Contents lists available atScienceDirect
Sensors and Actuators B: Chemical
journal homepage:www.elsevier.com/locate/snbCalix[4]arene based a NIR-fluorescent sensor with an enhanced stokes shift
for the real-time visualization of Zn(II) in living cells
Serkan Erdemir
a,*
, Sait Malkondu
baSelcuk University, Science Faculty, Department of Chemistry, Konya, 42031, Turkey
bGiresun University, Faculty of Engineering, Department of Environmental Engineering, Giresun, 28200, Turkey
A R T I C L E I N F O Keywords: NIR Fluorescence Sensor Bioimaging Zinc A B S T R A C T
Zinc(II) plays key roles actively involved in fundamental biological functions in the body. However, zinc dys-metabolism results in a number of human diseases. Therefore, miscellaneous techniques are needed to under-stand the complete biological role of Zn(II) in tissues and cells. Here, we introduce a novel calix[4]arene de-rivative (CIP), appended with two isophorone units via a 4-bromophenol linker for the fluorescence sensing Zn (II). CIP showed high fluorogenic selectivity for Zn(II) over a wide range of biologically and environmentally relevant metal ions in DMSO/H2O (v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4) and has superior sensitivity of (6.75 nM) for Zn(II). Free CIP has very low emission but demonstrated the distinct “turn-on” emission enhancement response toward Zn(II) at near infrared region. CIP allowed the fabrication of a rapid and convenient TLC strip sensor for Zn(II)-selective detection. Moreover, this is the first report on a calix[4]are derivative used as a NIR fluorescence probe that monitors changes in intracellular Zn(II) levels and can penetrate into living cells.
1. Introduction
Cations is known to play significant roles in a great variety of che-mical reactions, containing biological processes and also many other processes [1,2]. Therefore, great effort has been dedicated to the design of convenient probes for the purpose of quantitative determination and detection of cations [3–5]. Zn(II) after iron is the most abundantD-block metal ion in living organisms. Moreover, it is an environmentally me-tallic contaminant: high levels of zinc may suppress the soil biological processes leading to phytotoxic outcomes [6,7] and it is a common pollutant in food and agricultural wastes [8]. Zinc is a very significant trace element for human health and diet [9,10]. It is found as a cofactor in carbonic anhydrase enzyme which regulates the equilibrium between water, carbon dioxide and carbonic acid [11]. In 1947, Bradfield con-firmed the occurrence of carbonic anhydrase in plants [12]. Renal di-peptidase is another zinc dependent metalloenzyme which has the ability to hydrolyze imipenem [13]. Zinc in the cell membrane prevents the cell from being damaged by oxidative reactions [14]. The total level of free Zn(II) in human body can range from nanomolar levels in certain cells to milimolar levels in neuronal vesicles [15]. Low bioavailability of zinc, which is involved in DNA, growth hormones and the synthesis of enzyme in the cell, leads to the diminished appetite, taste and smell perception, and delay in wounds healing [16,17]. Besides, zinc can show cytotoxic effect, in part due to its ready interaction with
endogenous ligands such as peptides and proteins [18]. Extraordinary levels of Zn(II) ion has been monitored in breast carcinoma tissue to be 700% higher than that in normal ones [19]. There exists also a number of neurological disorders where disruption of zinc homeostasis often develops Alzheimer’s disease, epilepsy and Parkinson’s disease [20,21]. Although Zn(II) play essential cellular roles, a lot still needs to be learnt about the cellular balance of Zn(II) with regard to other common metal ions likewise Na(I), K(I), Ca(II), etc. Hence, certain tools for measuring Zn(II) in biological systems are being developed to elucidate some of the details regarding its physiological importance [22–24]. Currently, several methodologies such as flame atomic absorption spectrometry [25], acid-base titrimetry using glass pH electrode [26], induced plasma-atomic emission spectrometry [27] and differential pulse stripping anodic voltammetry [28]. However, these methods possess some defects that restrict the application in practice, for in-stance, need of the pre-treatment methods, high cost and sophisticated instrumentation, time-consuming procedures and are not convenient for bioimaging and large-scale monitoring. So, it is essential to discover simple, high sensitive, portable and low cost methods for the determi-nation of Zn(II) ions. Fluorescence method is a promising candidate for this purpose and has been provided as an efficient technique to sense metal ions in the recent years [1,29,30]. Its most important advantages are high sensitivity and specificity, non-destruction of sample, on-line monitoring, quick response and non-requirement of huge and complex
https://doi.org/10.1016/j.snb.2019.127574
Received 22 October 2019; Received in revised form 10 December 2019; Accepted 11 December 2019 ⁎Corresponding author.
E-mail address:serdemir82@selcuk.edu.tr(S. Erdemir).
Available online 12 December 2019
0925-4005/ © 2019 Elsevier B.V. All rights reserved.
instrumentation [31–35].
