Antibacterial and Antifungal Activities of Arum maculatum L. Leaves Extracts
Ferdağ ÇOLAK1* Filiz SAVAROĞLU2 Semra İLHAN21Department of Biology, Faculty of Science and Art, Dumlupinar University,Kütahya-TURKEY
2Department of Biology, Faculty of Science and Art, Eskişehir Osmangazi University,Eskişehir-TURKEY
*Corresponding Author Recevied : July 23, 2009
e-mail: fcolak@dumlupinar.edu.tr Accepted : September 16, 2009
Abstract
This study was designed to evaluate the antimicrobial activity of three extracts of the leaves of the Arum maculatum L. plant belonging to the Aroidae family collected from Kahramanmaraş, Turkey. Many efforts have been made to discover new antimicrobial compounds from a variety of sources such as micro-organisms, animals and plants. One such source is folk medicine. Systematic screening of them may result in the discovery of novel effective compounds.These extracts were first prepared with petroleum ether, methanol and ethyl acetate solvents by Soxhlet extraction. The antimicrobial activity of the extracts was then assessed using the agar-well diffusion method against gram-positive, gram-negative bacteria, and yeast and moulds. All extracts of Arum maculatum L. showed good activity against some type of bacteria (Bacillus cereus, Micrococcus luteus, Pseudomonas phaseolicola, Yersinia enterocolitica and Enterobacter aerogenes) and a mold (Aspergillus niger). The activity was compared with known antibiotics such as vancomycine, tetracycline and amphotericin B.
Key words: Arum maculatum, agar diffusion method, antimicrobial activity
INTRODUCTION
Turkey is an important floristic center internationally because of its geographic location, climate and the presence of nearly 10.000 natural plant species. The characteristics of plants that both inhibit microorganisms and are important for human health have been researched in laboratories since 1926. Traditional medical treatments used in daily life are now being used with empirical methods. Plants synthesis is a vast and diverse assortment of secondary metabolites. Various plants that humans have been using medicinally for hundreds of years can be attributed to secondary metabolites [1,2].
The Arum family, Aroidae, which numbers nearly 1.000 members, mostly tropical, and many of them marsh or water plants, is represented in this country by a sole species, Arum maculatum L., familiarly known as Lords and Ladies, or Cuckoo-pint. Being a herbaceous perennial, this is a plant which can grow year after year. In herbaceous perennials, such as the buttercup or daisy, the above-ground part of the plant dies away in the winter, but under the ground, part of the plant survives, producing new growth the following spring. In a woody perennial, such as a tree, the above-ground structure survives through the winter, from which new growth springs every spring [3]. Arum maculatum is widely grown throughout Turkey. It is popularly known in the Kahramanmaraş region as Tirşik. The leaves of the plant are used commonly by the local people as a vegetable. The roots of the Arum maculatum species are used in such treatments as diaphoretics, expectorants, and vermifuges [4].
The A. maculatum is known to contain alkaloid, saponin, cyanogenic glycosides [5,6] 2-heptanone, indole, pcresol, (E)-caryophyllene, a few monoterpenes,
[7,8] lectin [9].and two unidentified sesquiterpenes [10]. Saponins are a major family of secondary metabolites that occur in a wide range of plant species. Saponins are steroid and triterpen glycosides so named because of their soaplike properties [11]. Terpenes or terpenoids are active against bacteria, fungi, viruses, and protozoa. A terpenoid constituent, capsaicin, has a wide range of biological activities in humans, affecting the nervous cardiovascular, and digestive system, as well as finding use as an analgesic [12]. Lectin, purified from edible Arum maculatum tuber, was analyzed through SDS− PAGE and studied for its agglutination property using rabbit erythrocytes. The insecticidal activity of the Arum maculatum tuber lectin was tested against two economically important sucking pests, Lipaphis erysimi and Aphis craccivor [9]. The antimicrobial activity of the root of A. maculatum was first reported by Uzun et al [13] .A. maculatum root extracts showed no significant inhibition zone to the microorganisms tested.
The human body is able to directly absorb different compounds, and due to the low concentration of these compounds, it has transpired that the plant particularly provides effective protection against infection. This protective property, either through the plant itself or by the compounds contained within, also has the effect of prolonging the shelf life of food in which it is used or prevents it being spoilt by microorganisms. Its current use is as a plant additive to other food and its being consumed by the public may be due to both its health-giving properties and taste.
