MaterialsChemistryandPhysics129 (2011) 701–704
ContentslistsavailableatScienceDirect
Materials
Chemistry
and
Physics
jo u r n al h om ep a ge : w w w . e l s e v i e r . c o m / l o c a t e / m a t c h e m p h y s
Materials
science
communication
Electrospun
polymeric
nanofibrous
composites
containing
TiO
2
short
nanofibers
Ali
E.
Deniz,
Asli
Celebioglu,
Fatma
Kayaci,
Tamer
Uyar
∗UNAM-InstituteofMaterialsScience&Nanotechnology,BilkentUniversity,Ankara,06800,Turkey
h
i
g
h
l
i
g
h
t
s
• PolymericnanofibrouscompositescontaininganataseTiO2shortnanofibers(TiO2-SNF)wereproducedviaelectrospinning. • PMMA,PAN,PETandPCelectrospunnanofiberscontainingTiO2-SNFwereobtained.
• ThenanofibrouscompositescontainingTiO2-SNFhasshownphotocatalyticactivity.
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19February2011
Receivedinrevisedform13May2011 Accepted12June2011 Keywords: Compositematerials Nanostructures Polymers Electronmicroscopy
a
b
s
t
r
a
c
t
Inthisstudy,polymericnanofibrouscompositescontaininganataseTiO2shortnanofibers(TiO2-SNF) weresuccessfullyproducedviaelectrospinning.Thefabricationofthenanofibrouscompositestructure includestwosteps.First,anataseTiO2nanofiberswereobtainedbycalcinationofelectrospunPVP/TiO2 nanofibersandthencrushedintoshortnanofibersrangingfromfewmicronsinlength.Second,these TiO2-SNFweredispersedintopolymersolutionsandthenelectrospunintonanofibrouscomposites.We obtainednanofiberscontainingTiO2-SNFfromdifferentpolymertypesincludingPMMA,PAN,PETand PC.TheSEMandTEMimagingindicatedthatsomeoftheTiO2-SNFwerefullycoveredbythepolymeric matrixwhereassomeTiO2-SNFwerepartiallycoveredand/orstickonthesurfaceofthefibers.The photocatalyticactivityofnanofibrouscompositescontainingTiO2-SNFwasevaluatedbymonitoringthe photocatalyticdecompositionofamodeldye(rhodamine-6G)underUVirradiation.
© 2011 Elsevier B.V. All rights reserved.
1. Introduction
Functional nanofibers can be easily produced from var-ious polymers, polymer blends, sol–gels, composites and metal oxides via electrospinning technique [1–8]. Electrospun nanofibers/nanowebshaveseveralremarkablecharacteristicssuch asaverylargesurfaceareatovolumeratioandnanoporous fea-turesalongwithphysical,chemicalandsurfacefunctionalization
[1–8].Inaddition,itisfairlyeasytoimprovethefunctionalityof theelectrospunnanofibersbyelectrospinningofpolymermatrix containing functional additives (e.g. nanoparticles) [2,3,9]. For instance,theincorporation ofmetaloxide nanoparticlessuchas TiO2 into electrospunpolymeric nanofibers were reported and
itwasshownthatthesefunctionalnanofibrouscompositeshave potentialstobeusedinfiltrationsinceTiO2 nanoparticlesshow
photocatalyticactivityand arevery effectivefor degradationof organicpollutantsand/orkillingbacteria[10–13].Yet,theleaching ofnanoparticlesfromthesepolymericcompositesandtheir
recov-∗ Correspondingauthor.Tel.:+903122903571;fax:+903122664365. E-mailaddress:tamer@unam.bilkent.edu.tr(T.Uyar).
eryisalwaysanenvironmentalissue[14,15].Recently,ithasalso beenreportedthatelectrospunTiO2 shortnanofibershavingfew
micrometersinlengthhaveshownbetterphotocatalyticactivity comparedtoTiO2nanoparticles[16].
