ContentslistsavailableatScienceDirect
Micron
jo u rn al h om epa g e :w w w . e l s e v i e r . c o m / l o c a t e / m i c r o n
Review
Microscopic
characterization
of
peptide
nanostructures
Rashad
Mammadov,
Ayse
B.
Tekinay,
Aykutlu
Dana,
Mustafa
O.
Guler
∗UNAM-InstituteofMaterialsScienceandNanotechnology,BilkentUniversity,Ankara06800,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received21April2011
Receivedinrevisedform7July2011 Accepted8July2011 Keywords: Peptide Nanofiber Imaging Nanomaterialscharacterization
a
b
s
t
r
a
c
t
Peptide-basednanomaterialshavebeenutilizedforvariousapplicationsfromregenerativemedicine toelectronics since theyprovide severaladvantagesincluding easy synthesis methods,numerous routes for functionalization and biomimicry of secondary structures of proteins which leads to designofself-assemblingpeptidemoleculestoformnanostructures.Microscopiccharacterizationat nanoscaleiscriticaltounderstandprocessesdirectingpeptidemoleculestoself-assembleandidentify structure–functionrelationshipofthenanostructures.Here,fundamentalstudiesinmicroscopic charac-terizationofpeptidenanostructuresarediscussedtoprovideinsightsinwidelyusedmicroscopytools.In thisreview,wewillencompasscharacterizationstudiesofpeptidenanostructureswithmodern micro-scopes,suchasTEM,SEM,AFM,andadvancedopticalmicroscopytechniques.Wewillalsomention specimenpreparationmethodsanddescribeinterpretationoftheimages.
©2011ElsevierLtd.Allrightsreserved.
Contents
1. Introduction... 69
2. Transmissionelectronmicroscopy(TEM). ... 70
3. Scanningelectronmicroscopy(SEM)... 74
4. Atomicforcemicroscopy(AFM). ... 76
5. Opticalmicroscopy-basedtechniques... 79
6. Conclusion. ... 82
References... 82
1. Introduction
Molecularmechanismsgoverningproteinfolding and
forma-tionofsupramolecularassembliesbyproteinsinspiredscientists
todevelop peptide-basednanomaterials witha wide varietyof
applicationsfromelectronicsandnanocatalysistotissue
engineer-ingandbiosensors(Gazit,2007;GulerandStupp,2007;Ulijnand
Smith, 2008). The versatilityof application areas is due tothe
flexibilityofpeptidedesign,whichusesaminoacidswith
differ-entchemicalfunctionalities.Moreover,self-assemblyofpeptide
moleculesledtoformationofnanostructureswhichcanbe
orga-nizedtoformdifferenthigher-orderstructuressuchashydrogels
(Cuietal.,2010;Gazit,2007),surfacecoatings(Adler-Abramovich
et al., 2010), drug deliverysystems (Sarikaya et al., 2003) and
one-dimensionaltemplatesforsynthesisofnanowires(Acaretal.,
2011;RechesandGazit,2003)andnanotubes(Gazit,2007).
Pro-∗ Correspondingauthor.Tel.:+903122903552;fax:+903122664365. E-mailaddress:moguler@unam.bilkent.edu.tr(M.O.Guler).
cessesthatgovernthestructuralorganizationareprogrammedby
non-covalentinteractionsbetweentheaminoacidresidues.The
side chainsoftheamino acids,which containvariouschemical
groups,determinethetypeofintramolecularandintermolecular
interactionsanddriveformationofpeptidicnanostructures.These
interactionsincludehydrogenbonding,electrostatic,hydrophobic,
–andvanderWaalsinteractions.Eachoftheseinteractions
con-tributesindesignandsynthesisofself-assemblednanomaterials
(Toksozetal.,2010;ToksözandGuler,2009).Aminoacidsequence
determinestypesofinteractionsbetweenpeptides,which
eventu-allyleadsformationofdifferentsecondarystructures.Forexample,
someaminoacidsfavor formationof ␣-helix,whilesomefavor
-sheetorrandomcoilstructures.Propertiesofthefinal
nanostruc-turesareaffectedbythetypeofdominatingsecondarystructureof
thepeptides.Peptidicnanostructureswerepreviouslydiscussedin
fourdifferentgroupsas␣-helicalbased,-sheetbased,amphiphilic
peptidebasedand collagen-likepeptidenanostructures(Toksöz
andGuler,2009).