Many works have been conducted with the aim of identifying the different antimicrobial and phytochemical constituents of medicinal plants and using them for the treatment of microbial infections (both topical
14 F. Çolak et al / JABS, 3 (2): 13-16, 2009
and systemic applications) as possible alternatives to chemically synthetic drugs to which many infectious microorganisms have become resistant. Antimicrobial compounds from plants may inhibit microbial growth through different mechanisms and may have significant clinical value in the treatment of resistant microbes [14].
This study was aimed at evaluating the in vitroiantimicrobial property of three extracts of the leaves of the Arum maculatum L.
MATERIALS and METHODS
Collection and identification of plant materials
A. maculatum L. was collected from Kahramanmaraş in January 2006. The voucher specimens were preserved at the Herbarium of the Department of Biology, Faculty of Science and Art, Eskişehir Osmangazi University, Eskişehir, Turkey (ESOGU).
Plant extracts
The aerial parts of the plant material were dried in shade at room temperature and then ground to a fine powder in a mechanic grinder. The powdered plant materials (30g) were then extracted with 250 mL of petroleum ether, 70% methanol and ethyl acetate solvent for 8 h by Soxhlet equipment. Following filtration with Whatman filter paper (No 1), all extracts were concentrated and evaporated to dryness in vacuo at 55 °C by rotary evaporator [15]. The yields from the different extracts were weighed and dissolved in 1mL 100 % dimethyl sulphoxide (DMSO). All extracts were maintained at +4°C until be used for agar well diffusion assay.
Test Microorganisms
The extracts inhibitory effects on a total of 17 microbial species including 10 bacteria, 6 molds and 1 yeast were used as test organisms in this study. Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028, Staphylococcus aureus ATCC 25923 and Aspergillus niger ATCC 10949 were obtained from American Type Culture Collection (ATCC, Rock-wille. MD,USA); Bacillus cereus NRRL 3711, Proteus vulgaris NRRL B-123, Micrococcus luteus NRRL B-1018, Enterobacter aerogenes NRRL B-3567, Aspergillus flavus NRRL 1957, Aspergillus parasiticus NRRL 465, Aspergillus fumigatus NRRL 163 and Candida albicans NRRL Y-12983 from Northern Regional Research Laboratory (NRRL, USDA Peoria, Illionis /USA); Fusarium graminearum (wild type), Fusarium solani (wild type) from Eskişehir Osmangazi University, Department of Biology Eskişehir/Turkey and Yersinia enterocolitica and Pseudomanas phaseolicola from Anadolu University, Department of Biology Eskişehir/Turkey. The bacterial and fungal cultures of test organisms were maintained on Nutrient Agar and Malt Extract Agar slants at 4°C, respectively, and were subcultured in petri dishes prior to use. DMSO alone was used as a control under the same condition for tested microorganisms. Vancomycine (Bioanalyse, 30µg/disc)
and tetracycline (Bioanalyse, 30µg/disc) were used as a positive control for bacteria, and Amphotericin B (Sigma) dissolved in DMSO (10µg/well) was used as a positive control for yeast and fungi. The tests were carried out in triplicate. Antimicrobial activity was evaluated by measuring the zone of inhibition (mm) against the test microorganisms.
Determination of antimicrobial activity
The antimicrobial activities of the petroleum ether, methanol and ethyl acetate extracts from the plant sample were evaluated by means of the agar-well diffusion assay [16,17] with some modifications. In short, fifteen milliliters of the specified molten agar (45 °C) were poured into sterile Petri dishes (Ø 90 mm). The cell suspensions containing 108 CFU/ml cells for bacteria, 107 CFU/ml cells for yeasts, and 105 spore/mL of fungi were prepared and evenly spread onto the surface of the agar plates of Mueller-Hinton agar (Oxoid, UK) for bacteria, or Sabouraud dextrose agar (Oxoid, UK) medium for yeasts and fungi using sterile swab sticks. Once the plates were dried aseptically, 10 mm wells were bored using a sterile cork borer. Extracts (100 µl) were placed into the wells and the plates were incubated at 37°C for 24 h for bacterial strains, 48 h for yeasts, and 72 h for fungi at room temperature; tetracycline and vancomycine (30µg/disc) were used as positive controls for bacteria, and amphotericin B (10 µg/well) for yeasts and fungi. The tests were carried out in triplicate. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test organism.
