Inhibition effects of different toothpastes on demineralisation of incipient enamel lesions
Tam metin
(2) Altan et al. Fig 1. Toothbrushing simulator.. amounts of carbonated apatite. The reaction between hydroxyapatite and low concentrations of fluoride has been postulated to be an ionic exchange, in which fluoride replaces hydroxyl ions in the crystal lattice structure. The replacement of hydroxyl groups with the smaller fluoride ions should result in a more stable apatite structure. If an OH- ion in pure hydroxyapatite is completely replaced by a fluoride ion (F-), the resulting mineral is fluorapatite Ca10(PO4)6F2.10,18 In addition to the use of fluoride dentifrices, new agents such as Pro-Relief technology have been suggested as a means of stopping caries progression.3 Arginine is an amino acid naturally present in saliva. Arginine bicarbonate is comprised of an amino acid complex with calcium carbonate particles. It adheres to the surface of negatively-charged dentin and interacts with calcium carbonate; thus, it blocks the dentin tubules.4,12 Toothpaste containing a complex of arginine is used to control caries and increase the accuracy of treatment. Only a few studies have reported on the remineralisation potential in initial caries.3 Milk and milk derivatives have been proven to protect teeth.14,26,29,30 Casein phosphopeptide (CPP), which is a milk protein derived from cow’s milk using a selective deposition method, is obtained as a result of cleavage by trypsin. A nanocomplex of CPP-ACP consists of calcium, phosphate, and casein phosphopeptides. Studies have shown that the nanocomplex of CPP-ACP prevents enamel demineralisation and facilitates remineralisation of the enamel surface.14,26,30 The main function of casein phosphopeptides is to modulate the bioavailability of calcium phosphate by maintaining ionic phosphate and calcium supersaturation, which increases remineralisation.8,36 The role of ACP is also said to control the pre-. 180. cipitation of CPP with calcium and phosphate ions. The nanoclusters formed by CPP and ACP buffer the activities of free calcium and phosphate ions in the plaque fluid, helping to maintain supersaturation with respect to enamel mineral, and acting as a calcium-phosphate reservoir, thereby reducing enamel demineralisation and enhancing remineralisation.6,25 Bioactive glasses have been adopted as a potential bioactive material for bone healing.15,20 Based on this feature, studies have examined the effects of bioactive glass on remineralisation in dentistry. Bioactive glass is an inorganic compound consisting of silica calcium, sodium, and phosphate. When it comes in contact with saliva, it quickly releases calcium and phosphorus ions, which are necessary for remineralisation, into the saliva.1 NovaMin technology (GlaxoSmithKlein; Brentford, UK), which was first developed for the treatment of dentin hypersensitivity, treats the root surface of caries by preventing dentin demineralisation. When the active ingredient in NovaMin – inorganic chemical calcium sodium phosphorus silicate – comes in contact with saliva, water or any other bodily fluid, it binds to the tooth surface and initiates the remineralisation process on dental enamel.21,42 The present in vitro study aimed to assess the inhibition effect of pro-arginine technology on initial enamel lesions and compare its efficacy with fluoride toothpaste (Pronamel, Sensodyne GlaxoSmithKlein; Warren, NJ, USA), a tooth mousse containing CPP-ACP (Recaldent, GC; Tokyo, Japan), and a toothpaste (Restore, Dr Collins; Los Angeles, CA, USA) containing bioactive glass (NovaMin, GlaxoSmithKlein; Brentford, UK). The null hypothesis is that none of the tested toothpastes inhibit enamel demineralisation.. MATERIALS AND METHODS Enamel Specimen Preparation In this study, mandibular third molars were used. Malformed, hypoplastic and fractured teeth were excluded from the study. Tissue remnants were cleaned from 25 freshly extracted, caries-free wisdom teeth and stored in 0.2% thymol with distilled water at 4°C. The crowns of all teeth were removed 2 mm below the cementoenamel junction using a slow-speed diamond saw with water cooling (Isomet, Buehler; Lake Bluff, IL, USA). Crowns were sectioned off buccally and lingually, after which 3x3 mm (height x width) windows were prepared on the middle third of the enamel of the 50 samples obtained. Other tooth surfaces outside the windows were coated with nail varnish. Enamel surfaces were not abrasively polished. . Demineralisation Procedure All samples were incubated in demineralisation solution at 37°C for three days, and artificial carious lesions were produced. The demineralisation solution consisted of 2.0 mmol/l calcium, 2.0 mmol/l phosphate, and 75 mmol/l acetate at a pH of 4.3. All samples were rinsed with distilled water for 1 min and dried with air spray. Oral Health & Preventive Dentistry.