In regenerativemedicine,peptideassembliescanbeusedas
extracellular matrix (ECM) – mimicking materialsfor repairing
0968-4328/$–seefrontmatter©2011ElsevierLtd.Allrightsreserved. doi:10.1016/j.micron.2011.07.006
damagedtissues.Peptide-basedhydrogels havegreatadvantage
overothersyntheticmatricessincetheyallowversatilityfor
con-jugationofvariousbioactiveproperties(Dviretal.,2011).Peptide
amphiphile(PA)moleculeshavebeenusedextensivelyfor
regener-ativemedicinestudies(Webberetal.,2010).Inphysiologicalmedia,
duetopackingofhydrophobicalkyltails,theycanself-assemble
intocylindricalnanostructureswhichmakebundlesand
entangle-mentstoformhigherorderstructuresresultinginhydrogels(Cui
etal.,2010).Thesegelsarecomprisedofmeshworkofnanofibers
whicharecapableofencapsulatingupto99%water,resembling
nativeextracellularmatrices.ThePAnanofiberscanbeengineered
tocarrysignalssimilartothenativeenvironmentofcells,providing
anexcellentplatformfor inductionofcellularsignaling
mecha-nismseffectively(Stupp,2010).EpitopesderivedfromnaturalECM
proteinsorthatallowbindingtospecificbiologicalfactorswere
conjugatedtoPAnanofibersandresultingsyntheticmatriceswere
usedtoinduceangiogenesis(Rajangametal.,2006),neural(Silva
etal.,2004;Tysseling-Mattiaceetal.,2008),bone(Mataetal.,2010),
enamel(Huangetal.,2008)andcartilageregeneration(Shahetal.,
2010)andsurvivalofpancreaticislets(Chowetal.,2010).Inthese
studies,activationoftissue-specific responsewiththese
hydro-gelshasbeenshownbothinvitroandinvivo.Anti-cancerepitope
carryingPAshavebeenshowntobeinternalizedeffectivelyby
can-cercellswhileinhibitingtheirproliferation(Aulisaetal.,2009)or
destroyingthemspecifically(Standleyetal.,2010).Nanostructures
basedonpeptidemoleculeswithhydrophobicandchargedamino
acidsalsoformmatricesandmembranesinthephysiologicalmedia
(Zhangetal.,1993,1995).Thesematriceswerealsoshowntobe
promisingforregenerativemedicinestudiessuchaswound
heal-ing(Schneideretal.,2008)anddifferentiationofneuralstemcells (Gelainetal.,2006).
Peptidebasednanostructuresarealsopromisingcandidatesfor
productionofnanowiresforelectronicsapplications.Production
ofnanoelectronicdevices requiressynthesisof nanoscalewires.
Amyloid-like, -sheet-rich peptidenanofibers were extensively
usedfor this purpose. Amyloid-like nanofiberswere covalently
attachedthroughcysteineresiduestogoldparticles,where
result-ingnanowiresshowedconductiveproperties,similartoelectrical
wires (Scheibel et al., 2003). Histidine-rich peptide nanotubes
were used as templates for synthesis of conductivity-tunable
Cunanotubesthroughbiomineralization(Banerjeeet al.,2003).
Biomineralization of titania and silica on amyloid-like peptide
nanofiberswerealsoreportedrecently(Acaretal.,2011).
Bolaam-phiphilicpeptides,developedbyMatsuiandco-workers,arealso
suitable for nanoelectronics applications. They form nanotube
structures,which capturemetals andproduceinorganiccoating
aroundnanotube(Matsuietal.,2000).Metalloporphyrincoatingon
thesenanotubeshasbeenachieved,whichcanbeusedinnanoscale
sensorsorphotonics,duetoporphyrin’shighefficiencyinelectron
andenergytransfer(MatsuiandMacCuspie,2001).
Microscopiccharacterizationgivesvaluableinformationabout
morphology and size of nanostructures. Both of these features
are important in regenerative medicine, drug delivery,
biosen-sor development and other applications, since functionality of
designednanostructuresaresignificantlyaffectedbythese
param-eters.Forexample,inregenerativemedicinestudies,researchers
try to manipulate cells by designing materials mimicking the
nativeextracellularmatrix.TheECMhascharacteristicanisotropic
nanofibrillarstructure,whichhasimportantrolesincellular
behav-ior,includingmigration,signaling,proliferationanddifferentiation
(LutolfandHubbell,2005).Forbettercellularactivity,researchers
designECMmimickingsyntheticbiomaterialswithsimilar
nanofi-brousshapeandsize.Indrugdeliveryapplications,sizeandshape
ofnano-vehicles areimportant in determiningtheirhalf-life in
physiologicalenvironmentanduptakeintocells,whicharedirectly
relatedtoefficacyofthedeliverysystem(FarokhzadandLanger,
2009).Preciseinformationaboutsizeandshapeofnanostructures
synthesized for theabove mentioned purposes is crucial, since
nanoscaleaberrationsinthesepropertiesinterferewith
function-ality.
Advancementinmicroscopytechniqueshaspavedthewayfor
developmentofnanotechnology,materialsscience,molecular
biol-ogyandothergrowingfields.Forexample,improvementsinatomic
forcemicroscopy (AFM)now allows visualizationof
nanostruc-tures insolution,which enablesanalyzingthem in theirnative
environmentandavoidingartifactimagescausedbydryingeffect
duringspecimenpreparationandstrongcapillaryactionbetween
sampleandAFMprobe(Fotiadisetal.,2002).Moreover,imaging
nanoparticlesincells,understandingmechanicalfeaturesof
bio-logical molecules suchasnucleic acids,proteins and polymers,
andgeneratingstiffnessmapsofbiologicalsurfaceswereallmade
possiblewiththeuseofmodernAFMs(Dongetal.,2009;Husale
etal.,2009;Sahinetal.,2007;Tetardetal.,2008).Dip-pen
nano-lithographytechnique,whichbroughtsignificantadvancementin
functionalizationofsurfacesatnanoscale,isanotherachievement
madethroughimprovementofAFMprovingpotentialuseof
micro-scopesforpurposesotherthanimaging(Pineretal.,1999).