Calix[n]arene-based molecules as receptor building platform have been attracting special attention for sensing metal ions due to 3D structure, flexible template, wide possibility of chemical modifications and are suitable for bioimaging applications [4,36–39]. Calix[n]arenes display different conformations providing perfect cavities with special shapes and sizes for cations. Several strategies working with fluores-cence quenching mechanism [40–42] have been reported for the de-tection Zn(II). However, “turn on” mechanism are more desirable be-cause of wider detection range and the high selectivity [43]. Our approach has largely focused on “turn on” mechanism at the near in-frared (NIR) region. NIR fluorescent probes increase tissue penetration and reduce phototoxicity [44]. To date, a limited number of NIR fluorescent probes have been reported for the detection of Zn(II) ion [45–50]. Moreover, among these reported probes, NIR fluorescent probe based on the basis of calix[n]arene for the detection of Zn(II) has never been encountered in current literature. Therefore, the present study is the first report for the determination of Zn(II) with calixarene-based NIR fluorescent probe to best our knowledge. Clearly, the un-modified calix [4]arene has no specific interaction with metal ions. Introduction of isophorone-bromophenol moiety to calix [4]arene has greatly improved the selectivity of calix [4]arene for Zn(II) compared to other competing ions such as K(I), Na(I), Mg(II) and Ca(II) in the cell. It also gives a challenging platform for the future improvements of NIR probes for Zn(II) and other d-block metal ions.
2. Experimental
2.1. Materials and instruments
All chemicals used in experiments were provided from Sigma-Aldrich, Alfa Easer and ACROS. IR analysis were performed on a Bruker instrument using ATR method. 1H, 13C and APT NMR spectra were
taken on Varian 400 MHz spectrometer in DMSO-d6 as solvent.
Emission and UV–vis spectra were recorded on Perkin Elmer LS 55 and Shimadzu 1280 spectrophotometers, respectively. TOF-MS analysis was realized on an Agilent 6230 equipment.
2.2. Synthesis
As illustrated inScheme 1, the hydrazide derivative of calix [4] arene (4) was prepared from the reaction between the ester derivative of calix [4]arene and hydrazine hydrate according to known procedure [51]. Also, 3-dicyanomethylidene-1,5,5-trimethylcyclohex-1-ene (1) was synthesized according to previously reported method [52].
2.2.1. Synthesis of compound 2
A solution of 3-dicyanomethylidene-1,5,5-trimethylcyclohex-1-ene (0.372 g, 2.0 mmol) and 5-bromosalicylaldehyde (0.402 g, 2.0 mmol) in ethanol (10 mL) was refluxed in the presence of piperidine (20 μL) for 12 h. After this period, the mixture was treated with 1.0 M of HCl (100 mL), and then extracted with CH2Cl2(2 × 50 mL). The organic solvent
was evaporated and recrystallization of crude product from hexane affords a brown solid. Yield: 65%; Mp: 205−207 °C; FT-IR: 2222 cm−1
(CN);1H NMR (400 MHz, DMSO-d 6): δ 10.42 (s, 1 H), 7.87 (s, 1 H), 7.40 (d, 1H, Jab= 16.3 Hz), 7.30 (d, 1H, Jab= 16.3 Hz), 7.26–7.28 (m, 1 H), 6.82–6.84 (m, 2 H), 2.51 (s, 2 H), 2.43 (s, 2 H), 0.96 (s, 6 H).13C NMR (100 MHz, DMSO-d6): δ 27.05, 32.11, 38.46, 45.80, 76.84, 111.51, 113.49, 115.54, 118.20, 123.46, 125.45, 129.63, 130.38, 130.79, 132.71, 155.73, 156.75, 170.75. 2.2.2. Synthesis of compound 3
In trifluoroacetic acid (40 mL), 2 (0.4 g, 1.08 mmol) and hexam-ethylenetetramine (0.91 g, 6.48 mmol) were refluxed for 12 h. After this period, 1.0 M of HCI was mixed with the reaction mixture, and the resulting solution was stirred for 2 h at rt. The precipitate was filtered, washed with water and recrystallization from hexane/CH2Cl2produced
a yellow solid. Yield: 75%; Mp:152−153 °C; FT-IR: 1659 (HC = O), 2220 cm−1(CN);1H NMR (400 MHz, DMSO-d 6): δ 11.35 (s, 1 H), 10.03 (s, 1H, CHO), 8.26 (s, 1 H), 7.89 (s, 1 H), 7.56 (d, 1H, Jab= 16.27 Hz), 7.33 (d, 1H, Jab= 16.27 Hz), 6.90 (s, 1 H), 2.59 (s, 2 H), 2.48 (s, 2 H), 0.99 (s, 6 H).13C NMR (100 MHz, DMSO-d 6): δ 27.86, 28.21, 32.13, 38.43, 42.73, 78.40, 110.74, 113.33, 115.23, 124.20, 124.89, 128.02, 129.47, 131.82, 135.44, 136.63, 154.23, 157.28, 170.65, 194.83.
Scheme 1. The synthetic route for probe CIP. Reagents and conditions: (i) 3-dicyanomethylidene-1,5,5-trimethylcyclohex-1-ene (1), ethanol, 12 h, reflux (ii) TFA,
2.2.3. Synthesis of probe CIP
A solution of compound 3 (0.41 g, 1.03 mmol) in ethanol (10 mL) was mixed with a solution of hydrazide derivative of calix [4]arene (0.40 g, 0.50 mmol) in ethanol (30 mL) and the resulting mixture was refluxed for 24 h. The precipitated product was filtered off, washed with ethanol, and recrystallized from a mixture of CH2Cl2/hexane (2:1).