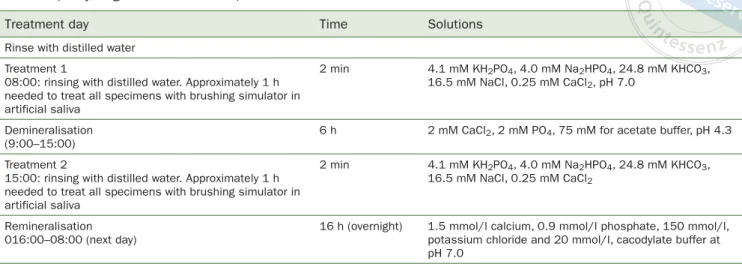
(3) Altan et al. Table 1 pH cycling and treatment steps Treatment day. Time. Solutions. Treatment 1 08:00: rinsing with distilled water. Approximately 1 h needed to treat all specimens with brushing simulator in artificial saliva. 2 min. 4.1 mM KH2PO4, 4.0 mM Na2HPO4, 24.8 mM KHCO3, 16.5 mM NaCl, 0.25 mM CaCl2, pH 7.0. Demineralisation (9:00–15:00). 6h. 2 mM CaCl2, 2 mM PO4, 75 mM for acetate buffer, pH 4.3. Treatment 2 15:00: rinsing with distilled water. Approximately 1 h needed to treat all specimens with brushing simulator in artificial saliva. 2 min. 4.1 mM KH2PO4, 4.0 mM Na2HPO4, 24.8 mM KHCO3, 16.5 mM NaCl, 0.25 mM CaCl2. Remineralisation 016:00–08:00 (next day). 16 h (overnight). 1.5 mmol/l calcium, 0.9 mmol/l phosphate, 150 mmol/l, potassium chloride and 20 mmol/l, cacodylate buffer at pH 7.0. Rinse with distilled water. SEM-Energy Dispersive Spectroscopy (SEM-EDX) Analysis At the end of demineralisationprocedure, the mineral contents (weight percentage) of demineralised enamel were measured with SEM-EDX (LEO 440, K61n, Leike Zeiss; Cambridge, UK). The SEM was operated in the conventional highvacuum mode (20 Kv). Samples were not gold-sputter coated, so that wet sample measurements could be performed. SEM-EDX analysis was carried out at three different points on the samples, and %wt calcium (Ca), %wt phosphorus (P), %wt sodium (Na), and %wt silica (Si) were measured.. pH Cycling, Simulated Toothbrushing The specimens were randomly assigned to the following groups (n = 10/group): 1: no treatment (control); 2. toothpaste containing arginine (ProRelief, Colgate Palmolive; New York, NY, USA); 3. fluoride toothpaste (Pronamel, Sensodyne GlaxoSmithKlein; Warren, NJ, USA); 4. tooth mousse containing CPP-ACP (Recaldent, GC; Tokyo, Japan); 5. toothpaste (Restore, Dr. Collins) containing bioactive glass (NovaMin, Glaxosmithkline). Group 2 to 5 samples were treated for 2 min with the remineralising dentifrices. A custom-made toothbrushing machine was employed with circular (clockwise) motion (Fig 1). The head of a toothbrush with soft bristles was attached to the machine, along with a 125 g load to provide constant contact. During brushing, the brush head was positioned parallel to the demineralised enamel surface. Each sample received 16,000 brush strokes (800 cycles/ min), as described by Ozalp and Tulunoglu.23 Each sample was covered with 20 ml of toothpaste and brushed twice a day for five days.35 The toothpaste was renewed after 5000 and 10,000 brush strokes. Artificial saliva was used to dilute the toothpastes (5 ml dentifrice, 15 ml saliva) used for brushing. Artificial saliva was prepared at pH 7 (4.1 mM KH2PO4, 4.0 mM Na2HPO4, 24.8 mM KHCO3, 16.5 mM NaCl, 0.25 mM CaCl2). Vol 17, No 2, 2019. After the first brushing, each sample was rinsed with distilled water, then immersed in 20 ml of demineralisation solution (2 mM CaCl2, 2 mM PO4, 75 mM for acetate buffer, pH 4.3) for 6 h at 37°C. After the demineralisation challenge, the enamel samples were rinsed with distilled water for 1 min and then treated again with toothpastes for 2 min. Samples were subsequently rinsed with distilled water for 1 min. Then, each crown was immersed in 20 ml of remineralisation solution for 16 h. The remineralisation solution contained 1.5 mmol/l calcium, 0.9 mmol/l phosphate, 150 mmol/l, potassium chloride and 20 mmol/l, cacodylate buffer at pH 7.0 (Table 1). The samples were stored in de/re-mineralisation solutions in an incubator at 37°C. The de-/remineralisation solutions and artificial saliva were changed daily.9. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDX) analysis After de-/remineralisation cycling, samples were completely dried and coated with gold film, then examined in SEM in conventional high-vacuum mode (20Kv). SEM images (LEO 440, Leike Zeiss) were obtained to evaluate the surfaces at 1000X and 2500X magnification. The mineral contents (%wt) of demineralised enamel were measured with SEMEDX (LEO 440, Leike Zeiss).. Statistical Analysis The results were analysed statistically using one-way ANOVA and Tukey’s honest significant differences (HSD) tests at p = 0.05. SPSS 21.0 (IBM; Armonk, NY, USA) was used for the statistical analysis of the results.. RESULTS SEM-EDX Analysis The weight percentages of mineral changes for calcium, phosphorus, sodium and silica are presented in Table 2.. 181.
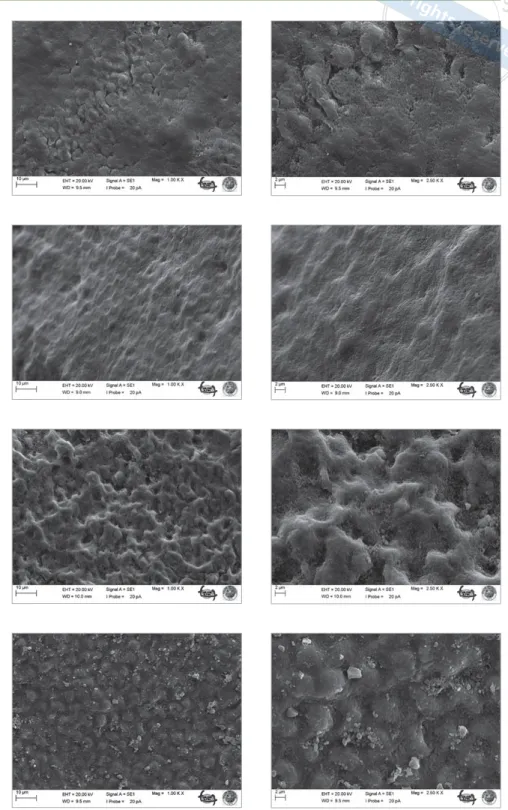
(4) Altan et al. Table 2 Weight percentage of mineral changes (Δ) and standard deviations Groups. ΔCa. ΔP. ΔNa. ΔSi. 1: control. -1.69 ± 1.02a. -0.56 ± 0.08a. -0.04 ± 0.03a. -0.01 ± 0.01a. 2: arginine toothpaste. 1.13 ± 0.85b. 1.11 ± 0.13b. 0.21 ± 0.09b. 0.08 ± 0.04a. 3: fluoride toothpaste. 1.34 ± 0.04b. 1.19 ± 0.23b. 0.24 ± 0.05b. 0.11 ± 0.05a. 4: CPP-ACP toothpaste. 2.46 ± 0.48c. 3.04 ± 0.14c. 0.04 ± 0.23c. -0.01 ± 0.0a. 5: bioglass toothpaste. 0.63 ± 0.39b. 0.6 ± 0.18b. 0.26 ± 0.18b. 0.54 ± 0.21c. Different superscript letters in each column indicate statistically significant differences within the tested materials (p < 0.05).. In Group 4 (CPP-ACP containing toothpaste), the enamel surface treated with CPP-ACP toothpaste had a inhomogeneous appearance, with some pits not filled with mineral deposits. In contrast, globules of deposits were observed on the enamel surface (Fig 5).41 In Group 5 (toothpaste containing bioglass), mineral deposition took place selectively on enamel prisms. Mineral deposition was homogeneous and all pits and voids were covered. Small toothpaste particles were observed on the enamel surface (Fig 6).. DISCUSSION. Fig 2. Demineralised enamel surface (2500X magnification).. Levels of Ca and P increased statistically significantly in all groups compared to the control group (p < 0.05). The levels of Ca and P in group 4 (CPP-ACP) increased statistically significantly compared with groups 1, 2, 3, and 5. In group 5 (NovaMin), there was also a statistically significant increase in Na and Si.. SEM Analysis In group 1 (control group), interprismatic structures and porosities created by the different treatments are visible on enamel surfaces. Ordered and disordered distributions of hydroxyapatite crystallites are apparent in the incipient lesion area on the enamel surface (Fig 2). In group 2 (toothpaste containing arginine), partial coverage was observed on the enamel surface. The treated enamel surfaces retained calcified deposits but holes were evident on the enamel (Fig 3). Group 3 (fluoride toothpaste) showed enamel surface changes after pH cycling and brushing with toothpaste. The enamel surface was filled with mineral deposits, and appeared smooth and uniformly flat (Fig 4).. 