Modernsensitivemicroscopestogetherwithaccuratespecimen
preparationtechniquesallowustodeterminesizeandshapeof
nanostructuresaccurately.Thereareseveralimagingtechniques
thatcanbeusedforcharacterizationofnanostructuresandeach
techniquehasadvantagesanddisadvantages(limitations),soitis
importanttounderstandtheseandchoosetherightoneforthe
desiredcharacterization.Acomparisonofthesetechniquesis
pro-videdinTable1.Inthisreview,weexaminemicroscopytechniques
usedforcharacterizationofpeptidenanostructuresinfourmain
parts:transmissionelectronmicroscopy(TEM),scanningelectron
microscopy (SEM), atomic force microscopy (AFM) and optical
microscopy-basedtechniques.Sample preparationmethods and
informationobtainedfromimagesarealsoemphasized.
2. Transmissionelectronmicroscopy(TEM)
Analysisofsizeandmorphologyofpeptidenanostructureswith
highresolutionatascaleofafewnanometersorevenbelowcan
beperformedbyTEM.Thistechniqueinvolvestransmissionofan
electronbeamthroughthespecimen,whichinteractswith
inter-nalstructuresthuscollectinginformation forimagegeneration.
Sincewavelengthofelectronbeamissmallerthanthatoflight,
TEMallowsimagingwithhighermagnificationandresolutionthan
lightmicroscopyandenablesobservationofinternalstructuresof
materials.DetailedworkingmechanismofTEMcanbefoundin
ref-erenceEgerton(2005).TEMisusedtoidentifytheverydetailsabout
nanometerscalestructuresandeventoperformatomicresolution
characterizationofmaterials,whichinvolvesnotonlystructural
characterizationbutalsoidentificationofelementalcomposition
andchemicalbonding(Urban,2008).
Thistechniqueisbasedontransmissionofelectronsthrough
specimen,whichshouldbethintogetgoodqualityimages.
Imag-ingofbulkysamplessuchashydrogelswithTEMneedsspecial
sample preparation techniques in order to preventimage
arti-facts.The easiest wayis to directlyapply gel sampleson TEM
grid(cuppergrid).Inpreviousstudies,1wt%peptideamphiphile
(PA)nanofibergelwasappliedontocuppergridsandstainedwith
phosphotungsticacid (PTA) (Guleret al., 2005).Thenanofibers
withinthe gelwere 7nmin diameter and severalhundredsof
nanometersinlength.Inanotherstudy,bolaamphiphilicpeptide
nanostructureswereinvestigatedsimilarly,withanexceptionthat
bothnegative (PTA)and positive(uranylacetate)stainingwere
performed(Claussenetal.,2003).Here,nanofiberswith5–8nm
Table1
Comparisonofmicroscopytechniques.
Properties TEM SEM AFM Opticalmicroscopy
Advantages Providesveryhigh(even atomic)resolutionand magnification Enablesvisualizationof detailedstructuressuchas subcellularstructures
Allowsimagingbulkysamples andthreedimensionalsurfaces
Allowsthreedimensional imaging
Easysamplepreparation Allowsimagingatwet conditions,enableslive specimenobservation
Fluorescentandpolarized imagingoptions Colorcanbeobserved Lessexpensive Allowsimagingoflive specimens
Disadvantages Requireslaborioussample preparation,resinembedding andsectioningforbulky samples
Duetothinandtwo dimensionalspecimen requirements,notsuitablefor imagingthreedimensional arrangementsof nanostructuresinbulky samples
Doesnotallowlivespecimen imagingduetovacuum
Limitedresolutionatnanoscale Givesinformationonlyabout surfaceofthespecimen Doesnotallowlivespecimen imagingduetovacuum
Imagegenerationisslow Sizeofobservedstructures dependsontipradius,iftip radiusisnotlowenoughwrong sizemeasurementscanbe madefornanoscalestructures
Lowresolution
Resolutionlimit 0.2–0.5nmwithconventional TEM,1 ˚AwithHR-TEM
∼10nm Dependsontipradius,couldbe smallerthan1nm
∼200nm Sampletype Nanofibers,nanoparticles,
fixedcellsandsubcellular structures
Threedimensionalstructureof hydrogels,nanofibernetworks, cellsencapsulatedin biomaterials
Nanoparticles,nanofibers, livingorfixedcellsand subcellularstructures
Fluorescentlylabeledproteins, amyloidfibrilsetc.
Samplepreparation Stainingisrequiredfororganic structures(e.g.PTAoruranyl acetate)
Lessdestructivemethodsfor threedimensionalstructures suchascriticalpointdrying, freezedryingfollowedby coatingwithafewnanometers ofAu/Pd
Airdryingorafewmin incubationissufficientfor imagingintheair
Immobilizationisrequiredfor wetimaging
Fluorescentlabelingwith antibodiesorchemicals
Samplefixation 2%glutaraldehydeandOsO4 2%glutaraldehydesolution(3%
sucroseinPBS)
2%glutaraldehyde 4%PFA,acetone,methanol
Fig.1.TEMimagestakenfromslidesofresin-embeddedPAnanofibergel.Differentstagesofmineralizationareshown.(A)After2daysofmineralization.Fiberswerestained withcalciumphosphatepremineral.(B)and(C)After5daysofmineralization,nucleationofhydroxyapatite(HA)crystalsstarted.Crystalnuclei(␣)andaggregatesofstacked crystalplates()areobservableatthisstage.(D)After11daysofmineralization,advancedcrystalgrowthonPAnanofibersisobserved.Growingcrystalseed(␣),ellipsoidal aggregate()andtwobundledmineralizingnanofibers(␥)arethemostnotablefeaturesobservedatthisstage.(E)and(F)Highermagnificationviewsoffeaturesobservedin (D).Formationofmineralaroundnanofiberexteriors(E)andbetweenbundlednanofibers(F).Arrowsindicatemineralgrowthatthejunctionsbetweenbundlednanofibers. ReprintedwithpermissionfromSpoerkeetal.(2009).Copyright©2009JohnWiley&Sons,Inc.