Yield: 72%; Mp: 260−262 °C; FT-IR: 1661 (C = N), 1708 (NHC = O), 2219 cm−1(CN);1H NMR (400 MHz, DMSO-d 6): δ 12.26 (2 H), 12.00 (s, 2 H), 8.31 (s, 2 H), 8.18 (s, 2 H), 7.72 (s, 2 H), 7.58 (s, 2 H), 7.12–7.33 (m, 12H), 6.85 (s, 2 H), 4.77 (s, 4 H), 4.28 (d, 4H, J = 12.80 Hz), 3.50 (d, 4H, J = 12.80 Hz), 2.61 (s, 4 H), 2.47 (s, 4 H), 1.16 (s, 18 H), 1.13 (s, 18 H), 1.02 (s, 12 H).13C NMR (100 MHz, DMSO-d 6): δ 15.14, 22.41, 27.90, 31.34, 31.80, 32.09, 34.11, 34.57, 38.71, 42.78, 43.63, 60.20, 72.42, 76.71, 111.76, 112.79, 113.85, 120.53, 122.90, 126.08, 126.48, 127.84, 128.89, 130.44, 133.53, 142.79, 147.97, 148.21, 149.82, 149.99, 156.01, 165.29, 171.26. TOF-MS: calcd. for C88H94Br2N8O8+(M+H)+: 1551.5500, found:1551,5621
2.3. Analytical procedures and bio-imaging studies
The stock solution of CIP (10.0 mM) was prepared in analytical grade DMSO, and then diluted to a desired concentration in DMSO/H2O
(v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4). Stock solutions (10 mM) of cations (perchlorate salts) and anions (tetrabutylammonium salts) in water were prepared. The concentration of CIP was selected as 10.0 μM or 20.0 μM for fluorescence or UV–vis experiments, respectively. Fluorescence spectra were recorded from 525 to 900 nm under ex-citation at 512 nm at room temperature.
Human colon cell line DLD-1 was obtained from ATCC (American Type Culture Collection) and was cultured in RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine, and 100 units mL-1 Penicillin G, and 100 μg mL-1 streptomycin at 37 °C in a humidified incubator containing 5% of CO2. To determine cellular localization, 1
× 105of DLD-1 cells were seeded into glass bottom dark 24-well plates
(Corning, USA) and incubated at 37 °C for 24 h. Then the medium was removed and cells were washed with 10.0 mM of PBS for three times. The cells were pretreated with 5.0 μM of the probe CIP and incubated at 37 °C for 45 min. Then the probe CIP was removed and the cells were washed with 10 mM of PBS for three times to remove excess CIP. The cells were treated with an equal volume of Zn(II) and incubated at 37 °C for 45 min. The excess Zn(II) was removed and the cells were washed. The color formation was monitored by using fluorescence microscope (Bio-Rad, USA).
3. Results and discussion
3.1. Production of probe CIP
The synthesis of CIP is shown inScheme 1. Firstly, compound 2 as a fluorophore, which has π-conjugation system was produced by Knoe-venagel condensation between 3-dicyanomethylidene-1,5,5-tri-methylcyclohex-1-ene (1) and 5-bromosalicylaldehyde in 65% of yield. Then, it was converted to the corresponding aldehyde derivative (3) by using HMTA in TFA. Another part of probe, a hydrazide derivative of calix [4]arene (4) was obtained according to a known method [51] in 90% of yield. Among the various ion receptors, calixarenes containing appropriate groups are found to be noteworthy recognition properties for cations owing to their highly preorganized architecture for the binding processes. In addition, the 1,3-disubstitution at the lower rim of calix [4]arene is of high attraction because keeping cone conformation which advances receptor's cation-binding capability. Therefore, the compound 3 was condensed with the hydrazide derivative of calix [4] arene (4) on the 1,3-position to yield the probe CIP as a Schiff base (imine) which provides excellent binding-sites for cations. The syn-thesized molecules were characterized by1H,13C, APT NMR, FT-IR, and
TOF-MS (Supporting information, Figs S1-S9)
3.2. Sensing properties of probe CIP towards Zn(II) ion
The sensing characteristics of CIP were surveyed by using fluores-cence and UV–vis spectrometers in presence or absence of Zn(II) in DMSO/H2O (v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4). As depicted in Fig.1, the absorption bands of CIP were observed at 292 and 416 nm. By addition of Zn(II) (2.0 equiv.), the absorptions of these two bands were lowered and a band at 512 nm was newly emerged. Under ex-citation at 512 nm, CIP showed weak emission at 667 nm. However, the fluorescence intensity of CIP at 667 nm significantly increased in pre-sence of Zn(II) (2.0 equiv), which demonstrates that CIP can be em-ployed as a highly selective fluorescence probe for Zn(II). Classical fluorophores including rhodamine [53], fluorescein [54], cyanine [55,56] and nile red dyes [57] demonstrate low Stokes shifts (Δλ ≤ 70 nm). However, two near-infrared fluorescent probes reported by Sta-siuk and coworkers for sensing Zn(II) ions in living cells show large Stokes shifts of 111 and 101 nm in ethanol [46]. Upon chelation of Zn (II) with Gd-1 displaying a ratiometric fluorescent response, a large Stokes shift (90 nm) was observed towards the red in emission, from 410 to 500 nm, and a 300 % increase in fluorescence amplitude [58]. Excited-state intramolecular proton transfer (ESIPT) in a series of 2-(2′-benzene-sulfonamidophenyl) benzimidazole derivatives was inhibited with the interaction of Zn(II). In the absence of Zn(II) at neutral pH, the fluorophores undergoing ESIPT yield a highly Stokes shifted emission (120−122 nm) from the proton-transfer tautomer [59]. In comparison to mentioned studies, the considerably large Stokes shift of CIP was found to be ∼155 nm, which supports that CIP can eliminate the ex-citation light backscattering effect for fluorescence imaging.
