http://dx.doi.org/10.1080/01694243.2015.1087256
© 2015 Taylor & francis
Does 2% chlorhexidine digluconate cavity disinfectant or
sodium fluoride/hydroxyethyl methacrylate affect adhesion
of universal adhesive to dentin?
Mahmut Kusdemira , Ali Rıza Çetinb, Alev Özsoya, Tuğba Toza, Funda Öztürk Bozkurta and Mutlu Özcanc
adepartment of restorative dentistry, university of Medipol, istanbul, Turkey; bdepartment of restorative
dentistry, university of selçuk, Konya, Turkey; cdental Materials unit, center for dental and oral Medicine, clinic
for fixed and removable Prosthodontics and dental Materials science, university of Zurich, Zurich, switzerland
ABSTRACT
The objectives of this study were to investigate the adhesion of a universal adhesive used either in total-etch (TE) or self-etch (SE) mode with and without 2% chlorhexidine digluconate cavity disinfectant (CHX) or sodium fluoride/hydroxyethyl methacrylate (NaF/HEMA) to dentin. Dentin surfaces of extracted human non-carious third molar teeth (N = 18) were exposed and randomly assigned to two groups. Half of the teeth were conditioned with TE and the others with SE adhesive mode. The teeth were then randomly divided into two groups where half were cleaned with 2% CHX (Cavity Cleanser, Bisco, CC) and the other half with NaF/HEMA (Aqua Prep F, Bisco, APF). Control groups in TE (C1) and SE (C2) adhesive system did not receive any cavity disinfectant. Dentin surfaces were conditioned with universal adhesive (Single Bond Universal, SBU) and resin composite blocks (3 M Z550) were bonded incrementally on the conditioned dentin using a mold. The teeth were stored in water for 48 h, and from each tooth, beam-shaped specimens (1 mm2) were prepared (n = 14, per group). Microtensile bond strength
(MTBS) was measured using a universal testing machine (1 mm/min). Data (MPa) were analyzed using one-way ANOVA and Tukey’s test (α = 0.05). Two-parameter Weibull distribution values including the
Weibull modulus, scale (m), and shape (0) values were calculated. Mean MTBS results (MPa) showed significant difference between the experimental groups (p = 0.001) and were in descending order as follows: C1-CC (32.8 ± 6.4)a < C1 (24.4 ± 5.2)b < C2 (21.1 ± 4.8)b
< C1-APF (19.3 ± 4.4)b < C2-CC (14.1 ± 4.1)c < C2-APF (8.1 ± 2.1)d. C1 and
C2 presented non-significant bond strength of the resin composite bonded with SBU (p > 0.05). CC application significantly increased the bond strength in TE mode, but significant reduction was observed when used in SE mode (p < 0.05). The use of APF did not significantly decrease the bond strength in TE mode, but significant reduction was observed when used in SE mode. Considering Weibull parameters, characteristics of adhesion seem to be less reliable for C2-CC (m = 3.86) and more reliable for C1-CC (m = 6.77). Failure types were predominantly adhesive between the dentin and the adhesive resin. Mixed failures were more common for both C1 and C2 and total etch-CC combination.
KEYWORDS
adhesion; adhesive resin; cavity disinfectant; chlorhexidine; universal bond system ARTICLE HISTORY received 6 July 2015 revised 13 august 2015 accepted 22 august 2015
CONTACT Mutlu Özcan mutluozcan@hotmail.com
Part of this study has been presented as a poster at the 46th annual Meeting of the international association for dental research (iadr) / continental european division (ced) in florence, italy, september, 4–7th, 2013.