Fig.2.(A)–(D)TEMimagesofnegativelystained,helicalterthiophenepeptidelipid(TTPL)nanofibers.(A)and(B)Singlehelicalnanofibershavingwidthof9±1nmand thehelicalpitchof65±6nm.(C)Widthofdoublehelicalnanofibersincreasedto17±1,whilehelicalpitchremainedsimilar(66±5nm).(D)TripleTTPLhelicesareshown (width25±2nmandhelicalpitch66±8nm).(E)TEMimagesofD-periodiccollagen-mimeticfibers.(F)SchematicviewofD-periodicbandingpatternincollagen-mimetic fibersandrelationshipbetweenpeptideoligomersformingthesefibers.EachD-periodincludesa“gap”zoneandalarger“overlap”zone.
FiguresA-DwerereprintedwithpermissionfromTsaietal.(2008).Copyright©(2008)Elsevier.FiguresE,FwerereprintedwithpermissionfromReleetal.(2007).Copyright ©(2007),AmericanChemicalSociety.
acetate,bindingpreferentiallytoacidgroupsonnanofibers,stained
bothcoreandperipheryofbolaamphiphilicnanofiberswith
car-boxylicacidgroupsextendingfrombothsidesofbolaamphiphiles
(Claussen et al., 2003). However, only periphery of the
nanos-tructureswasstainedwhencarboxylicacidgroupswerelocated
ononly oneside ofbolaamphiphiles. Anothermethodfor
sam-plepreparation isembeddinggelsintoresin.PAnanofibergels,
whichwereembeddedintoEPONTM(epoxy)resinandsectioned
withmicrotome,werevisualizedbyTEM(Hartgerinketal.,2001).
Bythismethod,itwaspossibletofollowdifferentstagesof
min-eralization,whileobservingaggregates ofstackedcrystalplates,
crystalnucleiandadvancedcrystalgrowthonPAnanofibers(Fig.1)
(Spoerkeetal.,2009).Analternativemethodisdilutinggeland
applyingdilutedsolutionontocoppergrid.Thismethodaimsto
avoidimagingofaggregatednanostructures,whileobserving
indi-vidualfeaturesonthesurface.Forthispurpose,1wt%PAgelswere
dilutedanddroppedontocarbongrids(Guleretal.,2006).Aftera
fewminutesexcesssolutionwasremovedwithfilterpaper,
nega-tivestainingwithPTAwasperformed,sampleswereairdriedand
imagedwith200kV.InanotherstudybyTsaietal.,self-supporting
gelswereformedanddiluted15-fold.Afterdryingofthesample
onTEMgrid,stainingwasperformedbyusinguranylacetate.This
methodallowedimaging helicalpeptidicnanostructures, which
haveapplicationsinnanoelectromechanicalsystems(NEMS),and
measuringimportantstructuralparameters,suchashelicalsizeand
pitch(Fig.2A–D)(Tsaietal.,2008).Single,doubleandtriple
heli-calstructureswereclearlyobservable(Tsaietal.,2008).Rajangam
etal.(2008)reportedanotherinterestingmethodforobservingthe
nanofibrousnatureofpeptidegelwherecarbongridwasdipped
into1wt%PAgelsuspensiontwicefor20sandthenstainedwith
PTA.
There are also numerous studies where TEM was used for
imaging peptidenanostructures in solutionform, which is less
challengingregardingpreparationofthinsamples.Inonestudy,
0.01wt% of peptidic thiophene molecules, which form
one-dimensionalnanostructureswithpotentialapplicationsinorganic
electronic devices, were dropped onto copper TEM grids and
dried.Sampleswerestainedwithuranylacetate for15minand
one-dimensionalnanostructureswith6–7nmwidthwereclearly
observedat100kV(Stoneetal.,2009).Dolphinetal.(2006)stained
amyloidprotofibrilswithasimilarmethodwhileusing2%uranyl
acetate.By usingsimilarmethod,peptidic fibrillarandmicellar
nanostructureswith5–20nmsizewereobserved(GulerandStupp,
2007).Releetal.(2007)designedcollagen-mimeticpeptideswith
D-periodicityand reportedthat0.5%uranylacetate stainingfor
10swassufficienttoprovidecontrastforTEMimaging(Fig.2E).