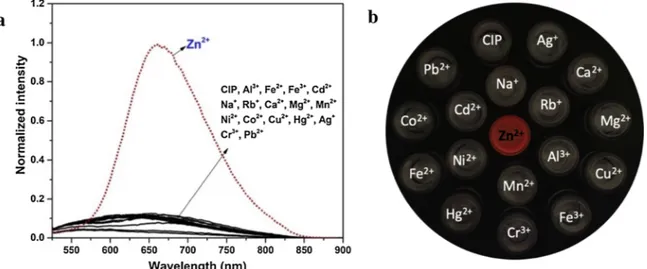
The selectivity of CIP, which is an important parameter for a fluorescent sensor, was tested by the interaction of a solution of CIP (10.0 μM) with a pool of metal ion (Al3+, Fe2+, Fe3+, Zn2+, Cd2+,
Na+, Rb+, Ca2+, Mg2+, Mn2+, Co2+, Ni2+, Cu2+, Ag+, Hg2+, Cr3+,
Pb2+). The changes in the fluorescence spectra are shown inFig. 2a.
Free CIP indicated a weak emission due to the PET (Photo-induced electron transfer) process and the excited stated of C]N isomerization when it was excited at 512 nm The addition of metal ions, except for Zn (II) had no marked effect on the emission profile of the CIP, whereas Zn (II) induced immediately a large enhancement (13-fold) in fluorescence intensity at 667 nm since both the PET processes and the excited stated C]N isomerization (free rotation) in CIP could be inhibited through the coordination of Zn(II) to CIP. In addition, the formed complex is a rigid and conjugated structure, giving rise to the CHEF (chelation en-hanced fluorescence) effect [60,61]. Consequently, colorless
Fig. 1. The emission and absorption spectra of CIP (red line) and CIP-Zn2+ (blue line) in DMSO/H2O (v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4).
fluorescence of the free probe CIP under UV light turns to a fascinating red color which could be clearly monitored only for Zn(II) (Fig. 2b). Otherwise, the sensitivity of CIP against Zn(II) was observed by the fluorescence and UV–vis titrations studies, and the results were illu-strated in Fig.3. The maximal absorption bands at 292 and 416 nm assigned to π-π* transitions were gradually decreased with increasing concentrations of Zn(II), and a new absorption band emerged at 512 nm by a red shift (Δλmax) of 96 nm. Also, the color of CIP solution changed
from yellow to somon pink (Fig. 3a, inset). As depicted inFig.3b, upon the gradual addition of Zn(II) (0–20.0 μM) into CIP, the fluorescence intensity at 667 nm linearly increased, and reached a maximum at about 10.0 μM concentration of Zn(II) (Fig. 3b, inset), showing a 1:1 of complexation ratio between CIP and Zn(II). This stochiometric ratio was also confirmed by TOF-MS (Fig. S9) and Job’s plot analysis (Fig. S10). From the fluorescence titration data, the limit of detection and the binding constant were determined as 6.75 nM and 2.60 × 105 M−1
according to 3σ/slope (Fig. S11) and the Benesi–Hildebrand equations (Fig. S12), respectively.
The time-dependent change in the fluorescence intensity of CIP was also tested in presence of Zn(II) (2.0 equiv.) to determine the reaction time. As shown in Fig. S13, the fluorescence intensity of CIP arrived a maximum value within 10 s, suggesting that CIP could be used as a sensor for the quick monitoring of Zn(II). In addition, the pH depen-dence of the CIP and CIP-Zn(II) was examined at various pH values
(3.0–11.0) to find the optimal sensing conditions. Free CIP could not display any fluorescence enhancement in the pH range of 3.0–11.0, while the fluorescence intensity of CIP-Zn(II) quickly was improved in the pH range of 5.0–8.0, indicating that CIP could be applied as Zn(II) sensor under neutral conditions, especially physiological conditions. (Fig. S14).
3.3. Competition and reversibility studies
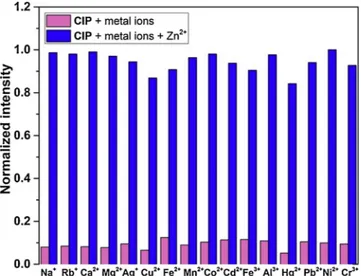
To further reveal the selectivity potential of CIP for Zn(II), a com-parative study of the emission response of CIP was tested toward wide range of metal ions (Fig.4). Specificity experiments revealed that Zn(II) can be discriminated from other competitive metal ions. The multi-coordination sites in the structure of CIP improve its Zn(II) detection selectivity compared to other metal ions. Only, Cu(II), Fe(II), Fe(III) and Hg(II) have a negligible effect on the detection of Zn(II). Besides, Zn(II) and Cd(II) ions demonstrates often identical spectral properties upon interaction with chemosensors since they are found in same group in the periodic table. Therefore, distinguish of them is still one of the main challenges in sensor area. The results indicate that CIP shows notable differences upon binding Zn(II) over Cd(II) and has proved to be re-markably selective for Zn(II).
To investigate the effects of anions on the emission of CIP-Zn(II) complex, series anions (I−, Br−, Cl−, F−, S2-, CN−, NO
3−, CH3COO−,
Fig. 2. (a) Fluorescence spectra of CIP (10.0 μM) and upon the addition of salts (2.0 equiv) of metal ions in DMSO/H2O (v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4); (b) Fluorescence colors of CIP in presence of various metal ions.