In this study,we used TiO2 short nanofibers(TiO2-SNF)for
producing polymeric nanofibrous composite structures. These polymericnanofiberscontainingTiO2-SNFweresuccessfully
pro-duced via electrospinning with the goal to develop functional nanofiltrationmaterialssinceTiO2isknowntobeveryeffectivefor
degradationoforganicpollutantsduetoitsphotocatalytic activ-ity.WeelectrospunnanofiberscontainingTiO2-SNFfromdifferent
polymer types which are commonly used as filtration materi-alssuchaspoly(methylmethacrylate)(PMMA),polyacrylonitrile (PAN),polyethyleneterephthalate(PET)andpolycarbonate(PC).As apreliminarystudy,thephotocatalyticactivityofPETnanofibers containingTiO2-SNFwasinvestigatedforphotocatalytic
decom-positionofrhodamine-6GunderUVirradiation.Tothebestofour knowledge,thisistheveryfirstreportshowingthatmetaloxide shortnanofiberscan beincorporatedintopolymeric nanofibers viaelectrospinning.Suchnanofibrouscompositestructurescanbe obtainedfromdifferentpolymertypesand willbeapplicablein variousareasdependingonthepolymertypeandthefunctionof metaloxides.
0254-0584/$–seefrontmatter © 2011 Elsevier B.V. All rights reserved. doi:10.1016/j.matchemphys.2011.06.018
702 A.E.Denizetal./MaterialsChemistryandPhysics129 (2011) 701–704
Fig.1.(a)RepresentativeSEMimage,(b)and(c)EDXelementalmapsand(d)EDXspectrumofelectrospunTiO2nanofibers,(e)SEMimageofTiO2-SNFobtainedafter
crushingtheelectrospunTiO2nanofibers.
2. Experimentaldetails
2.1. Materials
Titanium(IV)-isopropoxide(97%,Aldrich),glacialaceticacid(100%,Merck), ethanol(≥99.8%, Sigma–Aldrich),dimethylformamide(DMF)(Riedel, Pestenal), tetrahydrofuran(THF)(≥99.5%,Sigma),dichloromethane(DCM)(≥99%,Sigma), trifluoroaceticacid(TFA)(99%,Sigma–Aldrich),polyvinylpyrrolidone(PVP,Mw ∼1,300,000,Aldrich),poly(methylmethacrylate)(PMMA)(Mw∼350,000,Aldrich), polyacrylonitrile(PAN)(donatedbyAKSACo.,Turkey),polyethyleneterephthalate (PET)(donatedbyKorteksCo.,Turkey),polycarbonate(PC)(Mw∼64,000,Aldrich) wereusedas-received.
2.2. PreparationofTiO2nanofibers
Titanium(IV)-isopropoxide(2.88ml)precursorwasmixedwithglacialacetic acid(2ml)andethanol(2ml)solventsystem.Afterstirringfor10min,PVP(0.6g) dissolvedinethanol(3ml)wasaddedtothissolution.Theresultingmixturewas constantlystirredfor12h,andtheyellowishsol–gelwasobtained.ThePVP/TiO2
solprecursorwasplacedintoa1mlsyringehavingmetallicneedletip(inner diameter=0.4mm)forelectrospinning.Thesolutionwasfedbyasyringepump hor-izontally(Model:SP101IZ,WPI).Electricfieldwascomprisedbythehighvoltage powersupply(MatsusadaPrecision,AUSeries,Japan).Theelectrospinning param-eterswereasfollows:appliedvoltage=15kV,feedrate=0.5mlh−1,tip-to-collector
distance=10cm.Electrospunnanofibersweredepositedonagroundedstationary cylindricalmetalcollectorcoveredbyapieceofaluminumfoil.Theelectrospinning wascarriedoutinenclosedplexiglassboxandat23◦Cat26%relativehumidity.The electrospunnanofibersweredriedovernightunderthehoodandthencalcinatedat 500◦Cfor3hinanoveninordertoobtainpureTiO2nanofibers.