ImageresolutionwassufficienttomeasuresizeofD-periodson
carryinglight-sensitivemoietyself-assembledintodifferent
mor-phology upon light triggering and both of the nanosphere and
nanofiber forms were observed by air drying of the PA
solu-tion(0.4mM)onthesubstrateand stainingwithuranylacetate
(Muraoka et al., 2009). Anothermethodfor visualization of PA
nanofibersinvolvesinduction ofnanofiber formation ona TEM
grid.Hartgerinketal.placed10Lof0.01–0.02%solutionofPA
directlyonthegrid.Thegridwasthenplacedintoasealed
cham-berwithHClvaporsfor10minfornanofiberformationafterwhich
thegridswerewashedwithdeionizedwater.Samplewasstained
withphosphotungstic acidor uranyl acetate (Hartgerink et al.,
2002).In anotherstudy,1Lof 0.1wt%PAsolutioninaqueous
mediawasdropcastedontoacarbon-coatedcoppergrid.The
sam-plesonthegridwerestainedfor1–3mininphosphotungsticacid,
gently rinsedin water and blotted dry (Hsu et al., 2008).This
enabledobservationofnanofiberswithin5–8nmrange.
Vesicu-larstructures(50–450nm)formedbyself-assemblyofdipeptides
werevisualizedbynegativestainingwithuranylacetate(Mishra
et al.,2008).Authorsconcluded thatthere is a highdensity of
negativechargesonvesicles since there wasgrayscalecontrast
betweenvesiclesurfaceandgridbackground.Positivelycharged
vesicles showedlighterstainingthan background.Useof
nega-tivestaininghelpedtoclarifythesefinefeatures.Limetal.(2008)
observednanoribbonscomposedof-sheetformingand
bioac-tivepeptidesequencesdesignedforencapsulationofhydrophobic
drugsbyTEMimaging. After1mintreatmentof TEMgrid with
peptide solution, remaining solution was removed with filter
paperandpositivestainingwasperformedwithruthenium
tetrox-ide.
Sometimespre-fixationstepisnecessaryduringsample
prepa-rationtoconservedelicatestructuressuchassubcellularorganelles
and toprotect complex three-dimensionalnanostructuresfrom
effectsofsample preparation(e.g.drying).Beniashetal.(2005)
visualizedentrapmentofmammaliancellsinpeptideamphiphile
nanofibermatrixbyusingTEM.Fixationwithglutaraldehydeand
OsO4anddehydrationstepsprecededsamplepreparation,which
includedembeddinginepoxyresinandultrathinsectioning.A
sim-ilarmethodwasalsousedtovisualizepeptide-hyaluronicacidsac
structures(Capitoetal.,2008).
Metal-bindingpeptidenanofibrilsprovideagoodtemplatefor
tailored growth of metal particles on them, which is a smart
methodfornanowiresynthesis.Peptide nanofibrilswith
metal-bindingsiteshaveaffinitytodifferentmetalssuchassilver,gold
andplatinumdependingonaminoacidsequence(Kasotakisetal.,
2009).Metal-boundnanofiberswereobservedbyusingTEM
with-outanyneedforstainingtoincreasecontrast,sincemetalsprovide
contrast(Fig.3).Peptide amphiphilenanofibers areamong the
peptide-basednanostructureswhicharegoodtemplatesfor
min-eralization.Bright-fieldTEMimagingofvariousCd2+–PAmixtures
revealedthatCd2+ionsgrowhomogenouslyonPAnanofibers(Sone
and Stupp, 2004). Cd2+ ionsprovided necessary contrast
with-outfurtherstaining.Peptideamphiphiles,which weredesigned
asMRI contrastagents, formnanofibersandnanomicelles with
higherrelaxivitytimethanknownmonomericMRcontrastagents
(Bulletal.,2005).Thiscontributionisprobablycausedbyhigher
orderstructuresformedbymonomericpeptideamphiphileswhich
chelate Gd(III)ionseffectively. Visualizationof nanofiber
struc-tureswithGd(III)ionsdidnotneedanystainingeither,sinceGd
(III)ionsthemselvesprovidecontrast.Carnyetal.reportedthat
linkeraminoacidssuchascysteine(bearingthiolgroups)allowed
ordered organization of gold nanoparticles on diphenylalanine
peptidenanotubes.Thesegoldcoatednanotubeswerealsoimaged
byTEMwithhighcontrastwithoutanyfurtherstaining(Carnyetal.,
2006).Coatingnanostructureswithappropriatemetalsisa
conve-nientwayofincreasingcontrast.Contrastofnanofibrousstructures,
withadiameterof12nm,wasincreasedbyusingplatinumcoating
afterdryingofthesample(eithergelordilutedsuspension)onTEM
grid(Smeenketal.,2005).Ryadnovetal.(2003)observed
peptide-mediated assembly of gold nanoparticlesby using TEM, where
nanoparticleswereobservedtobeseparatedwithhomogenous
dis-tances(7nm)fromeachother.Inthisstudy,nanoparticle-peptide
solutionon carbonwas driedwith filterpaper withoutfurther
stainingforsamplepreparation.Althoughgoldnanoparticleswere
clearly observed,peptidenanostructuresholdingthem together
werenotobservable.
Inordertogetridofthenegativeeffectsofdrying,QFDE-TEM
protocolcan beusedfor samplepreparation. In thistechnique,
samplesarefrozenat−195◦C,afterwhichtheyarefracturedin
freeze-fracture apparatus. After etching at −95◦C, samples are
coatedwithplatinium/carbonmixture.Elongatednanostructures
formedbymixingpeptideamphiphileandoligo(phenylene
ethyny-lene)wereobservedbythismethod(Bulletal.,2008).