RESULTS
The plant material was subjected to an extraction process, with petroleum ether, methanol and ethyl acetate. The yields were 0.2 % for the petroleum ether extract, 0.4 % for the methanol extract, and 0.3 % for the ethyl acetate extract.
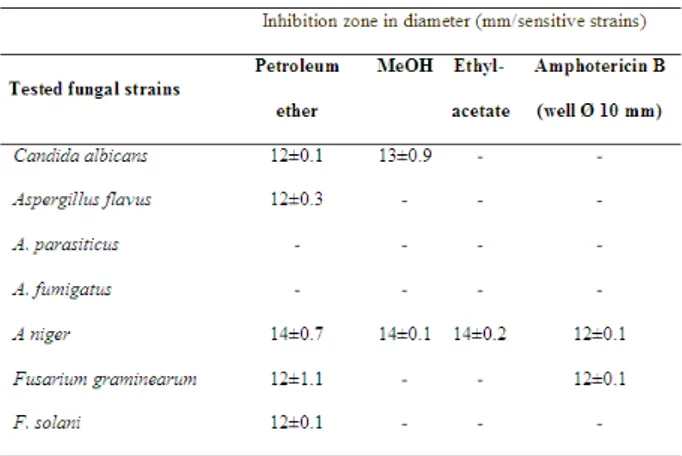
As shown in Table 1, 2 the extracts from A. maculatum showed antibacterial against some of the tested gram positive bacteria with the diameters of zone inhibition 15 and 42 mm. The most significant result was that the bacteria isolate B. cereus was inhibited by all of the plant extracts.
A. maculatum leaves extracts showed antibacterial properties against some of the tested gram negative bacteria with the diameters of zone inhibition 11 and 27 mm. In this study, extracts from the leaves of A. maculatum showed antibacterial against some of the tested yeast and fungi, with the diameters of zone inhibition 12 and 14 mm. A. parasiticus and A. fumigatus were not inhibited by all of the extracts. A. niger was the only fungus that was inhibited by the all of the extracts (14mm). The diameter of the inhibition zone around the active extracts was comparable with the standard antibacterial and antifungal antibiotics used as a positive control.
DISCUSSION
During the last ten years the pace of development of new antimicrobial drugs has slowed down while the prevalence of resistance (especially multiple) has increased astronomically. The increase in the number of antibiotic resistant bacteria is no longer matched by expansion in the arsenal of agents available to treat infections. Both the literature and ethno botanical records suggest that plants are the sleeping giants of the pharmaceutical industry. They may provide natural sources of antimicrobial drugs that will/or provide novel or lead compounds that may be employed in controlling some infections globally [18].
The most significant result was that the bacteria isolate B. cereus was inhibited by all of the plant extracts. Gram-negative bacteria are generally more resistant compared to the Gram-positive bacteria. The lack of activity of tested plant parts against Gram-negative bacteria could be attributed to the greater resistance of these bacteria, due to the presence of an extra outer membrane (polysaccharides, proteins, and phospholipids) in their cell wall acting as a foreign barrier for substances including antibiotics as well as to a different method of preparing extracts from that used by traditional healers, a process which leads to the separation or loss of some active principles. The later is also true for Gram-positive bacteria [19]. Both Bacillus cereus and Staphylococcus aureus produce toxins causing diarrhoea and vomiting. The illness occurs when people swallow the bacteria or
their spores which then multiply and produce toxins in the intestine, or from eating the toxins already produced in the food [19]. Arum maculatum root extracts were tested in vitro for antimicrobial activity against tested microorganisms using disc diffusion and microbroth dilution technique. This research showed that ethanol extracts of Arum maculatum showed antimicrobial activity aganist S. aureus, S. epidermidis and S. typhi, but petroleum ether extracts demonstrated no antimicrobial activity. This research showed that Arum maculatum, petroleum ether extracts had MIC values of 39.1µg/mL against Staphylococcus epidermidis [13]. The antifungal property of phytochemicals could involve cytocolic hyperacidity, breakage of electrons transport chain, H+-ATPase inhibition, channels inhibition, and intracellular enzymes synthesis inhibition [20]. Uzun et al [13] showed that the ethanol and petroleum ether extracts of Arum maculatum showed no anticandidal activity aganist C. albicans. In this study, the petroleum ether extract of Arum maculatum leaves showed antifungal activity against A. niger, A. flavus F.solani, F. graminearum and C. albicans. A. niger causes infections of the brain and other organs [21]. The differences observed in the bioactivity assay suggest the susceptibility of these microorganisms to various secondary metabolites present in this Arum maculatum. The composition of these secondary metabolites in turn varies according to the species, climatic conditions, and the physiological state of developments of the plants [22].