2.3. PreparationofpolymericnanofiberscontainingTiO2shortnanofibers
TiO2shortnanofibers(TiO2-SNF)rangingfrom∼1to∼10minlengthwere
obtainedbycrushingtheelectrospunTiO2nanofibersinamortar.The
concentra-tionofthepolymersolutionsandtheelectrospinningparameterswereoptimized inordertogetbead-freeuniformfibers.Thepolymersolutionswerepreparedas follows:PMMAandPANweredissolvedinDMFusing12.5%(w/v)and20%(w/v) polymerconcentration,respectively.Inaddition,20%(w/v)PETwasdissolvedin TFA:DCM(1:1,v/v),while25%(w/v)PCwasdissolvedinTHF:DMF(3:2,v/v) sol-ventsystem.Then,6%TiO2-SNF(w/w,withrespecttopolymer)inPMMAand
2%TiO2-SNF(w/w,withrespecttopolymer)inPAN,PETandPCsolutionswere
dispersedbystirringfor1h.The%loadingofTiO2-SNFwasaffectingthe
electro-spinning,therefore,TiO2-SNFloadingwaschosenforeachpolymersolutionthat
didnotaffecttheelectrospinningofbead-freenanofibers.Subsequently,PAN,PET, andPCsolutionswereelectrospunwiththefollowingparameters:applied volt-age=15kV,tip-to-collectordistance=10cmandfeedrate=1mlh−1.ForPMMA
Fig.2. XRDpatternofTiO2-SNF.
solutiontheparameterswereasfollows:appliedvoltage=10kV,tip-to-collector distance=15cmandfeedrate=1mlh−1.
2.4. PhotocatalyticactivityofpolymericnanofiberscontainingTiO2short
nanofibers
Thephotocatalyticactivitywasanalyzedbyphotodegradationof rhodamine-6G(Rh-6G)(2×10−5M)inaqueousmedium.ElectrospunPETnanowebcontaining
TiO2-SNF(PET-NF/TiO2-SNF)(weightofnanoweb:2mg,dimensionofthenanoweb:
1.0cm×1.0cm)wasputintoquartzcuvette(width:1cm,length:1cm,andheight: 5cm,Hellma)filledwithrhodamine-6Gsolution.Thecuvettewasplacedwitha distanceof5cmfromtheUVsource(8W,UVLMS-38EL)andkeptunderUV irra-diationat254nmwavelength.Atknowntimeintervals,thedyeconcentrationwas measuredbyusingUV-visspectrophotometer.DuringtheUV–vismeasurement,the PET-NF/TiO2-SNFnanowebwasfloatingonthetopofthedyesolutionandtherefore
thenanowebdidnotinterferewiththeUV–vismeasurement. 2.5. Measurementandcharacterization
Scanningelectron microscope (SEM) (FEI – Quanta 200 FEG) and trans-missionelectron microscope (TEM)(FEI – Tecnai G2F30) were usedfor the
A.E.Denizetal./MaterialsChemistryandPhysics129 (2011) 701–704 703
Fig.3.SEMimagesofnanofibrouscompositeswith(1)lowmagnificationand(2)highmagnification:(a1)and(a2)PMMA-NF/TiO2-SNF,(b1)and(b2)PAN-NF/TiO2-SNF,
(c1)and(c2)PET-NF/TiO2-SNFand(d1)and(d2)PC-NF/TiO2-SNF.