182. In contemporary dentistry, priority is given to non-invasively managing initial molecular changes on the enamel surface through remineralisation intervention to prevent the caries process.7 Caries progression involves a continual imbalance between pathological and protective factors that result in the dissolution of apatite crystals and loss of calcium, phosphate, and other ions from the enamel surface.33,38 In present study, calcium, phosphate, sodium and silica ions were analyzed with SEM-EDX to evaluate the in vitro efficacy of four different remineralising agents on incipient enamel lesions. It was noticed that all of the tested toothpastes used in this study inhibited demineralisation on enamel, so that the null hypothesis was rejected. Fluoride is the cornerstone of the non-invasive management of incipient enamel lesions.27 Fluoride is effective in promoting mineral deposition and inhibiting mineral dissolution.39 In particular, fluoride ions remineralise the demineralised enamel by penetrating the surface of the reactive crystals and increasing the diffuseability of calcium and phosphate. Fluoride ions promote the formation of fluorapatite in enamel in the presence of calcium and phosphate ions produced during enamel demineralisation by plaque bacterial organic acids. Fluoride ions can also drive remineralisation of previously demineralised enamel if enough salivary or plaque calcium and phosphate ions are available when the fluoride is applied.31 In the fluoride toothpaste group, SEM-EDX showed positive mineral changes (calcium, phosphate, sodium and silica) in the newly covered enamel layer. The demineralised enamel prisms (control group) conOral Health & Preventive Dentistry.
(5) Altan et al Fig 3 SEM images of toothpaste containing arginine (left: 1000X; right: 2500X).. Fig 4 SEM images of fluoride toothpaste (left: 1000X; right: 2500X).. Fig 5 SEM images of toothpaste containing CPP-ACP (left: 1000X; right: 2500X).. Fig 6 SEM images of toothpaste containing bioglass (left: 1000X; right: 2500X).. tained numerous voids, whereas the voids following remineralisation with fluoride toothpaste showed substantial occlusion. SEM images of the enamel surface revealed that homogeneous, thin, and completely closed interprismatic spaces were formed without including mineral precipitation. In this study, two main reasons may explain the deficiency of mineral precipitation on the enamel surface. The first reason is the solubility of the compound containing bioavailVol 17, No 2, 2019. able fluoride. 15 ml of artificial saliva was used to dilute the toothpastes used for brushing; this amount may be decreased to become more effective. The second reason is the adhesion of fluoride compound to the enamel surface.22 This low remineralisation potential may be due to the fact that the low solubility of fluoride toothpaste could lead to precipitation of calcium and phosphate ions, which makes it more difficult for these ions reach the tooth surface.. 183.