Introduction
Current dental adhesive systems and adhesive approaches seek to provide long-term bonding, while ensuring simplification of the technique.[1] Less application steps reduce manipulation time and technique sensitivity and may improve bonding effectiveness in routine clinical practice.[2,3] The current adhesive systems available on the market can be mainly classified as total-etch (etch-and-rinse) (TE) and self-etch (SE) adhesive strategies of three, two or one application step, respectively. In the TE adhesive strategy, the first step is the application of phosphoric acid to both enamel and dentin that removes the smear layer, exposes the collagen fibers in dentin, and increases the surface area and surface energy in enamel.[4,5] As the second step, a solvent-rich primer, hydrophilic functional monomer application, follows this step. Subsequently, adhesive resin, hydrophobic cross-linker resin, is applied as the third step separately in a single solution.[6] The main disadvantage of TE system is that there is a risk of collagen fiber collapse during drying the demineralized dentin that leads to a decrease in bond strength.[7,8] The incomplete impregnation of collagen fibers and the need to protect them against the degrading mechanisms led to the development of another category of adhesive system, namely SE adhesives.
SE adhesives were introduced with the goal of eliminating the highly sensitive step of acid etching. Acidic monomers in such adhesives simultaneously etch and infiltrate into the dentin [1,9] that excludes the problems associated with acid-demineralized dentin depth and resin infiltration of TE adhesives.[6] In the SE strategy, a distinction should be made between ‘mild’ and ‘strong’ SE adhesives. The underlying bonding mechanism of ‘strong’ SE adhesives is primarily diffusion-based, similar to the TE approach.[10] On the other hand, mild SE adhesives (pH 2) only partially dissolve the dentin. Phosphoric acid etching of dentin improves the interface infiltration morphology and removal of the smear layer and smear plugs by this acid application facilitate the adhesive penetration, especially in mild SE approach.[11] Manipulation has been further simplified by reducing the number of initial two solutions, an acidic primer followed by the application of a relatively hydro-phobic adhesive resin on the primed surface, to a one-step system, in which all components (etchant, primer, and adhesive resin) are incorporated into a single solution.[12] Adhesion of ‘all-in-one’ or ‘one-step self-etch’ adhesives to dentin has been progressively improved with respect to the first one-step SE adhesives by means of better chemical interaction [13] but adhesion to enamel still remains unsatisfactory. Hence, application of selective acid etching on enamel before SE adhesive application has been recommended, especially with the use of mild pH SE adhesives.[14] However, inadvertent pre-etching of dentin is a clinical risk as this can negatively affect bonding efficiency.[15,16] Nevertheless, the TE and SE adhesive systems are contemporary and are dividing the preference of clinicians, mainly when technical simplification vs. effectiveness of adhesion to different dentinal substrates is considered.
Recently, a new type of one-step SE adhesive resin has been introduced, classified as ‘univer-sal’ or ‘multi-mode’ adhesive that could be applied either with TE or SE technique.[17,18] These systems were introduced with manufacturer’s claims that one monomer solution could be used for either adhesive strategy, without compromising the bonding effectiveness and thereby, being able to replace existing simplified adhesive resins.[15,16] This versatile capability enables the clinician to apply the adhesive with the so-called selective enamel etching technique that combines the advantages of the TE technique on enamel. Universal adhesives work also with the simplified SE approach on dentin with additional chemical bonding on remnant carbonated apatite crystallites.[19]
Cavity disinfectant such as 2% chlorhexidine digluconate aqueous solution (CHX) or rewetting agents, an aqueous solution of sodium fluoride (NaF) and hydroxyethyl meth-acrylate (HEMA), are recommended for use upon completion of tooth preparation or etching, prior to sealing dentinal tubules with adhesive systems.[19] Cavity cleansers are intended for cleansing, moistening, and disinfecting cavity preparation. Through cleans-ing of cavity preparation, debri and bacteria are removed, decreascleans-ing the post-operative sensitivity. The use of an antimicrobial agent prior to placing restorations may thus help eliminating patient discomfort associated with antimicrobial activity.[20] The use of these rewetting agents on briefly air-dried, acid-etched dentin represents an alternative strategy to circumvent the shortcomings associated with the moist bonding technique. HEMA-containing rewetting agents especially help to rehydrate the collapsed collagen matrix caused by air-drying.[21] Such rewetting agents facilitate subsequent resin infiltration into interfi-brillar spaces of demineralised dentin. There is limited information to date on the adhesive performance of universal adhesives.[18,20]
The objectives of this study, therefore, were to investigate the adhesion of a universal adhe-sive used either in TE or SE mode with and without 2% CHX or rewetting agent to dentin. The null hypothesis tested was that adhesion of universal adhesive to dentin would not be affected by the application of 2% CHX or rewetting agent based on NaF and HEMA.