A. maculatum is known to contain saponin and a few monoterpenes. Monoterpenes and saponins have potent antifungal activity [23,24]. Saponin possesses antifungal activity in vitro with respect to Candida albicans, C. krusei, C. tropicalis, and S. cerevisia. In addition to this, activity was especially high against Gram-positive bacteria (Bacillus cereus, B. subtilis, Staphylococcus aureus and Enterococcus faecalis) with M. arabica being the species showing the broadest spectrum of action [25,26]. The antifungal properties of saponins are generally ascribed to the ability of these molecules to complex with sterols
Table 1. Antibacterial activity of three extracts from
Arum maculatum L. (well Ø 10 mm)
Table 2. Antifungal activity of three extracts from Arum
16 F. Çolak et al / JABS, 3 (2): 13-16, 2009
in fungal membranes, thus causing pore formation and loss of membrane integrity [27]. Cyanogenic glycosides release the well known poisonous gas hydrogen cyanide (HCN) (11). Hydrocyanic acid (HCN) or prussic acid is generally found in stressed plants and is formed by enzymatic action on compounds called cyanogenetic glucosides (dhurrin) when growth is adversely affected. A. maculatum leaves give off prussic acid when injured. There have been reports of antimicrobial properties associated with glucosides [3,28]. However, it is the presence of both glucosides and saponins in the leaf extracts of Arum maculatum that is the main reason for the antimicrobial activity demonstrated by the leaf extracts.
Extract from the leaves of Arum maculatum has showed significant antibacterial activities and could be used as an antimicrobial agent in new drug therapy.
REFERENCES
[1]. Kayacıoğlu A, Öner C. 1994. Bazı bitki ekstraktsiyonlarının antimutajenik etkilerinin Amest Salmonella test sistemi ile araştırılması. Turkish Journal of Botany. 18: 117-122.
[2]. Chaudhry NMA, Tarıq P. 2006. Antimicrobial activity of Cinnamomum cassia against diverse microbial flora with its nutritional and medicinal impacts. Pakistan Journal of Botany. 38: 169-174.
[3]. http://www.naturegrid.org.uk/biodiversity/plants/ fparum.html
[4]. http://www.indiana.edu/~ancmed/drugswp.html [5]. Balabanlı C, Albayrak S, Türk M, Yüksel O. 2006. Some toxic plants growing in rangelands of Turkey and their effects on animals. SDU Orman Fakültesi Dergisi. 2: 89-96.
[6]. http://www.botanical-online.com/ alcaloidesaroangles.html
[7]. Kite GC. 1995. The floral odour of Arum maculatum. Biochemical Systematics and
Ecology. 23: 343–354.
[8]. Kite CG, WLA Hetterscheid, MJ. Lewis, PC Boyce, J Ollerton, E Cocklin, A Diaz, MJ Simmonds. 1998. Inflorescence odours and pollinators of Arum and Amorphophallus (Araceae). In: ( ed. S.J. Owens &P.J. Rudall), Reproductive Biology. pp. 295-315. Royal Botanic Gardens, Kew.
[9]. Majumder P, Mondal H.A, Das S. 2005. Insecticidal activity of Arum maculatum tuber lectin and its binding to the glycosylated insect gut receptors. Journal Agriciculturel Food Chemistry. 53: 6725–6729.
[10]. Diaz A, Kite GC. 2002. A comparison of the pollination ecology of Arum maculatum and A. italicum in England. Watsonia. 24: 171–181.
[11]. Minale L, Pizza C, Riccio R, Zollo F. 1982. Steroidal gycosides from starfishes. Pure& Appiled Chemistry. 54: 1935-1950.
[12]. Cowan MM. 1999. Plant Products as Antimicrobial agents. Clinic Microbiology Rewiew 12:
564-582.
[13]. Ergin U, Sariyar G, Adsersen A, Karakoc B, Ötük G, Oktayoglu E, Pirildar S. 2004. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. Joıurnal Ethnopharmacology. 95: 287–296.
[14]. Zakaria Z, Sreenivasan S, Mohamad M. 2007. Antimicrobial activityof Piper ribesoides extract aganist Staphylocoocus aureus. Journal Applied Biology Sciences. 1: 87-90.
