with aquarium tank internal submersible pumping filters that helped in continuous oxygenation of stationary water of water-tanks. Plants acclimatized easily in the aquariums without showing any negative sign on growth.
4. Discussion
Water hyssop is an important medicinal aquatic plant that has been reported as potential endangered species in India due to extensive collection for medicinal purposes. Therefore, there is need to develop in vitro regeneration protocols for conservation of this plant. Besides that, plant metabolites can be altered and several reports provide evidence of altering secondary metabolites in different plants due to LEDs. Successful in vitro regeneration of Water hyssop has been reported using normal day light lamps or fluorescent lamps. Recently, KARATAS et al., [29] reported adventitious shoot regeneration of water hyssop using leaf explant under different LEDs lights. LED lights are also used for successful in vitro propagation of other important economic plants (LIAN et al., [5], BAQUE et al., [30]). The present study describes the successful use of four different R:B LED light combinations and white LED lights on shoot regeneration of water hyssop cultured on different concentrations of BA using shoot tip explants.
Results revealed the response of shoot tip explant to BA × LEDs that resulted in single shoot initiation within 7-10 days followed by callus initiation and multiple shoot induction which also support the findings of KARATAS et al., [20] and KARATAS & AASIM, [31]. They reported 100 % callus induction of water hyssop using different explants and plant growth regulators. Callus induction in response to different LEDs has also been reported in
13202 Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 Cymbidium orchid (HUAN et al., [32]), Anthurium (BUDIARTO, [33]) and Brassica napus (AFSHARI et al., [34]). Frequency of shoot regeneration displayed clear bearings of BA, LEDs or BA × LEDs which was recorded 100 %. KARATAS & AASIM [31] achieved 100 % shoot regeneration frequency from leaf explant cultured on different concentrations of BA or TDZ. Contrarily, VIJAYAKUMAR et al., [15] and MAHENDER et al., [35] obtained variable shoot regeneration frequency of B. monnieri using fluorescent light.
Results on shoots per explants and shoot length clearly showed the effects of BA, LEDs or BA × LEDs. Results revealed the importance of BA concentration on shoot proliferation and higher concentration of BA as more appropriate for gaining maximum number of shoots per explant. Furthermore, increase in BA concentration generated more number of shoots per explant in line with the findings of KARATAS & AASIM, [31]. Result on LEDs lighting system clearly revealed the importance of R:B LEDs compared to white LEDs for multiple shoot regeneration. The results further indicated that high blue light intensity with red (2:1 or 1:1) was more superior for maximum shoot regeneration. These results proved better efficiency and superiority of LEDs for gaining higher number of in vitro propagated plants. LI et al., [6] attained relatively larger plantlets with greater biomass of cotton under red LED provided with a quantity of blue LED light. They reported 1:1 R:B LEDs as best for in vitro growth of upland cotton plantlets. On the other hand, the results revealed the negative effects of reduced blue light intensity in the presence of red on shoots per explants. NHUT & al. [36] obtained maximum total fresh weight of plantlets under 80:20 % R:B LEDs. In other study on strawberry, NHUT et al., [37] reported maximum plantlet growth at 70:30% R:B LEDs. Whereas, CHANG et al., [38] reported better growth of calla lily under monochromatic red or blue LEDs. Interactive effects of BA × LEDs also featured the importance of growth conditions for maximum shoot proliferation. 3 fold more number of shoots per explants were scored under different 1.0 mg l-1 BA × R:B LEDs compared to 0.25 mg l-1 BA × R:B LEDs. Contrarily, BA × white LEDs
were the least effective to induce shoots per explant. LIAN et al., [5] reported higher number of bulblets along with bulblet growth, size, higher fresh or dry weight, dry matter percentage and roots per bulblet of Lilium under fluorescent light or R:B LEDs.
BA concentrations significantly affected the mean shoot length and higher concentrations of BA also enhanced the shoot length in accordance to the findings of VIJAYAKUMAR et al., [15]. Whereas, KARATAŞ & AASIM, [31] reported negative effects of BA concentrations on shoot length. Results on mean shoot length also showed the importance of R:B intensity. Equal intensity of R:B LEDs led to longer shoots compared to other R:B combinations and white LEDs. Contrarily, HEO et al., [4] reported negative effects of R:B LEDs on shoot length of grape. However, red LEDs promoted the shoot length which supports the findings of CHANG et al., [38], who reported increased shoot elongation of Zantedeschia albomaculata when cultures were kept under red or blue light.
Results revealed that BA and LEDs individually resulted in longer shoots at 1:1 R:B and 0.5 mg l-1 BA. Likewise, interaction of both factors that is 0.5 mg l-1 BA × 1:1 R:B LEDs
also induced longer shoots
.