Materials and methods Specimen preparation
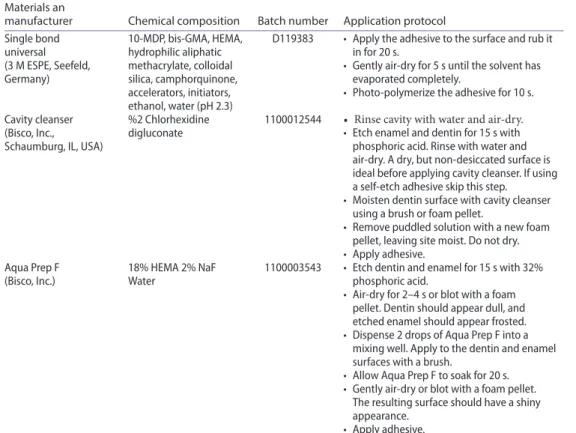
The brands, manufacturers, chemical compositions, and batch numbers of the materials used for the experiments are listed in Table 1. Schematic description of the experimental design is presented in Figure 1.
Extracted caries-free human third molar teeth (N = 18) were used in this study. After tis-sue remnants were removed with a scaler (H6/H7; Hu-Friedy, Chicago, IL, USA), teeth were stored in 0.5% Chloramin T and distilled water up to maximum six months after extraction. The roots were removed from the coronal parts using a diamond disc (IsoMet 1000, Buehler Ltd, USA) under water-cooling. The coronal part of teeth were embedded in a polyvinyl chloride (PVC) mold with their occlusal surfaces exposed using auto- polymerizing acrylic resin (Scandiquick, Scandia, Hagen, Germany).
A low-speed diamond saw (Isomet, Buechler Ltd, IL, USA) under water-cooling was used to remove the cusps and expose the dentin which was then ground finished using 600, 800, and 1000-grit silicon carbide abrasive papers under water-cooling for 5 s in sequence. The exposed dentin was inspected to ensure that all of the occlusal enamel had been removed and the pulp horns had not been perforated.
Experimental groups
The teeth were initially randomly assigned into two groups according to the different bonding strategies of the adhesive system. Half of the teeth were conditioned with TE and the other with SE adhesive technique. The teeth were then further randomly divided into two groups where half of them were cleaned with 2% CHX (Cavity Cleanser, Bisco Inc. Schaumburg, IL, USA, CC) and the other half with NaF/HEMA (Aqua Prep F, Bisco Inc.,
APF). Control groups in TE and SE adhesive system did not receive any cavity disinfectant. Dentin surfaces were conditioned with a universal adhesive (Single Bond Universal, 3 M ESPE, Seefeld, Germany, SBU). Application protocols of the materials according to the manufacturers’ instructions are presented in Table 1.
Restorative procedures
After the bonding procedures, resin composite (3 M Z550, 3 M ESPE, Seefeld, Germany) was built incrementally using a mold (height: 4 mm). Each increment was photo-polymerized for 20 s (Guilin Woodpecker Medical Instrument Co., Ltd, Guangxi, China) from a constant distance of 2 mm from the surface. The output of the polymerization unit was 1100 mW/cm2 verified by a radiometer (Demetron LC, SDS Kerr, Orange, CA, USA).
The bonded tooth-composite assemblies were stored in distilled water at 37 °C for 48 h, and the specimens were sectioned with a slow-speed diamond saw (Isomet, Buehler Ltd.) in order to obtain beams from a tooth with a cross-sectional area of approximately 1 mm2, measured with a digital caliper (Sylvac, Fred V. Fowler Co., Massachusetts, USA). Only the beams from the central region of each tooth were used for the bond tests.