[15]. Bradshaw LJ. 2001. Laboratory Microbiology. Saunders College Publishing Research 13: 122-124.
[16]. Güven K, Yücel E, Çetintas F. 2006. Antimicrobial activities of fruits of Crataegus and Pyrus species. Pharmaceutical Biology. 44: 79-83.
[17]. NCCLS, Performance standards for antimicrobial disk susceptibility tests. Approved Standarts NCCLS Publication M2-A4, Villanova, PA, 1990.
[18]. Akinpelu DA, Onakoya TM. 2006. Antimicrobial activities of medicinal plants used in folklore remedies in south-western. African Journal of Biotechnology. 5: 1078-1081.
[19].Maregesi S M, Pieters L, Ngassapa OD, Apers S, Vingerhoets R, Cos P, Dirk A. Berghe V, Vlietinck AJ. 2007.Screening of some Tanzanian medicinal plants from Bunda district for antibacterial, antifungal and antiviral activities. Journal Ethnopharmacology. 113: 457-470.
[20]. López Díaz T M, González C. J, Moreno B, Otero A. 2002. Effect of temperature, water activity, pH and some antimicrobials on the growth of Penicillium olsonii isolated from the surface of spanish fermented meat sausage. Food Microbiology. 19: 1-7.
[21]. Denning DW. 1998. Invasive aspergillosis. Clinic Infections Diseases. 26: 781-80.
[22].Mahomoodally MF, Gurib-Fakim A , Anvar HS . 2005.Antimicrobial activities and phytochemical profiles of endemic medicinal plants of mauritius. Pharmaceutical Biology. 43: 237-242.
[23].Garcia R, Alves ESS, Santos MP Aquıje GMFV, Fernandes AR, Santos RB, Ventura JA, Fernandes PMB. 2008. Antimicrobial activity and potential use of monoterpenes as tropical fruits preservatives. Brazilian Journal Microbiology. 39: 163-168.
[24].Yadava RN, Jharbade J. 2007. A new bioactive triterpenoid saponin from the seeds of Lactuca scariola Linn. Natural Product Research 21: 500-506.
[25].Mel’nichenko EG, Kirsanova MA, Grishkovets VI, Tyshkevich LV, Krivorutchenko L. 2003. Antimicrobial activity of saponins from Hedera taurica. Mikrobiology Z. 65: 8-12.
[26]. Osbourn AE. 2003.Saponin in cereals. Phytochemistry 62: 1-4.
[27].Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M. 2006. Antimicrobial activity of saponins from Medicago sp. structure-activity relationship. Phytotheraphy Resources. 20: 454-457.
Presence of Aflatoxin M1 Ewe’s Milk in the Northwest Region of Iran
Mohammad Hosein M29$66$*+1*Department of Food Hygiene and Food Quality Control, İslamic Azad University, Shabestar Branch, IRAN
*Corresponding Author Recevied : July 26, 2009
e-mail: Movassagh2@yahoo.com Accepted : September 19, 2009
Abstract
Aflatoxin M1 is the hydroxylated metabolite of aflatoxin B1 and may be found in milk or milk products obtained from livestock that have ingested contaminated feed. Iranian white cheese is made from ewe’s milk. The aim of this study was to evaluate Aflatoxin M1 contamination in ewe’s milk samples in Tabriz city (Iran) by ELISA (Enzyme Linked Immunosorbent Assay). Ten ewe milk samples from different cheese maker centers in Tabriz city were collected during 2 months (May to June 2009). AFM1 was found in 30% of the analyzed samples. Results show that in 3 samples (30%) the AFM1 concentrations were less than 5 ng/l. It can be concluded that AFM1 levels in the samples purchased in Tabriz city, appear to be safe at the moment.
Key words: Aflatoxin M1; Ewe milk; ELISA, Tabriz
INTRODUCTION
Milk is a good source of many nutrients. However it could be a source of toxic substances like Aflatoxin M1 (AFM1). Contaminated milk with AFM1 could be a threat for children are consumed milk and dairy products. Aflatoxin may be produced by three species of Aspergilus-A.flavus, A.parasiticus, and rare A.nomius – that contaminate plants and its products. A.flavus produces only B aflatoxins, while the others produce both B and G aflatoxins Aflatoxins M1 and M2 are the hydroxilated metabolites of aflatoxin B1 and B2 and may be found in milk products obtained from live stock that have ingested contaminated feed [1]..