Results further revealed that shoot length increased with increase in BA concentrations in the presence of all R:B LEDs. Contrarily, shoot length decreased with increased BA concentrations in the presence of white LEDs.In vitro rooting followed by successful acclimatization is of immense importance for the establishment of successful shoot regeneration protocol. It was not difficult to obtain 100 % rooting and successful acclimatization of water hyssop in this study. Our previous studies on water hyssop also presented easy rooting and acclimatization in aquariums (KARATAS et al., [20], KARATAS et al., [29], KARATAS & AASIM, [31]).
Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 13203
5. Conclusions
Lighting systems coordinate with plant photoreceptors, affects plant morphology and plant composition like secondary metabolites. The study is the first ever to check the efficacy of different Red and Blue LEDs combination on in vitro plant regeneration of water hyssop, an important medicinal aquatic plant which is also under threat of endangered species in India. Present observations are novel and show clear bearings of LED lights on shoot regeneration behavior. Further studies could lead to a better understanding about production of secondary metabolites contained in this plant, functional genomics of plant, understanding of the physiological and molecular events underlying different behavior of these plants.
References
1. P.H. QUAIL, Photosensory perception and signalling in plant cells: new paradigms? Curr. Opin. Cell Biol., 14, 180, 188 (2002).
2. M. CHEN, J. CHORY, C. FANKHAUSER, Light signal transduction in higher plants. Annu. Rev. Cell. Dev.
Biol., 38, 87, 117 (2004).
3. G. TAMULAITIS, P. DUCHOVSKİS, Z. BLIZNIKAS, K. BREIVÉ, R. ULINSKAITÉ, A. BRAZAITYTÉ, A. OVİCKOVAS, A. ZUKAUSKAS, High power light-emitting diode based facility for plant cultivation.
J. Physics D: Appl Phys., 38, 3182, 3187 (2005).
4. J.W. HEO, K.S. SHIN, S.K. KİM, K.Y. PAEK, Light quality affects in vitro growth of grape “Teleki 5BB”. J. Plant Bio., 49, 276, 280 (2006).
5. M.L. LIAN, H.N. MURTHY, K.Y. PAEK, Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’ Sci. Horti., 94, 365, 370 (2002).
6. H. LI, C. TANG, Z. XU, Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tiss. Org. Cult., 103, 155, 163 (2010).
7. H.T. WHELAN, E.V. BUCHMANN, A. DHOKALIA, M.P. KANE, N.T. WHELAN, M.T. WONG-RILEY, T.J. EELLS, L.J. GOULD, HAMMAMIEHR, R. DAS, M. JETT, Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J. Clin. Laser Med. Sur., 21, 67, 74 (2003).
8. H.C. WU, E.S. DuTOIT, In vitro organogenesis of Protea cynaroides L. shoot-buds cultured under red and blue light-emitting diodes. In: Embryogenesis. Sato K.I. (ed.). InTech China. (2012).
9. S.J. NAHAR, K. SHIMASAKI, S.M. HAQUE, Effect of different light and two polysaccharides on the proliferation of protocorm-like bodies of cymbidium cultured in vitro. ISHS Acta Hort., 956, 307, 313 (2012). 10. S.U. PARK, D.J. AHN, H.J. JEON, T.R. KWON, H.S. LIM, B.S. CJOI, K.H. BAEK, H. BAE, Increase in the
contents of Ginsenosides in raw ginseng roots in response to 450 and 470 nm light from Light-Emitting Diodes. J. Ginseng Res., 36, 198, 204 (2012).
11. R.C. MORROW, LED Lighting in Horticulture. HortScience, 43, 1947, 1950 (2008).
12. F.M. FLAIG, J. RINCK, M. STEPHAN, T. BOCKSROCKER, T. BRUNS, C. KÜBEL, A.K. POWELL, A.G. OZİN, U. LEMMER, Multicolor Silicon Light-Emitting Diodes (SiLEDs). Nano Lett., 13, 475, 480 (2013).
13. G. ALI, P.S. SRIVASTAVA, M. IQBAL, Morphogenic and biochemical responsesof Bacopa monnieri cultures to zinc toxicity. Plant Sci.,143, 187, 193 (1999).
14. D.G. MUKHERJEE, C.D. DEY, Clinical trial on Brahmi. J. Exper. Med. Sci., 10, 5, 11 (1996).
15. M. VIJAYAKUMAR, R. VIJAYAKUMAR, R. STEPHEN, In vitro propagation of Bacopa monnieri L., A multipurpose plant. Ind. J. Sci. Tech., 3, 781, 786 (2010).
16. S.B. VOHORA, T. KHANNA, M. ATHAR, Analgesi of activity of Bacosine a new triterpin isolated from
Bacopa monniera Fitoterapia., 68, 161, 365 (1997).
17. C. STOUGH, J. LLOYD, J. CLARKE, L. DOWNEY, C. HUTCHİNSON, T. RODGESS, P. NATHAN, The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects.
Psychopharacol., 156, 481, 484 (2001).