Table 1. The brands, manufacturers, chemical compositions, batch numbers, and application protocols of the materials used for the experiments.
bis-gMa: Bisphenol a glycol dimethacrylate; MdP: 10-methacryloyloxy methacrylate; heMa: 2-hydroxyethyl metacrylate; naf: sodium floride.
Materials an
manufacturer Chemical composition Batch number Application protocol single bond universal (3 M esPe, seefeld, germany) 10-MdP, bis-gMa, heMa, hydrophilic aliphatic methacrylate, colloidal silica, camphorquinone, accelerators, initiators, ethanol, water (ph 2.3)
d119383 • apply the adhesive to the surface and rub it in for 20 s.
• gently air-dry for 5 s until the solvent has evaporated completely.
• Photo-polymerize the adhesive for 10 s. cavity cleanser
(Bisco, inc., schaumburg, il, usa)
%2 chlorhexidine
digluconate 1100012544 • Rinse cavity with water and air-dry.• etch enamel and dentin for 15 s with phosphoric acid. rinse with water and air-dry. a dry, but non-desiccated surface is ideal before applying cavity cleanser. if using a self-etch adhesive skip this step.
• Moisten dentin surface with cavity cleanser using a brush or foam pellet.
• remove puddled solution with a new foam pellet, leaving site moist. do not dry. • apply adhesive.
aqua Prep f
(Bisco, inc.) 18% heMa 2% naf Water 1100003543 • etch dentin and enamel for 15 s with 32% phosphoric acid. • air-dry for 2–4 s or blot with a foam
pellet. dentin should appear dull, and etched enamel should appear frosted. • dispense 2 drops of aqua Prep f into a
mixing well. apply to the dentin and enamel surfaces with a brush.
• allow aqua Prep f to soak for 20 s. • gently air-dry or blot with a foam pellet.
The resulting surface should have a shiny appearance.
Microtensile bond strength test
The beams were attached to the testing apparatus (Bencor-Multi-T-testing, Danville Engineering, Danville, CA, USA) with cyanoacrylate adhesive (Zapit, Dental Ventures of America, Corona, CA, USA) and tensile load was applied using the universal testing machine (Instron 5566 series 5000, Instron Corporation, London, UK) at a crosshead speed of 1 mm/ min. The Microtensile bond strength (MTBS) data were derived by dividing the force imposed at the time of maximum load (N) by the bonded area (mm2). When specimens failed before actual testing, bond strength was considered as 0 MPa in the calculations. The mean Microtensile bond strength test for each group was calculated from 14 beams and expressed in MPa. Failure analysis and microscopy evaluation
Failure sites were initially observed using an optical microscope (x20) (Zeiss Supra V50, Carl Zeiss, Oberkochen, Germany) and classified as follows: Type I: Adhesive failure between the adhesive resin and the dentin; Type II: Mixed failure between the adhesive resin and the dentin with less than half of the adhesive remained on the dentin surface; Type III: Cohesive failure in the composite; Type IV: Cohesive failure in the dentin.
Statistical analysis
Kolmogorov–Smirnov and Shapiro–Wilk tests were used to test normal distribution of the data (SPSS Software V.21, Chicago, IL, USA). As the data (MPa) were normally distributed,
1-way analysis of variance (ANOVA) and Tukey`s test were applied to analyze possible dif-ferences between the groups. Maximum likelihood estimation without a correction factor was used for 2-parameter Weibull distribution, including the Weibull modulus, scale (m), and shape (0), to interpret predictability and reliability of adhesion (Minitab Software V.16, State College, PA, USA). p < 0.05 was considered to be statistically significant in all tests.
Results
Mean MTBS results (MPa) showed significant difference between the experimental groups (p = 0.001) and were in descending order as follows: TE-CC (32.8 ± 6.4)a<TE (24.4 ± 5.2)b<SE (21.1 ± 4.8)b<TE-APF (19.3 ± 4.4)b<SE-CC (14.1 ± 4.1)c<SE-APF (8.1 ± 2.1)d (Table 2).