Fig.4. TEMimagesofthePMMA-NF/TiO2-SNFfromtwodifferentfibersshowingthat(a)theTiO2-SNFwasembeddedinthePMMAfibermatrixand(b)thesmallTiO2-SNF
wasembeddedandthelargeTiO2-SNFwasstickingoutfromthePMMAfibermatrix.TiO2-SNFwasvisualizedasadarkobjectintheTEMimage.
morphologicalinvestigationofthenanofibers.Thefiberdiameterrangeandthe averagefiberdiameterweredeterminedfromtheSEMimagesandaround100 fiberswereanalyzed.EnergydispersiveX-ray(EDX)wasperformedforthe ele-mentalanalysisoftheTiO2nanofibers.TheX-raydiffraction(XRD)patternforTiO2
nanofiberswereobtainedbyusingPANanalyticalX’PertPowderdiffractometerwith CuK␣radiationinarange2Â=10–75◦.UV–vis–NIRspectrophotometer(VarianCary
5000)wasusedattherange400–600nmwavelengthtomonitorthephotocatalytic degradationofrhodamine-6Gdyesolution.
3. Resultsanddiscussion
Pure TiO2 nanofibers were produced via electrospinning of
PVP/TiO2 sol precursor followedby removal oforganic
compo-nent(PVP)bycalcinationat500◦Cfor3h.Duringthecalcination process of PVP/TiO2, thematerial maintained its fibrous
struc-tures and brittle TiO2 nanofibers were obtained. The scanning
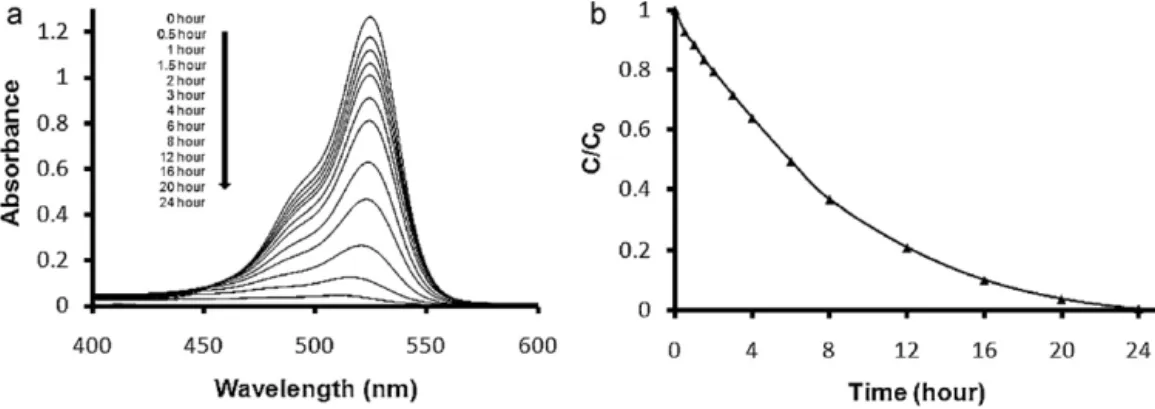
Fig.5.TheUV–visspectrumoftheRh-6GsolutioncontainingPET-NF/TiO2-SNF(a)asafunctionoftheUVirradiationtime,(b)therate(C/C0)ofRh-6Gdegradation.The
704 A.E.Denizetal./MaterialsChemistryandPhysics129 (2011) 701–704
electronmicroscopy(SEM)andtheenergydispersiveX-ray(EDX) spectroscopyanalysesofTiO2nanofiberswereperformed(Fig.1).
Bead-free TiO2 nanofibers having diameters in the range of
40–500nm with average fiber diameter of 200±120nm were obtained. The elemental mapping of TiO2 nanofibers by EDX
showed that titanium and oxygen are the main elements of thenanofibers(Fig.1(b)–(d)).Electrospun TiO2 nanofiberswere
crushed into short nanofibers (TiO2-SNF) ranging from ∼1 to
∼10minlength(Fig.1(e)).