High-resolutionfieldemissiongunTEM(HR-TEM)isanimaging
methodwhichenablesvisualizationofcrystallographicstructure
ofmaterialsatnanoscale.Resolutionthatcanbereachedwiththis
methodisbelow1 ˚A.Soneetal.usedHR-TEMtovisualizelattice
structureofCdSnanocrystalsgrownonPAnanofibers(Fig.4A).
HR-TEMwasalsousedtovisualizenegativelystained(uranylacetate)
peptidenanotubes(RechesandGazit,2003).Inthisexample,
HR-TEMprovidedindicationoftheregularstructuresofthetubewalls.
Moreover,silver-filledpeptide nanotubesfor nanowire
produc-tionwerevisualizedwithoutstaining,sinceagainsilverprovided
requiredcontrast.
Cryo-TEM isanother powerfultechnique toobserve peptide
nanostructuresintheirnativestate(Hartgerinketal., 2001).In
onestudy,hydrogels formedbyself-assemblyof-hairpin
pep-tidesweresnap-frozenbyliquidethaneandimagedbycryo-TEM
(Schneideretal.,2002).Cryogenicsystemholdstemperature
con-stantat−170◦C,preventingsublimationofthesample.Structures
wereimagedwhileunderfocusedinordertoenhancecontrastand
nanofibrousnatureofthescaffoldwasobservedbythismethod.
Inanotherstudy,morphologicaltransformationofnanostructures
fromtwistedribbonsintohelicalribbonswasobserved(Pashuck
andStupp,2010).Pashucketal.(2010)rapidlyfrozethinlayersof
peptideamphiphilesolutionsinliquidethanetopreserve
morpho-logicalstructuresandtoavoiddryingeffect.Byusingthismethod,
nanofiberswith8–10nmdiameterswereobserved(Fig.4C).
Cryo-TEMprovidesvaluableinformationwhichcannotbeobtainedwith
techniquesbasedonsampledrying.Cuietal.(2009)observedthe
flexiblestructure ofnanobeltswhichariseastilts,flippingsand
entanglementsinmorphology(Fig.4B).Inthismethod,contrast
relatedtodifferenttiltanglesofnanobeltswasobserved(Fig.4D).
At90◦ tiltangle(nanobelt surfaceisparalleltoelectronbeam),
electronstravelthelongestdistanceinnanobeltsandthehighest
contrastisachieved.
EF-TEM is an improved electron microscopy system, which
canfilterscatteredelectronsinaspecimenaccordingtoenergy,
besidesscatterangle.Differentenergywindowscanbeusedfor
filteringprocessandselectingelectrons(KohlandReimer,2008).
Energy-basedselectionofcontrastmightallowachieving
elemen-talcontrast.Thismethodallowsimagingwithoutfurtherstaining
withsufficientcontrast.Kogisoetal.(2000)usedEF-TEMfor
exam-inationoffinestructuresinhydrogelsandproducedhigh-contrast
imageswithoutstaining.
Insummary,TEMimagingallowssuccessfulimagingofpeptide
nanostructures,whichareafewnanometersinsize,byusing
differ-entspecimenpreparationtechniquesforincreasingimagecontrast.
ModernversionsofTEMcanevenvisualizenanostructuresin
solu-tion(cryo-TEM)orcrystallographicstructuresatangstromlevel
(HR-TEM).TEMcanbeusedfordetailedmorphologicaland
struc-turalanalysisofnanostructuresatascaleofafewnanometersor
Fig.3.TEMimagesofpeptidenanofibrilsincubatedinsilver(AandB),gold(CandD)andplatinum(EandF)solution.Nonegativestainingwasperformed.Metalson nanofibrilsprovidedsufficientcontrast.
ReprintedwithpermissionfromKasotakisetal.(2009).Copyright©(2009),JohnWiley&Sons,Inc.
3. Scanningelectronmicroscopy(SEM)
Whenspecimenis too thickfor TEMimaging, using SEM is
amoreappropriatechoicetogethighresolutionimages. Unlike
TEM,SEMuseselectronsreflectedfromsurfaceofsampleas
sig-nals for image generation.Detailed information aboutworking
principleofSEMisexplainedinreferencePease(2008).SEMcan
provideinformationaboutsurfacetopography,crystalline
struc-ture,chemical compositionandelectrical behaviorofanalmost
1msliceonthesurfaceofthesample(Vernon-Parry,2000).