(6) Altan et al. Arginine toothpaste (Pro Relief system) contains arginine amino acids and calcium carbonate.9 Its mechanism involves binding to the negatively charged dentin-enamel surface, creating a protective layer that covers surface defects and occludes the exposed dentinal tubules, consequently reducing sensitivity.9 Poggio et al24 and Lombardini et al17 reported that remineralisation of eroded enamel is supported by toothpaste containing arginine. When the tested groups were compared, fluoride toothpaste (group 3) was found to have an inhibition demineralisation potential similar to that of arginine toothpaste (group 2) according to SEM-EDX analysis; however, SEM images were different. Arginine toothpaste is a combination of arginine and an insoluble calcium compound. Despite similar fluoride ratios in groups 2 (1450 ppm F-) and 3 (1450 ppm F-), differences in SEM images and less increase in calcium and phosphate concentration compared to the fluoride group may be due to inhibitory effects between arginine protein and fluoride ions in toothpaste (group 3). CPP-ACP can infiltrate into the porosities of enamel lesions and diffuse down concentration gradients into the body of the lesion.7 The mechanism of action of the CPPACP nanocomplex effectively increases the level of bioavailable calcium and phosphate ions.22 In the present study, the highest mineral changes were found in the CPP-ACP group. SEM images clearly showed accumulation of CPPACP, a salient feature of remineralisation. The CPP-ACP mousse can be used alone in the treatment of initial enamel caries; however, in our opinion, it can also be combined with other toothpastes that have a low fluoride percentage. Doing so increases the remineralisation effect of the agents and protects from adverse systemic side effects by decreasing the amount of fluoride that is used. Bioglass rapidly exchanges with hydrogen cations (H3O+), bringing about the release of calcium and phosphate ions from the glass.13 When bioglass particles are attached to the surface of teeth, the number of sodium ions increases, and the pH of the local environment also increases. The increase in the number of silica ions on the enamel surface plays a key role in the collapse of hydroxyapatite in the early stages of caries and the creation of nucleation sites.11,16 When the mineral changes of surface enamel were examined in this study, the highest increase in the amount of sodium and silica ions was observed in the toothpaste containing bioactive glass. In the present study, it was found that toothpaste containing bioactive glass has a relatively high remineralisation potential compared to the other agents used. A limitation of this study is that enamel itself is not conductive, which is a problem when using EDX baseline assessment in high vacuum conditions without any coating.. 184. CONCLUSION The application of the tested toothpastes showed a preventive effect against enamel demineralisation to varying degrees. Among the materials tested, the toothpaste containing bioactive glass appears to be more effective in incorporating into prismatic and interprismatic structure of demineralised enamel.. REFERENCES 1. 2. 3. 4.. 5. 6. 7.. 8. 9. 10.. 11.. 12.. 13.. 14.. 15.. 16.. Allan I, Newman H, Wilson M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials 2001;22:1683–1687. Amaechi BT, van Loveren C. Fluorides and non-fluoride remineralisation systems. Monogr Oral Sci 2013;23:15–26. Amaechi BT. Remineralisation therapies for initial caries lesions. Current Oral Health Reports 2.2 2015;2:95–101. Ayad F, Ayad N, Delgado E, Zhang YP, DeVizio W, Cummins D, et al. Comparing the efficacy in providing instant relief of dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride to a benchmark desensitizing toothpaste containing 2% potassium ion and 1450 ppm fluoride, and to a control toothpaste with 1450 ppm fluoride: a three-day clinical study in Mississauga, Canada. J Clin Dent 2009;20:115–122 Buzalaf M, Hannas A, Kato M. Saliva and dental erosion. J App Oral Sci 2012;20:493–502. Cochrane NJ, Reynolds EC. Calcium phosphopeptides–mechanisms of action and evidence for clinical efficacy. Adv Dent Res 2012;24:41–47. Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res 2008;42:88–97. Cross KJ,Huq NL, Reynolds EC. Casein phosphopeptides in oral healthchemistry and clinical applications. Curr Pharm Des 2007;13:793–800. Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent 2009;20:1–9. Cury JA, Tenuta LM. Enamel remineralisation: controlling the caries disease or treating early caries lesions? Braz Oral Res 2009;23(suppl 1): 23–30. Diamanti I, Koletsi-Kounari H, Mamai-Homata E, Vougiouklakis G. Effect of fluoride and of calcium sodium phosphosilicate toothpastes on presoftened dentin demineralisation and remineralisation in vitro. J Dent 2010;38:671–677. Docimo R, Montesani L, Maturo P, Costacurta M, Bartolino M, Zhang YP, et al. Comparing the efficacy in reducing dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride to a benchmark commercial desensitizing toothpaste containing 2% potassium ion: an eight-week clinical study in Rome, Italy. J Clin Dent 2009;20:137–143. Du Min Q, Bian Z, Jiang H, Greenspan DC, Burwell AK, Zhong J, et al. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am J Dent 2008;21:210–214. Harper D, Osborn J, Hefferren J, Clayton R. Cariostatic evaluation of cheeses with diverse physical and compositional characteristics. Caries Res 1986;20:123–130. Hench LL, Splinter RJ, Allen W, Greenlee T. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mat Res 1971;5: 117–141. LaTorre G, Greenspan DC. The role of ionic release from NovaMin (calcium sodium phosphosilicate) in tubule occlusion: an exploratory in vitro study using radio-labeled isotopes. J Clin Dent 2010;21:72–76.. Oral Health & Preventive Dentistry.
(7) Altan et al 17. Lombardini M, Ceci M, Colombo M, Bianchi S, Poggio C. Preventive effect of different toothpastes on enamel erosion: AFM and SEM studies. Scanning 2004;36:401–410. 18. Lussi A, Hellwig E, Klimek J. Fluorides – mode of action and recommendations for use. Schweiz Monatsschr Zahnmed 2012;122:1030–1042. 19. Mattousch TJ, van der Veen MH, Zentner A. Caries lesions after orthodontic treatment followed by quantitative light-induced fluorescence: a 2-year follow-up. Eur J Orthod 2007;29:294–298. 20. MerolliA, Leali PT, Guidi PL, Gabbi C. Comparison in in-vivo response between a bioactive glass and a non-bioactive glass. J Mater Sci Mater Med 2000;11:219–222. 21. Milly H, Festy F, Andiappan M, Watson TF, Thompson I, Banerjee A. Surface pre-conditioning with bioactive glass air-abrasion can enhance enamel white spot lesion remineralisation. Dent Mat 2015;31:522–533. 22. Narayana SS, Deepa VK, Ahamed S, Sathish ES, Meyappan R, Satheesh Kumar KS. Remineralisation efficiency of bioactive glass on artificially induced carious lesion an in-vitro study. J Indian Soc Pedod Prev Dent 2014;32:19–25. 23. Ozalp S, Tulunoglu O.SEM–EDX analysis of brushing abrasion of chitosan and propolis based toothpastes on sound and artificial caious primary enamel surfaces. Int J Paed Dent, 2014;24:349–57. 24. Poggio C, CeciM, BeltramiR, LombardiniM, Colombo M. Atomic force microscopy study of enamel remineralisation. Ann Stomatol 2014;5:98–102. 25. Prestes L, Souza BM, Comar LP, Salomao PA, Rios D, Magalhaes AC. In situ effect of chewing gum containing CPP–ACP on the mineral precipitation of eroded bovine enamel – a surface hardness analysis. J Dent 2013; 41:747–751. 26. Pulido MT, Wefel JS, Hernandez MM, Denehy GE, Guzman-Armstrong S, Chalmers JM, et al. The inhibitory effect of MIpaste, fluoride and a combination of both on the progression of artificial caries-like lesions in enamel. Oper Dent 2008;33:550–555. 27. Rao A, Malhotra N. The role of remineralising agents in dentistry: a review. Compend Contin Educ Dent 2011:32:27–34. 