ThecrystallinestructureofTiO2-SNFwasidentifiedbyX-ray
diffraction(XRD).Fig.2hasshowntheXRDofTiO2-SNFwithseries
ofdiffractionpeakscorrespondingtoanatasephasewhichisknown foritsphotocatalyticactivity[17–19].Thesalientpeakat2Â=25.4◦ correspondstothe(101)planeoftheanataseTiO2structureand
theotherpeaksat2Â=38.1◦,48.4◦,54.3◦,55.3◦,63.0◦,69.1◦,70.3◦ correspondtothediffractionof(004),(200),(105),(211),(204), (116),and(220)planes,respectively[20].XRDpatternoftheTiO2
-SNFhasnodiffractionpeakat2Â=27.5◦whichischaracteristicfor therutile phasesignifying thatcalcinationprocessyieldedTiO2
nanofibershavingpureanatasestructure.
Thefabricationofelectrospunpolymericnanofibers contain-ing TiO2-SNF includes two steps. In the first step, PVP/TiO2
electrospunnanofiberswerecalcinated in order toobtainpure anataseTiO2 nanofibersand thentheywerecrushedintoshort
nanofibers ranging from ∼1 to ∼10m in length (Fig. 1(e)). These TiO2-SNF were blended with polymer solutionand then
electrospunintonanofibrouscompositestructures.Inthisstudy, weelectrospunnanofibersfromseveraldifferentpolymertypes including poly(methyl methacrylate) (PMMA), polyacrylonitrile (PAN),polyethyleneterephthalate(PET) andpolycarbonate(PC) containingTiO2-SNF.Theseelectrospunnanofibrouscomposites
wereabbreviatedas:PMMA-NF/TiO2-SNF,PAN-NF/TiO2-SNF,
PET-NF/TiO2-SNFandPC-NF/TiO2-SNF.TherepresentativeSEMimages
ofthesenanofibrouscompositesaredepictedinFig.3.Weobserved thattheincorporationofTiO2-SNFdidnotaffectthe
electrospin-ningofthesepolymersandbead-freenanofiberswereobtained inallcases.Wefurtheranalyzedthesenanofibrouscompositesby transmissionelectronmicroscopy(TEM).TherepresentativeTEM imagesofthePMMA-NF/TiO2-SNFcompositenanofibersaregiven
inFig.4.SEMandTEManalysesrevealedthatsomeoftheTiO2-SNF
werefullycoveredbythepolymericmatrixwhereassomeTiO2-SNF
werepresentonthesurfaceofthepolymericmatrixand/or stick-ingoutfromthepolymericmatrix.Asmentionedabove,TiO2-SNF
hasanatasestructurewhichisknownforitsphotocatalytic activ-ity.Asapreliminarystudy,wealsoinvestigatedthephotocatalytic activityofPET-NF/TiO2-SNF.
Thephotocatalyticdegradationofrhodamine-6G(Rh-6G)dye solutionwasusedasamodelphotoreaction,anditsdegradation wasfollowedbytheUV–vismeasurementofthesolution contain-ingPET-NF/TiO2-SNF.ThechangeintheabsorbanceontheUV–vis
spectrumoftheRh-6GsolutioncontainingPET-NF/TiO2-SNFasa
functionoftheUVirradiationtimeisshowninFig.5.Thechangeof theabsorptionpeakpointofRh-6Gat526nmwasusedfor calcu-latingtherate(C/C0)ofRh-6GdegradationwhereC0andCindicate
theinitialconcentrationofRh-6GbeforeUVirradiationandafterUV irradiationattimet,respectively.AsthephotodegradationofRh-6G
occurredwithrespecttoUVirradiationtime,theabsorbanceofthe Rh-6GsolutionintheUV–visspectrumdecreasedgradually,and theredcolorofthesolutiondisappearedtotallyafter24hofUV irra-diationindicatingthatalmost100%oftheRh-6Gwasdecomposed. ThisresultconfirmedthephotocatalyticdegradationofRh-6Gby PET-NF/TiO2-SNFnanofibrouscomposite.