Fig. 3. (a) UV–vis titration spectra of CIP (20 μM) and (b) fluorescence titration spectra of CIP (10 μM) in the presence of Zn2+at different concentrations in DMSO/ H2O (v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4).
H2PO4−, ClO4−and HSO4−) were treated with the complex solution.
High emission of CIP-Zn(II) complex was almost quenched with the addition of S2-(Fig. S15a). However, other tested anions could not
re-markably influence the emission of CIP-Zn(II) complex. This reverse effect is due to the detachment of Zn(II) from CIP-Zn(II) complex through S2-and the releasing of free CIP, with the response of emission
quench. Exploiting the present displacement behavior, reversibility of from CIP-Zn(II) complex with S2-was achieved with alternate cycle of
addition of Zn(II) to CIP followed by the addition of S2-. The
reversi-bility character make CIP act as a Zn(II) and S2-controlled “OFF–ON”
fluorescent switch. Through the alternate addition of Zn(II) and S
2-allowed to perform at least five cycles with slight emission loss (Fig.S15b).
3.4. Test kits and bio-imaging applications
The practical usage of the probe CIP against Zn(II) was tested on a silica gel TLC strip. For this, a solution of CIP (0.5 mM) was dropped on a TLC plate, and dried in air. Then, the solution of Zn(II) in the various concentrations (0.05-0.5 mM) was added to each spot containing the probe CIP on the TLC plate. As depicted inFig.5, the fluorescence color of the CIP immobilized on TLC strip changed immediately from color-less to red under 365 nm UV light, suggesting that the proposed probe could be effective as testers in practical applications. Similar results were also obtained on the test papers (Fig. S16).
To demonstrate the practical application of CIP in living cells, the DDL-1 cells were incubated with CIP (5.0 μM) at 37 °C under an at-mosphere of 5% CO2 for 1 h, and no fluorescence was observed
(Fig. 6a). However, the addition of Zn(II) (5.0 μM) to the cells pre-treated with CIP was induced to strong red fluorescence emission (Fig.6b), demonstrating that the CIP possessed well cell penetrability and could readily detect to intracellular Zn(II).
3.5. The proposed interaction mechanism between CIP and Zn(II)
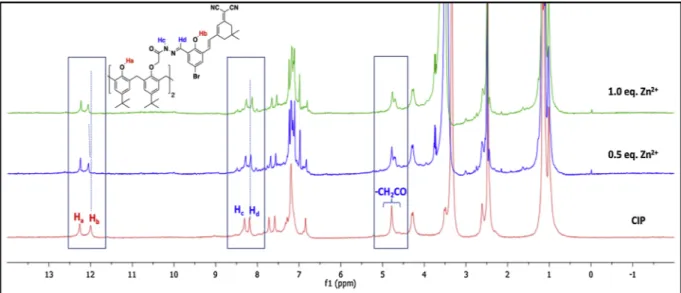
The complexation mechanism between CIP and Zn(II) was in-vestigated by1H NMR titration study in DMSO-d
6(Fig. 7). The different
amounts of Zn(II) was added to a solution of CIP (0.038 M) in DMSO-d6,
and then spectrum were taken. Interaction of Zn(II) (1.0 equiv) with CIP induced slight changes in1H NMR spectra, and while the
phenolic-OH (Hb) proton downfield shifted to 12.08 ppm (Δδ = 0.08), the amide
NH (Hc) and imine CH (Hd) protons upfield shifted to 8.25 ppm (Δδ =
Fig. 4. Bar diagram representing Fluorescence responses of CIP (10 μM) at 667
nm containing (10 μM) of Zn2+and other interfering metal ions (50 μM) in DMSO/H2O (v/v, 8/2, PBS buffer, 0.5 mM, pH 7.4).
Fig. 5. Fluorescence changes visualized on test strip of CIP treated with of Zn2+ at different concentrations under 365 nm UV light.
0.06) and 8.11 ppm (Δδ = 0.07), respectively. Also, the carbonyl linked -OCH2protons at 4.77 ppm was observed as two peaks possibly owing
to the interaction between Zn(II) and the carbonyl group in probe CIP. These data suggest that the phenolic-OH, imine and carbonyl groups are the most reasonable binding sites in the complex formation.
To further understand the recognition mechanism of CIP with Zn (II), the DFT calculations were performed. For this purpose, the para-meters in PM6 method using Gaussian 16 software were optimized due to it predicts the present large molecule more accurately as a whole [62].Fig. 8. depicts the molecular orbital diagrams of CIP and CIP-Zn (II) complex. In CIP-Zn(II) complex, CIP exploits nitrogen atom of imine and phenolic hydroxyl group to coordinate with Zn(II). For CIP and CIP-Zn(II) complex, the frontier molecular orbitals have not been distributed on the entire molecule, however HOMO of CIP is mainly centered on calixarene and its LUMO is dominantly spread over the
bromofenol linker and the isophorone fluorophore. On the contrary, HOMO of CIP-Zn(II) complex is chiefly restricted bromo phenol and ethyl acetate hydrazide linkers and its LUMO on the isophorone unit. The energy gap between HOMO-LUMO of CIP (ΔE =3.97 eV) was re-markably decreased to 3.08 eV of CIP-Zn(II) complex, which suggest that binding of CIP with Zn(II) caused to the suppression of PET process and distinct difference in emission output. Besides, another reason for the emission enhancement in CIP-Zn(II) complex is the restriction of free rotation around C]N bond in excited state. Therefore, theoretical data are in agreement with the complex formation between CIP and Zn (II) supported by1H NMR analysis.