In 1987 the WHO classified the aflatoxins as Group 1 carcinogens. Aflatoxins are potent liver carcinogens and DNA-damaging agents from natural source[2].
It has been stated, in fact, that the contamination of milk and milk products with AFM1 displayed variations according to geography, country and season[3,4,and 5]. AFM1 is resistant to thermal inactivation; pasteurization, autoclaving and other varieties of food processing procedures are not effective in the reduction of this toxin [6,7].
In Iran, the cheese was produced from ewe milk traditionally, and it is important to determine the levels of Aflatoxin M1 in ewe milk. There is no information on ewe milk contamination by AFM1 in Iran. For the first time, this study was carried out to evaluate the prevalence of ewe milk contamination with AFM1 in Tabriz city (northwest region of Iran). This is the first report, as far as we are aware, of AFM1 contamination of ewe’s milk in the northwest region of Iran.
MATERIALS AND METHODS
A total of 10 ewe milk samples from different cheese maker centers in Tabriz city were collected randomly during 2 months (May to June 2009).
The milk samples were centrifuged in 10 ◦ C for 10 min with 3500 × g. After centrifugation, upper cream layers were completely discarded and the lower phases were freezed for the quantitative test. The quantity
of AFM1 was determined by I’ screen aflatoxin M1 test (Tecna, Italy) which is a competitive enzyme immunoassay based on antigen–antibody reaction. Sample solutions of 100 µl were added to the wells to occupy the binding sites proportionately then mixed gently and incubated for 45 min at room temperature (20-25 ◦ C). The liquid was poured out of the wells and the wells filled with 250 µl washing buffer and poured out the liquid again. This washing step repeated four times. In the next stage 100 µl of enzyme conjugate were added to occupy the remaining free binding sites and incubated for 15 min at room temperature and repeated washing step. Then 100 µl of developing solution was added to each well and incubated for 15 min at room temperature. By using a multichannel pipette, 50 µl of stop solution was added to each well. The measurement of AFM1 was done photometrically at 450 nm against air blank within 60 min in ELISA reader (Sunrise, USA). Then data were analyzed by chi square test [1].
RESULTS
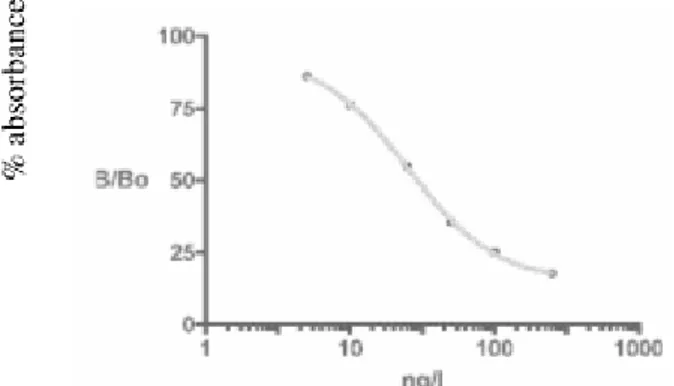
The standard curve for AFM1 detection by competitive ELISA is given in fig 1. As can be seen from the figure, the calibration curve was found virtually linear in the 5-250 ng/l range. The detection limit was found to be 5 ng/l. AFM1 was found in 30% of the analyzed samples. Results show that in 3 samples (30%) the AFM1 concentrations were less than 5 ng/l.
18 M. H. Movassagh / JABS, 3 (3): 17-19, 2009
DISCUSSION
In the Iranian food standard(Anonymous, 2002), AFM1 levels in raw milk were limited to 50 ng/l, similar to EC regulations. Aflatoxin M1 was found in 30% of examined ewe milk samples[1].
According to the results obtained by Bognanno and et al in Italy, Aflatoxin M1 was found in 81% of examined ewe milk samples and 1.25% of all samples were over the legal limits (50 ng\l) [8]. In Greece, ewe’s milk and the produced curd and feta cheese samples were examined
for presence of AFM1 and levels of AFM1 in milk were found far below the tolerance level(highest value 18.2 ng\l) which was parallel to our results [9]. Montagna and et al (2008) reported AFM1 contamination in 12.9% of cheese (made by ewe’s milk) samples in Italy [10].. We are not aware of other surveys on the AFM1 content of ewe milk, so that comparison of results is not possible. As compared to other studies on cow’s milk, which is shown in Table 1, AFM1 contamination in ewe’s milk is lower than cow’s milk in Iran.