More-over,samplepreparationtechniquesforSEMarelessdestructive
thanTEM.Therefore,itisbettertouseSEMforobservingbulky
sam-ples(Vernon-Parry,2000).ECM-mimickingbiomaterialsformedby
non-covalentinteractionsofpeptidenanofiberscanbeconsidered
asexamplesforthistypeofbulkysamples.Inordertoincrease
contrastandresolutionofimagingofthesehydrogels,specimen
preparationisacriticalstep.Foreffectivespecimenpreparation,
thefirstrequirementisdehydrationofthesamplewithout
destruc-tingthethree-dimensionalstructureofthehydrogel.Airdrying
ofhydrogels causescollapsingofthenanofiber network, which
hinders our understanding of three-dimensional structure and
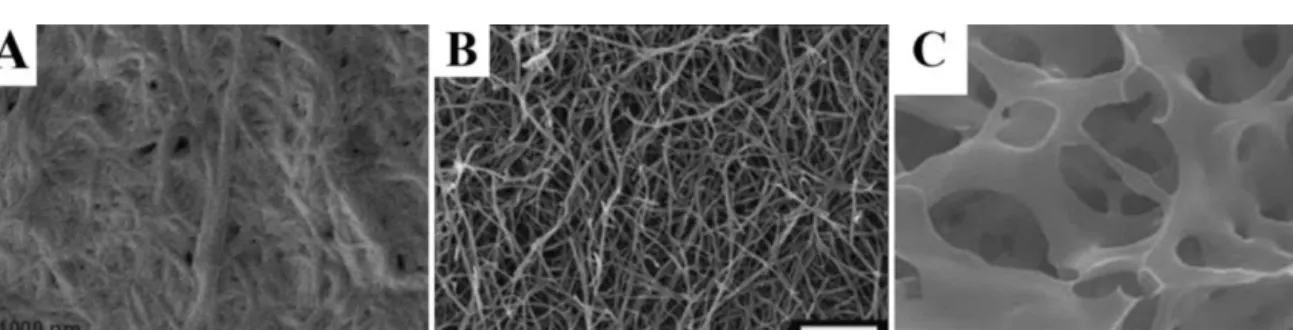
porosityofthematerial(Mahleretal.,2006)(Fig.5A).Network
dehydrationbyethanoltreatmentandcriticalpointdryinghelpin
solvingthisproblem(Spoerkeetal.,2009).Thismethodenabled
observationofthree-dimensionalnanofiber networkcomprising
peptideamphiphilegels(Fig.5B)(Rajangametal.,2006;Spoerke
etal.,2009).Inthesestudies,coatingspecimenswithaverythin
layerof(3nm)gold–palladiumalloyincreasedcontrastof
nanos-tructuresandqualityofimages.Anothermethodfordehydrationof
hydrogelsisfreeze-drying.Althoughthree-dimensionalstructures
mightbedistortedthismethod,XuandKopecek(2008)achievedto
getfineimagesofmeshworkstructureformedbyself-assembled
triblockpolypeptides(Fig.5C).Thismethodincludessnap-freezing
ofhydrogelsampleswithliquidnitrogen,freeze-dryingoffrozen
hydrogelandcoatingofsampleswithgold.
Adler-Abramovichetal.(2006)visualizednanotubestructures
formedbyself-assemblyofdiphenylalanineunitsbySEM(5kV).
Forsamplepreparation,peptidesolutionwasdroppedontoaglass
coverslip,air-dried and coated withgold.Individual nanotubes
reaching80nminsizewereobservedbythis method.Ryadnov
etal.visualizedindividualpeptidenanofibers,which are50nm
Fig.4. CharacterizationofnanostructuresbythehelpofadvancedTEMmodules.(A)HR-TEMimageshowingcrystallatticestructureofmineralsgrownonPAnanofibers. (B)Cryo-TEMimageshowsmechanicalflexibilityofpeptideamphiphilenanobelts.(C)Vitroeusicecryo-TEMimagingofPAnanofibers.(D)Illustrationofcontrastgeneration mechanismincryo-TEMimages.Excellentcontrastinnanobeltimages(B)isassociatedwithdifferenttiltanglesofnanobelts.Whennanobeltistilted90◦,electronstravel
maximumdistanceinnanobeltandhavethehighestpossibilitytobescattered(darkerlinesinimage).
FigureAwasreprintedwithpermissionfromSoneandStupp(2004).Copyright©(2004),AmericanChemicalSociety.FigureB,DwerereprintedwithpermissionfromCui etal.(2009).Copyright©(2009),AmericanChemicalSociety.FigureCwasreprintedwithpermissionfromPashucketal.(2010).Copyright©(2010),AmericanChemical Society.
Fig.5. SEMimagesof3Dhydrogelswithdifferentsamplepreparationmethods.(A)Air-driedFmoc–diphenylalaninegel.(B)Critical-pointdriedpeptideamphiphilenanofiber gel.(C).Freeze-driedtriblockpolypeptidehydrogel.
FigureAwasreprintedwithpermissionfromMahleretal.(2006).Copyright©2006WILEYVCHVerlagGmbH.FigureBwasreprintedwithpermissionfromSpoerkeetal. (2009).Copyright©(2009),JohnWiley&Sons,Inc.FigureCwasreprintedwithpermissionfromXuandKopecek(2008).Copyright©(2008),Springer.
carbon-coatedgridsbyairdryingandvisualizedbySEMafter
stain-ingwithuranylacetate(RyadnovandWoolfson,2003).Branching
ofnanofibers,whichself-assembledfrombranchedpeptides,could
beidentifiedthroughthismethod.
Peptide membranes, which are formed by adding peptide
solutioninhyaluronicacid(orhyaluronicacid/heparin)solution,
provideanotherexampleforhigher orderstructuresformedby
peptidenanofibers.Here,pre-fixationwith2%glutaraldehyde
solu-tion(3%sucroseinPBS)beforenetworkdehydrationandcritical
pointdryingstepsarerequiredinordertopreservemembraneand
sacstructures(Capitoetal.,2008;Chowetal.,2011).Cross-section
ofmembranescanbeobservedbycuttingsacsinhalf.PAnanofiber
–Tifoamhybridstructureswerealsopre-fixedwithglutaraldehyde
andformaldehydesolutions(Sargeantetal.,2008a).