To reveal the potential of CIP to detect Zn(II) in practical applica-tion, the real-environment assays were performed. Because real water samples do not contain zinc residues, tap water samples were spiked with the known amounts of Zn(II) ions, then the recoveries was cal-culated. The results are given in Table S1. Satisfactory recoveries for Zn (II) were obtained in the range of % 92.5 and % 103.0 Therefore, the results validate that CIP can be practically used for Zn(II) detection in water samples.
4. Conclusion
A new NIR fluorescent sensor on the basis of calix [4]arene (CIP) for Zn(II) was developed in this study. CIP showed a strong specific and selective turn-on fluorometric detection and NIR emission for Zn(II) over other cations with an extraordinary Stokes shift (∼155 nm), very low detection limit (6.75 nM) and fast response time (10 s). The com-plexation between CIP and Zn(II) was clarified by spectrometric, NMR and MS analyses and was supported through DFT calculations. CIP was successively used for fabrication of test strips that can easily detect Zn (II) in aqueous samples. In addition, the utility of CIP as a bio-analytical molecular tool was successfully illustrated by fluorescence monitoring of Zn(II) in biological processes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influ-ence the work reported in this paper.
Fig. 7. Changes in1H NMR spectra of CIP upon addition of Zn2+in DMSO-d
6at room temperature.
Fig. 8. The frontier molecular orbitals (HOMO and LUMO) of CIP and
Acknowledgments
Authors are thankful to the Research Foundation of Selcuk University (BAP) for financial assistance. Also, we acknowledge the help from Assoc. Prof. Serdar Karakurt, Biochemistry Department of Selcuk University-Turkey, in living cell imaging studies.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.snb.2019.127574. References
[1] B. Valeur, I. Leray, Design principles of fluorescent molecular sensors for cation recognition, Coord. Chem. Rev. 205 (2000) 3–40.
[2] A. Bianchi, K. Bowman-James, E. Garcìa-España, Supramolecular Chemistry of Anions: Vch Pub, (1997).
[3] A.P. De Silva, H.N. Gunaratne, T. Gunnlaugsson, A.J. Huxley, C.P. McCoy, J.T. Rademacher, et al., Signaling recognition events with fluorescent sensors and switches, Chem. Rev. 97 (1997) 1515–1566.
[4] J.S. Kim, D.T. Quang, Calixarene-derived fluorescent probes, Chem. Rev. 107 (2007) 3780–3799.
[5] A.P. De Silva, D.B. Fox, A.J. Huxley, T.S. Moody, Combining luminescence, co-ordination and electron transfer for signalling purposes, Coord. Chem. Rev. 205 (2000) 41–57.
[6] A. Voegelin, O. Jacquat, S. Pfister, K. Barmettler, A.C. Scheinost, R. Kretzschmar, Time-dependent changes of zinc speciation in four soils contaminated with zincite or sphalerite, Environ. Sci. Technol. 45 (2010) 255–261.
[7] J. Mertens, F. Degryse, D. Springael, E. Smolders, Zinc toxicity to nitrification in soil and soilless culture can be predicted with the same biotic ligand model, Environ. Sci. Technol. 41 (2007) 2992–2997.
[8] E. Callender, K.C. Rice, The urban environmental gradient: anthropogenic influ-ences on the spatial and temporal distributions of lead and zinc in sediments, Environ. Sci. Technol. 34 (2000) 232–238.
[9] G. Lee, D. Williams, G. Cartwright, A. Prasad, D. Oberleas, Trace Elements in Human Health and Disease vol. 1 Zinc and Copper, New york: Academic press, 1976.
[10] J.D. Deshpande, M.M. Joshi, P.A. Giri, Zinc: The trace element of major importance in human nutrition and health, Int. J. Med. Sci. Public Health 2 (2013) 1–6. [11] G.K. Walkup, S.C. Burdette, S.J. Lippard, R.Y. Tsien, A new cell-permeable
fluor-escent probe for Zn2+, J. Am. Chem. Soc. 122 (2000) 5644–5645. [12] J. Bradfield, Plant carbonic anhydrase, Nature 159 (1947) 467.
[13] F.M. Kahan, H. Kropp, J.G. Sundelof, J. Birnbaum, Thienamycin: development of imipenem-cilastatin, J. Antimicrob. Chemother. 12 (1983) 1–35.
[14] T.M. Bray, W.J. Bettger, The physiological role of zinc as an antioxidant, Free Radic. Biol. Med. 8 (1990) 281–291.
[15] K.-L. Chang, T.-C. Hung, B.-S. Hsieh, Y.-H. Chen, T.-F. Chen, H.-L. Cheng, Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells, Nutrition 22 (2006) 465–474. [16] A.S. Prasad, Discovery of human zinc deficiency: its impact on human health and
disease, Adv. Nutr. 4 (2013) 176–190.
[17] S. Chan, B. Gerson, S. Subramaniam, The role of copper, molybdenum, selenium, and zinc in nutrition and health, Clin. Lab. Med. 18 (1998) 673–685.
[18] J.L. Vinkenborg, M.S. Koay, M. Merkx, Fluorescent imaging of transition metal homeostasis using genetically encoded sensors, Curr. Opin. Chem. Biol. 14 (2010) 231–237.