Table-1 the prevalence of cow’s milk contamination in other studies
It is known that contamination of AFM1 in milk is a result of exposure of AFB1 to ewes through feed-stuffs [17]. The wide variations in AFM1 levels among studies could be related to geographic and climatic differences but also to differences in feeding systems, and farm management practices. This study showed that contamination with AFM1 in Tabriz is lower than standard levels. In Iran, especially in Tabriz, ewe milk is used for cheese making.
It is concluded that consuming of ewe milk is safe for people in Tabriz, and the level of AFM1 in cheese should be lower than standard levels.
ACKNOWLEDGMENTS
The author gratefully acknowledges the contribution of Dr. Yaghobei, Mr. A. Ghorbanei, and Tabriz Blood Transfusion Organization to this work, and Islamic Azad University, Shabestar Branch for founding this study as a research project.
REFERENCES
[1]. Movassagh Ghazani MH. 2009. Aflatoxin M1 contamination in pasteurized milk in Tabriz (northwest of Iran), Food and Chemical Toxicology, 47: 1624-1625
[2]. Daniels JM, Liu L, Stewart RK, Massey TE. 1990. Carcinogenesis, 11: 823-827
[3]. Galvano FV, Galofaro G. 1996. Occurrence and stability of aflatoxin M1 in milk and milk products, a worldwide review, Journal of Food Protect, 59: 1079-1090.
[4]. Pittet, A. 1998. Natural occurrence of mycotoxins in foods and feeds, an updated Review, Revue De Medicine Veterinaire, 149: 479-492.
[5]. Celik TH, Sarimehmetoglu B, Kuplulu O. 2005. Aflatoxin M1 contamination in pasteurized milk, Veterinarski Arhiv, 75 (1), 57-65.
[6]. Deshpandeh SS. 2002. Fungal toxins, In: Handbook of food toxicology, S.S. Deshpandeh New York, pp.387-456.
[7]. Park DL. 2002. Effect of processing on aflatoxin, Advances in Experimental Medicine and Biology, 504: 173-179.
[8]. Bognanno M, La Fauci L, Ritieni L, Tafuri A, De Lorenzo A, Micari P, Di Renzo L, Ciappellano S, Sarullo V, Galvano F. 2006. Survey of the occurrence of aflatoxin M1 in ovine milk by HPLC and its confirmation by MS, Mol. Nut. Food Res, 50: 300-305.
[9]. Kaniou-Grigoriadou I, Eleftheriadou A, Mouratidou T, Katidou P. 2005. Determination of aflatoxin M1 in ewe’s milk samples and the produced curd and feta cheese, Food Control, 16: 257-261. [10]. Montagna MT, Napoli C, De Giglio O, Iatta R, Barbuti G. 2008. Occurrence of aflatoxin M1 in dairy products in southern Italy, Int. J. Mol. Sci. 9: 2614-2612. [11]. Gholampour Azizi I, Khoushnevis SH, Hashemi SJ. 2007. Aflatoxin M1 level in pasteurized and sterilized milk of Babol city, Tehran University Medical Journal, 65: 20-24.
[12]. Nakajima M, Tabata S, Akiyama H, Itoh Y, Tanaka T, Sunagawa H, Tyonan T, Yoshizawa T, Kumagai S. 2004. Occurrence of aflatoxin M1 in domestic milk in Japan during the winter season, Food Additives and Contaminants, 21: 472-478.
[13]. Karimi G, Hassanzadeh M, Teimuri M, Nzari F, Nili A. 2007.Aflatoxin M1 contamination in pasteurized milk in Mashhad, Iran, 3(3):153-156.
[14]. Nuryano N, Agus A, Wedhastri S, Maryudani YB, Sigit Setyabudi FMC, Bohm J. 2009.A limited survey of aflatoxin M1 in milk from Indonesia by ELISA, Food Control, 20: 721-724.
[15]. Alborzi S, Pourabbas B, Rashidi M, Astaneh B. 2006. Aflatoxin M1 contamination in pasteurized milk in Shiraz (south of Iran), Food Control, 17, 582-584. [16]. Hussain I, Anwar J. 2008.A study on contamination of aflatoxin M1 in raw milk in Punjab province of Pakistan, Food Control, 19: 393-395. [17]. Unusan N. 2006. Occurrence of aflatoxin M1 in UHT milk in Turkey, Food and Chemical Toxicology, 44: 1897-1900.