28. Reynolds EC. The prevention of sub-surface demineralisation of bovine enamel and change in plaque composition by casein in an intra-oral model. J Dent Res 1987;66:1120–1127. 29. Reynolds EC, Johnson I. Effect of milk on caries incidence and bacterial composition of dental plaque in the rat. Arch of Oral Biol 1981;26:445–451.. Vol 17, No 2, 2019. 30. Reynolds EC. Remineralisation of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res 1997; 76:1587–1595. 31. Reynolds, EC. Calcium phosphate-based remineralisation systems: scientific evidence? Aust Dent J 2008;53:268–273. 32. Robinson C, Shore RC, Brookes SJ, Strafford S, Wood SR, Kirkham J. The chemistry of enamel caries. Crit Rev Oral Biol Med 2000;11:481–495. 33. Silverstone LM, Wefel JS, Zimmerman BF, Clarkson BH, Featherstone MJ. Remineralisation of natural and artificial lesions in human dental enamel in vitro. Effect of calcium concentration of the calcifying fluid. Caries Res 1981;15:138–157. 34. Stookey GK, DePaola PF, Featherstone JD, Fejerskov O, Moller IJ, Rotberg S, et al. A critical review of the relative anticaries efficacy of sodium fluoride and sodium monofluorophosphate dentifrices. Caries Res 1993;27: 337–360. 35. Stookey GK, Featherstone JD, Rapozo-Hilo M, Schemehorn BR, Williams RA, Baker RA, et al. The Featherstone laboratory pH cycling model: a prospective, multi-site validation exercise. Am J Dent 2011;24:322–328. 36. Schüğbach P, Neeser JR,Golliard M, Rouvet M, Guggenheim B. Incorporation of caseinoglycomacropeptide and caseinophosphopeptide into the salivary pellicle inhibits adherence of mutans streptococci. J Dent Res 1996;75:1779–1788. 37. Struzycka I. The oral microbiome in dental caries. Pol J Microbiol 2014; 63:127–135. 38 Cate JM, Arends J. Remineralisation of artificial enamel lesions in vitro: III. A study of the deposition mechanism. Caries Res 1980;14:351-358. 39. Ten Cate JM, Featherstone JDB. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med, 1991;2: 283–296. 40. van der Veen MH, Mattousch T, Boersma JG. Longitudinal development of caries lesions after orthodontic treatment evaluated by quantitative light-induced fluorescence. Am J Orthod Dentofacial Orthop 2007;131: 223–228. 41. Vyavhare S,Sharma DS, KulkarniVK. Effect of three different pastes on remineralisation of initial enamel lesion: an in vitro study. J Clin Pediat Dent 2015;39:149–160. 42. Wang Z, Jiang T, Sauro S, Wang Y, Thompson I, Watson TF, et al. Dentine remineralisation induced by two bioactive glasses developed for air abrasion purposes. J Dent 2011;39:746–756.. 185.
(8)
Şekil


Benzer Belgeler
However, we also saw that these two theorists were no less reductive in their respective displacements. In Austins case, polysemy was reduced by its proper context, defined
Due to her ongoing symptoms, computed tomography coronary angiography was performed which revealed right coronary artery (RCA) originating from the left coronary sinus and,
We evaluated the association between serum GGT, Ca, inor- ganic phosphorus (Pi) levels and short-term cardiac mortality with variables such as age, gender, DM, HT, serum high
14 noktalı uğur böceği Propylea quatuordecimpunctata örnekleme yapılan bütün habitatlardan elde edildi. Edirne civarındaki farklı habitatlardan yapılan
Spinal kord ve nöral köklerde bas›ya neden olan kompresif tip VH’ lar ise daha çok T3-T9 seviyeleri aras›ndaki torakal ver- tebralarda görülmektedir (13). BT ve MRG ile
This review included current studies using saliva samples for the detection of SARS-CoV-2, comparing its sensitivity, cycle threshold, and specificity with those of NP swab.. In
Araştırmada, “kü tüphanecilik ve enformasyon bilimi ” alanında en çok atıf alan iki dergiye dikkat çekilmiştir: Scientometrics (%23.3) ve Journal of the American
Özellikle temel unsurları sağlama bakımından demokratik olarak niteleyebileceğimiz ülkelerde, devlet ile sivil toplum arasındaki ilişkide; gerek devlet gerekse sivil