[19] I.L. Mulay, R. Roy, B. Knox, N.H. Suhr, W.E. Delaney, Trace-metal analysis of cancerous and non-cancerous human tissues, J. Natl. Cancer Inst. 47 (1971) 1–13. [20] Z. Paolo, Metal Ions And Neurodengenerative Disorders: World Scientific; (2003). [21] K.J. Barnham, A.I. Bush, Metals in Alzheimer’s and Parkinson’s diseases, Curr. Opin.
Chem. Biol. 12 (2008) 222–228.
[22] K.R. Gee, Z.-L. Zhou, W.-J. Qian, R. Kennedy, Detection and imaging of zinc se-cretion from pancreatic β-cells using a new fluorescent zinc indicator, J. Am. Chem. Soc. 124 (2002) 776–778.
[23] P. Zalewski, I. Forbes, R. Seamark, R. Borlinghaus, W. Betts, S. Lincoln, et al., Flux of intracellular labile zinc during apoptosis (gene-directed cell death) revealed by a specific chemical probe, Zinquin, Chem. Biol. 1 (1994) 153–161.
[24] T. Budde, A. Minta, J. White, A. Kay, Imaging free zinc in synaptic terminals in live hippocampal slices, Neuroscience 79 (1997) 347–358.
[25] N. Altunay, A. Elik, C. Bulutlu, R. Gürkan, Application of simple, fast and eco-friendly ultrasound-assisted-cloud point extraction for pre-concentration of zinc, nickel and cobalt from foods and vegetables prior to their flame atomic absorption spectrometric determinations, Int. J. Environ. Anal. Chem. 98 (2018) 655–675. [26] E.G.C. ERGÜN, A. Kenar, Simultaneous determination of copper (II) and zinc (II) via
simple acid-base titrimetry using glass pH electrode, Turk. J. Chem. 42 (2018) 257–263.
[27] N. Ozbek, S. Akman, Method development for the determination of calcium, copper, magnesium, manganese, iron, potassium, phosphorus and zinc in different types of breads by microwave induced plasma-atomic emission spectrometry, Food Chem. 200 (2016) 245–248.
[28] U. Celik, J. Oehlenschläger, Determination of zinc and copper in fish samples col-lected from Northeast Atlantic by DPSAV, Food Chem. 87 (2004) 343–347. [29] M.H. Lee, J.S. Kim, J.L. Sessler, Small molecule-based ratiometric fluorescence
probes for cations, anions, and biomolecules, Chem. Soc. Rev. 44 (2015) 4185–4191.
[30] K. Rurack, Flipping the light switch ‘ON’–the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions, Spectrochimica Acta Part A, Mol. Biomol. Spectrosc. 57 (2001) 2161–2195. [31] X. Xie, C. Yin, Y. Yue, J. Chao, F. Huo, Fluorescent probe detect distinguishly
sul-fite/hydrogen sulfide and thiol via two emission channels in vivo, Sens. Actuators B Chem. 277 (2018) 647–653.
[32] Y. Yue, F. Huo, P. Ning, Y. Zhang, J. Chao, X. Meng, et al., Dual-site fluorescent probe for visualizing the metabolism of Cys in living cells, J. Am. Chem. Soc. 139 (2017) 3181–3185.
[33] W. Zhang, F. Huo, C. Yin, Photocontrolled single-/Dual-Site alternative fluorescence probes distinguishing detection of H2S/SO2 in vivo, Org. Lett. 21 (2019) 5277–5280.
[34] Y. Yue, F. Huo, C. Yin, Noradrenaline-Specific, Efficient Visualization in Brain Tissue Triggered by Unique Cascade Nucleophilic Substitution, Anal. Chem. 91 (2018) 2255–2259.
[35] Y. Yue, F. Huo, P. Yue, X. Meng, J.C. Salamanca, J.O. Escobedo, et al., In situ ly-sosomal cysteine-specific targeting and imaging during dexamethasone-induced apoptosis, Anal. Chem. 90 (2018) 7018–7024.
[36] R. Kumar, A. Sharma, H. Singh, P. Suating, H.S. Kim, K. Sunwoo, et al., Revisiting fluorescent calixarenes: from molecular sensors to smart materials, Chem. Rev. 119 (2019) 9657–9721.
[37] I. Leray, B. Valeur, Calixarene‐based fluorescent molecular sensors for toxic metals, Eur. J. Inorg. Chem. 2009 (2009) 3525–3535.
[38] M. AnthonyáMcKervey, Calixarene-based sensing agents, Chem. Soc. Rev. 25 (1996) 15–24.
[39] K. Sharma, P. Cragg, Calixarene based chemical sensors, Chem. Sens. 1 (2011) 1–18.
[40] N. Aksuner, E. Henden, I. Yilmaz, A. Cukurovali, Development of a highly sensitive and selective optical chemical sensor for the determination of zinc based on fluorescence quenching of a novel schiff base ligand, Sens. Lett. 8 (2010) 684–689. [41] Y. Hu, Q. Li, H. Li, Q. Guo, Y. Lu, Z. Li, A novel class of Cd (II), Hg (II) turn-on and Cu (II), Zn (II) turn-off Schiff base fluorescent probes, J. Chem. Soc. Dalton Trans. 39 (2010) 11344–11352.
[42] A. Hens, A reversible turn-off fluorescence probe (HNAPP) for Zn (ii) ion and in-organic phosphate ions (H 2 P and HP) at physiological pH, RSC Adv. 5 (2015) 54352–54363.