TE and SE presented no significant difference in MTBS of the resin composite bonded with universal adhesive (p > 0.05). CC application significantly increased the bond strength in TE mode but significant reduction was observed when used in SE mode (p < 0.05). The use of APF did not significantly decrease the bond strength in TE mode but significant reduction was observed when used in SE mode.
Weibull distribution presented lower shape (0) for TE (4.79), SE (5.16), TE-CC (6.77), TE-APF (5.99), SE-CC (3.86) and SE-APF (4.27) (Figure 2).
Failure types were predominantly adhesive between the dentin and the adhesive resin (Type I) with and without CC or APF, except for etch and rinse groups, where mainly Type II
Table 2. MTBs (mean ± standard deviation) of resin composite bonded with universal adhesive on den-tin after cavity cleansing methods and ething modes, maximum, minimum and confidence intervals (95%).
same uppercase letters in each column indicate no significant differences (p > 0.05). see figure 1 for group descriptions. Experimental groups nbeam Mean (SD) Minimum Maximum
Confidence interval Lower bound Upper bound
Te-cc 14 32.8 ± 6.4a 17.4 41.5 29.18 36.56 Te 14 24.4 ± 5.2b 18 35.2 21.41 27.47 se 14 21.1 ± 4.8b 14.5 29.4 18.32 23.84 Te-aPf 14 19.3 ± 4.4b 8.5 23.1 11.83 16.56 se-cc 14 14.1 ± 4.1c 27.7 52.2 36.92 47.26 se-aPf 14 8.1 ± 2.1d 5.3 52.2 21.06 26.53
Figure 2. Probability plot with Weibull curves (95% ci) using maximum likelihood estimation, scale and shape values for all groups.
failures were observed (Figure 3). Cohesive failure in the dentin was not observed in any of the groups.
Discussion
This study investigated the adhesion of a universal adhesive used either in TE or SE mode with and without 2% CHX or rewetting agent based on NaF and HEMA to dentin. Based on the results of this study, due to significant effect of the cavity disinfectants on the results, the null hypothesis could be rejected.
The use of CHX-containing products as a cavity disinfectant has gained popularity; however, studies have reported that adhesion of the restorative materials could be impaired by the application of disinfectants. Results of laboratory studies found in the literature is controversial regarding whether or not to use this agent and there is not much informa-tion on how these agents may affect the bond of composite resin materials. In previous studies, the morphology of the adhesive interface has been studied to in order to identify the hybridization patterns provided by adhesive systems under many different conditions. [22,23] The collapse in collagen fibers, caused by dentin hydration [24], limits the possibility of the micromechanical retention of the adhesive system in primed dentin. However, when the collagen fibers are re-expanded, there is an improvement in the bond strength of the sub-sequent adhesive and the composite resin. The depth of demineralization promoted by the phosphoric acid determines the thickness of the hybrid layer, as the application of acid prior to the application of the primer or primer/adhesive removes the smear layer, demineralizing the dentin structure and consequently exposing collagen fibers. This procedure then forms the hybrid layer.[24,25] The maintenance of the collagen fibers in acid-etched dentin makes the infiltration of hydrophilic monomer easier.[26]
When shear bond test was used, other studies also showed that the application of CHX did not have a negative effect on the bond strength of adhesive systems.[27,28] On the other hand, one study even reported increased shear bond strength when CHX was used.[29] De Castro et al. [28] reported that 2% CHX solution, applied before or after acid etching of the dentin, did not interfere with the μTBS of composite resin to the dentin treated with different
Figure 3. frequencies of failure modes in percentages. Type i: adhesive failure between the adhesive resin and the dentin; Type ii: Mixed failure between the adhesive resin and the dentin with less than half of the adhesive remained on the dentin surface; Type iii: cohesive failure in the composite; Type iV: cohesive failure in the dentin. see figure 1 for group abbreviations.