18. N. BASU, K. WALIA, The chemical investigations of the leaves of Herpestis monniera. Indian J. Pharm., 4, 84, 85 (1994).
19. A. SHAKOOR, M. AKRAM, C.M. ASHARAF, M.R. SIDDIQUI, Pharmagonistic study and chemical / pharmacological evaluation of Brahmi-buti. Hamdard Medicus, 37, 92, 109 (1994).
13204 Romanian Biotechnological Letters, Vol. 23, No. 1, 2018 medicinal aquatic plant water hyssop (Bacopa monnıeri L. PENNELL) using different internodes. Arch. Biol.
Sci. Belgrade, 65, 297, 303 (2013).
21. A. TANVIR, M. KHAN, F. SHAH, In vitro micropropagation of Brahmi-Bacopa monniera (L.) Pennell – A step for conservation. Nanobiotechnica Universale, 1, 139, 150 (2010).
22. K.N. TIWARI, J. SINGH, Effective organogenesis from different explants of Bacopa monnieri L.(Wettst.)-An important medicinal plant. BFIJ, 2, 18, 22 (2010).
23. A.M. SHOHAEL, M.B. ALI, K.W. YU, E.J. HAHN, R. ISLAM, K.Y. PAEK, Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem., 41, 1179, 1185 (2006).
24. B.H. GANGADHAR, R.K. MISHRA, G. PANDIAN, S.W. PARK, Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. HortScience, 47, 1729, 1735 (2012).
25. J.H. JEONG, Y.S. KIM, H.K. MOON, S.J. HWANG, Y.E. CHOI, Effects of LED on growth, morphogenesis and eleutheroside contents of in vitro cultured plantlets of Eleutherococcus senticosus Maxim. Kor. J. Med.
Crop Sci., 17, 39, 45 (2009).
26. S.J. KIM, E.J. HAHN, J.W. HEO, K.Y. PAEK, Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic., 110, 143, 151 (2004).
27. T. MURASHIGE, F. SKOOG, A revised medium for rapid growth and bioassays with tobacco tissue cultures.
Physiol. Plant. 15, 473, 497 (1962).
28. G.W. SNEDECOR, W.G. COCHRAN, Statistical methods. The Iowa State University Press, Iowa. USA. (1997).
29. M. KARATAŞ, M. AASIM, M. Dazkirli, Influence of Light-Emitting Diodes and Benzylaminopurin on adventitious shoot regeneration of water hyssop (Bacopa monnieri (L.) PENNELL) in vitro. Arch. Biol. Sci., 68, 501,508 (2016).
30. A. BAQUE, Y.K. SHIN, T. ELSHMARI, E.J. LEE, K.Y. PAEK, Effect of light quality, sucrose and coconut water concentration on the microporpagation of Calanthe hybrids (‘Bukduseong’ × ‘Hyesung’ and ‘Chunkwang’ × ‘Hyesung’). Aust. J. Crop Sci., 5, 1247, 1254 (2011).
31. M. KARATAŞ, M. AASIM, Efficient adventitious shoot regeneration of medicinal aquatic plant water hyssop (Bacopa monnieri L. Pennell). Pak. J. Agri. Sci., 51, 665, 670 (2014).
32. L.V.T. HUAN, M. TANAKA, Effects of Red and Blue Light-Emitting Diodes on Callus Induction, Callus Proliferation, and Protocorm-Like Body Formation from Callus in Cymbidium Orchid. Environ. Cont. Biol., 42, 57, 64 (2004).
33. K. BUDIARTO, Spectral quality affects morphogenesis on Anthurium plantlet during ın vitro culture.
Agrivita, 32, 234, 240 (2010).
34. R.T. AFSHARI, R. ANGOSHTARI, S. KALANTARI, Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. POJ, 4, 60, 67 (2011).
35. A. MAHENDER, B. MALLESHAM, K. SRINIVAS, G.K. KUMAR, K.V. RAO, Y. RAJESH, P. ZHANG, A. SADANANDAM, A rapidand efficient method for in vitro shoot organogenesis and production of transgenic Bacopa monnieri L. mediated by Agrobacteriumtumefaciens. In Vitro Cell. Dev. Biol. Plant., 48, 153, 159 (2012).
36. NHUT DT, HONG LTA, WATANABE H, GOİ M, TANAKA M Growth of banana plantlets cultured ın vıtro under red and blue lıght-emıttıng dıode (led) ırradıatıon source. ISHS Acta Hort., 575, 117, 124 (2002). 37. D.T. NHUT, T. TAKAMURA, H. WATANABE, K. OKAMATO,M. TANAKA, Responses of strawberry
plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tiss. Org.
Cult., 73, 43, 52 (2003).
38. H.S. CHANG, D. CHARKABARTY, E.J. HAHN, K.Y. PAEK, Micropropagation of calla lily (Zantedeschia