[43] I.A. Bagatin, E.S. de Souza, A.S. Ito, H.E. Toma, Mixed 8-oxyquinolinecalix [4] arene/phenanthroline receptors as luminescence sensors for zinc (II) ions, Inorg. Chem. Commun. 6 (2003) 288–293.
[44] H.-W. Yeh, O. Karmach, A. Ji, D. Carter, M.M. Martins-Green, H. Ai, Red-shifted luciferase–luciferin pairs for enhanced bioluminescence imaging, Nat. Methods 14 (2017) 971.
[45] A. Gogoi, G. Das, NIR sensing of Zn (II) and subsequent dihydrogen phosphate detection by a benzothiazole functionalized ninhydrin based receptor, RSC Adv. 4 (2014) 55689–55695.
[46] S. Zhang, R. Adhikari, M. Fang, N. Dorh, C. Li, M. Jaishi, et al., Near-infrared fluorescent probes with large Stokes shifts for sensing Zn (II) ions in living cells, ACS Sens. 1 (2016) 1408–1415.
[47] M. Fang, S. Xia, J. Bi, T.P. Wigstrom, L. Valenzano, J. Wang, et al., Detecting Zn (II) ions in live cells with near-infrared fluorescent probes, Molecules 24 (2019) 1592. [48] Q.-Q. Zhang, B.-X. Yang, R. Sun, J.-F. Ge, Y.-J. Xu, N.-J. Li, et al., A near-infrared phenoxazinium-based fluorescent probe for zinc ions and its imaging in living cells, Sens. Actuators B Chem. 171 (2012) 1001–1006.
[49] B. Tang, H. Huang, K. Xu, L. Tong, G. Yang, X. Liu, et al., Highly sensitive and selective near-infrared fluorescent probe for zinc and its application to macrophage cells, Chem. Commun. (2006) 3609–3611.
[50] K. Kiyose, H. Kojima, Y. Urano, T. Nagano, Development of a ratiometric fluor-escent zinc ion probe in near-infrared region, based on tricarbocyanine chromo-phore, J. Am. Chem. Soc. 128 (2006) 6548–6549.
[51] S. Erdemir, S. Malkondu, O. Kocyigit, O. Alıcı, A novel colorimetric and fluorescent sensor based on calix [4] arene possessing triphenylamine units, Spectrochimica Acta Part A, Mol. Biomol. Spectrosc. 114 (2013) 190–196.
[52] X. Zhang, Y. Chen, Synthesis and fluorescence of dicyanoisophorone derivatives, Dye. Pigment. 99 (2013) 531–536.
[53] M. Beija, C.A. Afonso, J.M. Martinho, Synthesis and applications of Rhodamine derivatives as fluorescent probes, Chem. Soc. Rev. 38 (2009) 2410–2433. [54] Y. Duan, M. Liu, W. Sun, M. Wang, S. Liu, Q.X. Li, Recent progress on synthesis of
fluorescein probes, Mini. Org. Chem. 6 (2009) 35–43.
[55] A.P. Gorka, R.R. Nani, M.J. Schnermann, Cyanine polyene reactivity: scope and biomedical applications, Org. Biomol. Chem. 13 (2015) 7584–7598.
[56] J.L. Bricks, A.D. Kachkovskii, Y.L. Slominskii, A.O. Gerasov, S.V. Popov, Molecular design of near infrared polymethine dyes: a review, Dye. Pigment. 121 (2015) 238–255.
[57] J. Jose, K. Burgess, Benzophenoxazine-based fluorescent dyes for labeling biomo-lecules, Tetrahedron 62 (2006) 11021–11037.
[58] G.J. Stasiuk, F. Minuzzi, M. Sae‐Heng, C. Rivas, H.P. Juretschke, L. Piemonti, et al., Dual‐modal magnetic Resonance/Fluorescent zinc probes for pancreatic β‐Cell mass imaging, Chem. Eur. J. 21 (2015) 5023–5033.
[59] Y. Wu, P.V. Lawson, M.M. Henary, K. Schmidt, J.-L. Bredas, C.J. Fahrni, Excited state intramolecular proton transfer in 2-(2 ‘-Arylsulfonamidophenyl) benzimida-zole derivatives: insights into the origin of donor substituent-induced emission
energy shifts, J. Phys. Chem. A 111 (2007) 4584–4595.
[60] S. Erdemir, S. Malkondu, A novel “turn on” fluorescent sensor based on hydroxy-triphenylamine for Zn2+ and Cd2+ ions in MeCN, Sens. Actuators B Chem. 188 (2013) 1225–1229.
[61] S. Erdemir, S. Malkondu, A simple triazole-based “turn on” fluorescent sensor for Al3+ ion in MeCN–H2O and F− ion in MeCN, J. Lumin. 158 (2015) 401–406. [62] M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, et al.,
Gaussian 16, Revis. A J. Conscious. Transform. 3 (2016).
Serkan Erdemir received his Ph.D. from Selcuk University. Currently, he is a Professor in the Department of Chemistry at Selcuk University, Turkey. His research interests are organic synthetic chemistry, organic material science, supramolecular chemistry and molecular sensors.
Sait Malkondu is now an Associate Professor in Environmental Engineering, Giresun University. He got his Ph.D. in Selcuk University, Turkey. Currently his research interest focuses on the synthesis and properties of new fluorescent materials for the detection of environmental pollutants and biologically important analytes.