adhesive resins (Prime&Bond NT, Single Bond, or Clearfil SE Bond). However, Meiers and Kresin [30] found that use of CHX-based cavity disinfectant after tooth preparation, and before the application of a dentin-bonding agent might be material specific regarding their interactions with the sealing ability of various dentin-bonding systems. In another study, 2% CHX cavity disinfectant application, before or after etching, decreased the shear bond strength of composite resin to dentin but rinsing the cavity disinfectant before adhesive resin application to dentin did not affect the bond strength.[31]
In this study, SBU used in TE mode after CC application exhibited significantly higher bond strength values than those of the other application modes. SBU contains 10-MDP in its composition where MDP can chemically bond to Ca++ ions and form stable MDP-Ca salts. According to the ‘adhesion-decalcification’ concept, these salts deposit at the adhesive interface, forming ‘self-assembled nano-layers’, may be responsible for the good perfor-mance of MDP-containing adhesives on dentin.[16] Previous studies have confirmed that 10-MDP is the best acidic functional monomer, showing stable and durable interaction with hydroxyapatite for both enamel and dentin.[9,32,33] Thus, the selective etch technique is especially recommended for MDP-containing universal adhesives. However, our results are in contrast with a previous study on 10-MDP-based adhesives reporting that phosphoric acid etching of dentin prior to adhesive application significantly decreased the bond strength to dentine.[17] The results of this study clearly indicated that the performance of SBU was dependent on the adhesive strategy. The results of MTBS test showed higher resin–dentin bond strengths when the universal adhesive was used in TE mode with and without CC or APF compared to SE mode. It also has to be noted that in SE mode, both CC and APF demonstrated significantly lower MTBS values indicating that the disinfectant regimen or rewetting agents perform better when dentin is acid etched separately most probably due to the removal of the smear layer.
A previous study that tested the hypothesis that CHX could inhibit the degradation of resin–dentin bonds by blocking the matrix metalloproteinase concluded that CHX stabilized the bond strengths of the treated dentin surfaces.[34] Our study concluded that APF, based on NaF and HEMA, does have a negative effect on the bond strength of universal adhe-sive used in SE mode. HEMA, a methacrylate derivative, is a component of many current hydrophilic adhesives due to its ability to promote adhesion [30] as it infiltrates into the intertubular dentin during absorption, thus facilitating the diffusion of resin monomers and the formation of hybrid layer.[34] The ambiphilic nature makes HEMA a very convenient component of adhesives since it acts as a link between the hydrophilic dentin surface and the hydrophobic restorative resins.[35,36] In this study, application of APF with universal adhesive resulted in significant reduction in MTBS values. This could be explained on the grounds that APF is composed of 35% HEMA, and HEMA is the main absorption path of universal adhesive tested. The high concentration of hydrophilic components, due to the combination of APF and universal adhesive, in this case SBU, decreased the bond strength values, as it was difficult for the hydrophobic component of the bonding agent to penetrate in the dentin tubules, which is responsible from the hybrid layer resistance.[37]
As the specimens were tested only after 48 h water storage, this study simulates an early bonding scenario. Maximum polymerization with these cements may take up to 24 h [38], and during this time, the patients need to function and consequently early debondings may occur. The extended storage time in water or challenging the interfaces in thermocycling after initial bonding of the resin composite could be taken into account in future studies. However, it has to be noted that during thermocycling process, with some cement systems
further polymerization and thereby increased degree of conversion could be observed. For this reason, short- and long-term aging in the same study may bring additional information on the adhesion behavior of resin composite to dentin.
In our study, the teeth were stored in 0.5% Chloramin T and water before using for testing procedures. Chloramin T is a close analog to sodium hypochlorite, but unlike bleach, it does not affect collagen network.[38] Among other storage media used for teeth, bond strength of resin materials to dentin is not affected by storage in Chloramin T.[39]
Briefly, considering adhesion results, failure types and Weibull parameters, the use of CC, 2% chlorhexidine digluconate, was more effective on improving the bond strength of the universal adhesive tested, compared to the use of APF in both adhesive strategies.