4. Conclusion
Here,wesummarizedtheproductionofpolymericnanofibrous compositescontaininganataseTiO2 shortnanofibers(TiO2-SNF)
viaelectrospinningtechnique.Wehaveshownthat nanofibrous compositescontainingTiO2-SNFfromdifferentpolymerssuchas
PMMA,PAN,PETandPCcaneasilybeobtainedvia electrospin-ning.TheSEMandTEMstudiesrevealedthatsomeTiO2-SNFwere
fullyinside thepolymericmatrix whereassomeTiO2-SNFwere
partiallycoveredandstickonthefibersurface.Thephotocatalytic activityofthesenanofibrouscompositescontainingTiO2-SNFwas
evaluatedbythephotocatalyticdecompositionofrhodamine-6G underUVirradiation.Ourpreliminaryresultsindicatedthatthese nanofibrouscompositeshavephotocatalyticactivityandcouldbe usefulfortreatmentoforganicpollutants.Moreover,such nanofi-brouscompositestructurescanalsobeapplicableinotherareas dependingonthepolymertypeandthefunctionofmetaloxides.
Acknowledgements
StatePlanningOrganization(DPT)ofTurkeyisacknowledged forthesupportofUNAM-InstituteofMaterialsScienceand Nano-technology. Dr. Uyar acknowledges Marie Curie International Reintegration Grant (IRG) for funding NANOWEB (PIRG06-GA-2009-256428) project. A. Celebioglu and F. Kayaci thank to TUBITAK-BIDEBfornationalgraduatestudyscholarships.
References
[1]A.Greiner,J.H.Wendorff,Angew.Chem.Int.Ed.46(2007)5670. [2]W.E.Teo,S.Ramakrishna,Comp.Sci.Technol.69(2009)1804. [3]X.Lu,C.Wang,Y.Wei,Small5(2009)2349.
[4]S.Agarwal,J.H.Wendorff,A.Greiner,Macromol.RapidCommun.31(2010) 1317.
[5]V.Thavasi,G.Singh,S.Ramakrishna,EnergyEnviron.Sci.1(2008)205. [6]S.Agarwal,A.Greiner,J.H.Wendorff,Adv.Funct.Mater.19(2009)2863. [7]K.Yoon,B.S.Hsiao,B.Chu,J.Mater.Chem.18(2008)5326.
[8]J.Y.Chen,H.C.Chen,J.N.Lin,C.Kuo,Mater.Chem.Phys.107(2008)480. [9]J.Bai,Q.Yang,M.Li,S.Wang,C.Zhang,Y.Li,Mater.Chem.Phys.111(2008)
205.
[10]M.Roso,S.Sundarrajan,D.Pliszka,S.Ramakrishna,M.Modesti, Nanotechnol-ogy19(2008)285707.
[11] H.R.Pant,M.P.Bajgai,K.T.Nam,Y.A.Seo,D.R.Pandeya,S.T.Hong,H.Y.Kim,J. Hazard.Mater.185(2011)124.
[12]P.Zhao,J.Fan,J.Membr.Sci.355(2010)91.
[13]T.He,Z.Zhou,W.Xu,F.Ren,H.Ma,J.Wang,Polymer50(2009)3031. [14]D.R.Reinhart,N.D.Berge,S.Santra,S.C.Bolyard,WasteManage.30(2010)
2020.
[15]R.Kaegi,B.Sinnet,S.Zuleeg,H.Hagendorfer,E.Mueller,R.Vonbank,M.Boller, M.Burkhardt,Environ.Pollut.158(2009)2900–2905.
[16]S.K.Choi,S.Kim,S.K.Lim,H.Park,J.Phys.Chem.C114(2010)16475. [17]X.Chen,S.S.Mao,Chem.Rev.107(2007)2891.
[18]A.L.Linsebigler,G.Lu,J.T.Yates,Chem.Rev.95(1995)735. [19]R.Liu,H.Ye,X.Xiong,H.Liu,Mater.Chem.Phys.121(2010)432. [20]P.K.Khanna,N.Singh,S.Charan,Mater.Lett.61(2007)4725.