Conclusions
From this study, the following could be concluded:
(1) Adhesion of the resin composite bonded with universal adhesive showed no signif-icant difference after application of either TE or SE adhesives.
(2) Cavity disinfectant, 2%CHX, significantly increased the bond strength in TE mode but significant reduction was observed when used in SE mode.
(3) The use of cavity rewetting agent based on NaF/HEMA did not significantly decrease the bond strength in total etch mode but significant reduction was observed when used in SE mode.
(4) Characteristics of adhesion seems to be less reliable for the use of universal adhesive in the SE mode in combination with 2%CHX and more reliable for TE mode in combination with 2%CHX.
Clinical relevance
Based on bond strength data, failure types, the use of 2% CHX cavity disinfectant may be beneficial when universal adhesive is used in TE mode but in the SE mode neither 2% CHX nor NaF/HEMA could be advised.
Disclosure statement
The authors did not have any commercial interest in any of the materials used in this study. ORCID
Mahmut Kusdemir http://orcid.org/0000-0003-3460-4382
References
[1] Wagner A, Wendler M, Petschelt A, et al. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014;42:800–807.
[2] Van Meerbeek B, De Munck J, Yoshida Y, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper. Dent. 2003;28:647–660. [3] Cho BH, Dickens SH. Effects of the acetone content of single solution dentin bonding agents on
[4] Van Meerbeek B, Inokoshi S, Braem M, et al. Morphological aspects of the resin–dentin interdiffussion zone with different dentin adhesive systems. J. Dent. Res. 1992;71:1530–1540. [5] Van Meerbeek B, Perdiago J, Lambrechts P, et al. The clinical performance of adhesives. J.
Dent. 1998;26:1–20.
[6] Seziando A. Looking for the ideal adhesive–a review. Rev. Port Estomatol. Med. Dent. Cir. Maxillofacial. 2014;55:194–206.
[7] Tay FR, Gwinnett JA, Wei SH. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free acetone based, single bottle primer/adhesives. Dent. Mater. 1996;12:236–244.
[8] Spencer P, Swafford JR. Unprotected protein at the dentin–adhesive interface. Quintessence Int. 1999;30:501–507.
[9] Van Meerbeek B, Yoshihara K, Yoshida Y, et al. State of art of self-etch adhesives. Dent. Mater.
2011;27:17–28.
[10] Krithikadatta J. Clinical effectiveness of contemporary dentin bonding agents. J. Cons. Dent.
2010;13:173–180.
[11] Oliviera SS, Pugach MK, Hilton JF, et al. The influence of smear layer on adhesion: a self etching primer vs. a total etch system. Dent. Mater. 2003;19:758–767.
[12] Breschi L, Mazzoni A, Ruggeri Jr A, et al. Dental adhesion review: aging and the stability of the bonded interface. Dent. Mater. 2008;24:90–101.
[13] Yoshida Y, Yoshihara K, Nagaoka N, et al. Self-assembled nano-layering at the adhesive interface. J. Dent. Res. 2012;91:376–381.
[14] Peumans M, De Munck J, Van Landuyt KL, et al. Eight year clinical evaluation of a 2-step self-etch adhesive with and without selective enamel etching. Dent. Mater. 2010;26:1176–1184. [15] Torii Y, Itou K, Nishitani Y, et al. Effect of phosphoric acid etching prior to self-etching primer application on adhesion of resin composite to enamel and dentin. Am. J. Dent. 2002;15:305– 308.
[16] Van Landuyt KL, Peumans M, De Munck J, et al. Extension of a one-step self-etch adhesive into a multi-step adhesive. Dent. Mater. 2006;22:533–544.
[17] Hanabusa M, Mine A, Kubochi T, et al. Bonding effectiveness of a new multi-mode adhesive to enamel and dentin. J. Dent. 2012;40:475–484.
[18] Perdiago J, Seziando A, Monteiro PC. Laboratory bonding ability of a multi-purpose dentin adhesive. Am. J. Dent. 2012;25:153–158.
[19] Marchesi G, Frasetto A, Mazzoni A, et al. Adhesive performance of a multi-mode adhesive system: 1-year in vitro study. J. Dent. 2014;42:603–612.
[20] Hiraishi N, Yiu CK, King NM, et al. Effect of 2% chlorhexidine on dentin microtensile bond strengths and nanoleakage of luting cements. J. Dent. 2009;37:440–448.
[21] Itthagarun A, King NM, Wefel JS, et al. The effect of fluoridated and non-fluoridated rewetting agents on in vitro recurrent caries. J. Dent. 2001;29:255–273.
[22] Elhabashy A, Swift Jr EJ . Bonding to etched, physiologically hydrated dentin. Am. J. Dent.
1994;7:50–52.
[23] Susin AH, Vasconcellos WA, Saad JRC, et al. Tensile bond strength of self-etching versus total-etching adhesive systems under different dentinal substrate conditions. Braz. Oral Res.
2007;21:81–86.
[24] Nakabayashi N, Sami Y. Bonding to intact dentin. J. Dent. Res. 1996;75:1706–1715.
[25] Prati C, Chersoni S, Mongiorgi R, et al. Resin-infiltrated dentin layer formation of new bonding systems. Oper. Dent. 1998;23:185–194.
[26] Spohr AM, Conceicao EN, Pacheco JFM. Tensile bond strength of four adhesive systems to dentin. Am. J. Dent. 2001;14:247–251.
[27] Botelho MG. Inhibitory effects on selected oral bacteria of antibacterial agents incorporated in a glass ionomer cement. Caries Res. 2003;37:108–114.
[28] de Castro FL, Andrade MF, Duarte Junior SL, et al. Effect of 2% chlorhexidine on microtensile bond strength of composite to dentin. J. Adhes. Dent. 2003;5:129–138.
[29] Erdemir A, Ari H, Gungunes H, et al. Effects of medications for root canal treatment on bonding to root canal dentin. J. Endod. 2004;30:113–116.
[30] Meiers JC, Kresin JC. Cavity disinfectants and dentine bonding. Oper. Dent. 1996;21:153–159. [31] Yiu CK, Hiraishi N, Tay FR, et al. Effect of chlorhexidine incorporation into dental adhesive
resin on durability of resin–dentin bond. J. Adhes. Dent. 2012;14:355–362.
[32] Van Landuyt KL, Yoshida Y, Hirata I, et al. Influence of the chemical structure of functional monomers on their adhesive performance. J. Dent. Res. 2008;87:757–761.
[33] Iwai H, Nishiyama N. Effect of calcium salt of functional monomer on bonding performance. J. Dent. Res. 2012;91:1043–1048.
[34] Carrilho MR, Geraldeli S, Tay F, et al. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007;86:529–533.
[35] Nakabayashi N, Takarada K. Effect of HEMA on bonding to dentin. Dent. Mater. 1992;8: 125–130.
[36] Xu J, Stangel I, Butler IS, et al. An FT-Raman spectroscopic investigation of dentin and collagen surfaces modified by 2-hydroxy-ethylmethacrylate. J. Dent. Res. 1997;76:596–601.
[37] Soares CJ, Santos Filho PCF, Barreto BCF, et al. Effect of previous desensitizer and rewetting agent application on shear bond strength of bonding system to dentin. Cienc. Odontol. Bras.
2006;9:6–11.
[38] Vrochari AD, Eliades G, Hellwig E, et al. Curing efficiency of four self-etching, self-adhesive resin cements. Dent. Mater. 2009;25:1104–1108.
[39] Mobarak EH, El-Badrawy W, Pashley DH, et al. Effect of pretest storage conditions of extracted teeth on their dentin bond strengths. J. Prosthet. Dent. 2010;104:92–97.