Peptidenanoparticlesareanotherexampleofnanostructures
whichcanbeobservedbySEM.Liuetal.usedfieldemissionSEM
inordertoimageantimicrobialpeptidenanoparticleswithsizes
oflessthan150nm.Forsamplepreparation,nanoparticlesolution
wasdroppedontoasiliconwaferandair-driedatroom
tempera-ture.Driedsampleswerecoatedwithplatinumbeforeimaging(Liu
etal.,2009).
ModernSEMinstrumentsincludemoresophisticatedversions
suchas HR-SEM (high resolution SEM), E-SEM (environmental
SEM)andcryo-SEM(cryogenicSEM)whichareusedforvarious
purposes.Mahleretal.(2006)reportedthatFmoc-diphenylalanine
(FF)unitsformgelsathigherconcentrations(1wt%)which
con-sistoffibrousnetworks.HR-SEMandE-SEMwereusedtoanalyze
three-dimensionalmorphologyof thesehydrogels.Flexibility of
individualfiberscanberecognizedfromHR-SEMimages.Toimage
non-conductingsamples,E-SEMseemstobeabetterchoice,since
itdoesnotincludeanytreatments(stainingorcoating)for
sam-plepreparation.UnlikeconventionalSEM,sampleislocatedina
chamberwithhighpressureratherthanvacuum.ImagesofFFgel
obtainedbyE-SEMconfirmedexistenceofafibrillarnetworkunder
humidconditions(Mahleretal.,2006).
Inaddition,peptidenanostructurescanalsobevisualizedby
using cryo-SEM. Cryo-SEMinvolves snap-freezingof sample in
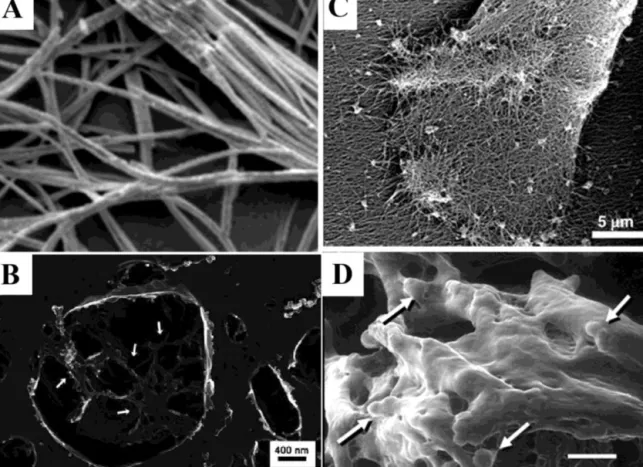
ordertoobservenanostructuresinsolutionform.Nanofibergels
formedbyFmoc-dipeptideswereobservedbycryo-SEMand
diam-etersofnanofibersweremeasured(Fig.6A).Sincethisvaluewas
wellabovethediameterofFmocdipeptidebuildingblock,authors
concludedthatobservednanofiberswerebundlesof
supramolec-ularaggregatesofdipeptides(Jayawarnaetal.,2006).
Visualization of supramolecular structures encapsulated in
membranous structures suchas liposomes can be achieved by
usingaspecialtechniqueforspecimenpreparation,called
quick-freeze/deep-etch(QFDE).Light-inducedPAnanofibersinliposomes
werevisualizedwithSEMimagingbyusingthissamplepreparation
method(Leeetal.,2008).Thistechniqueproducesreplicasfromthe
fracturedsurfaceofarapidlyfrozensample.Afterfracture,the
etch-ingprocesssublimateswaterfromthesurface,exposingstructures
otherwisehiddeninice.ThePAnanofibernetworkwasobserved
fromfracturedpartsofsurfaces(Fig.6B).
Imagingcellsinnanofibergelsisanotherissuethatwassolved
byspecial sample preparation protocolsand sophisticated SEM
techniques.To imagecellsonPAnanofiber scaffolds,fixationis
necessary.Shahetal.usedethanoldehydrationandcriticalpoint
dryingafter fixation.Mesenchymal stemcells adhered onto PA
nanofibernetworkwerevisualizedbyusingthismethod(Fig.6C)
(Shah et al., 2010). E-SEM is alsoa good instrument for
imag-ingbiologicalentitiessuchascellsentrappedinnanofibermatrix.
Chondrocytesculturedin gelwere imagedin theirnativeform
(hydrated)byusingE-SEM(Fig.6D)(Jayawarnaetal.,2006).
SEMisamicroscopytechniquewidelyusedforobservationof
thickersamplessuchasthree-dimensionalstructureofhydrogels,
cellsentrappedinnanofibermatricesorrelativelybiggerindividual
nanostructures.Thus,itshouldbeexploitedtoanalyzethebulky
natureofhigher-orderstructuresformedbypeptide
nanostruc-tures.
4. Atomicforcemicroscopy(AFM)
AFMinvolvesscanningofsurfacewithacantilever,typically
madeofsiliconnitrideforbiologicalapplications,whose
deflec-tionsarerecordedbycomputertogenerateimage(Engeletal.,
1999).MoreinformationaboutthebasicmechanismofAFMcan
befoundinreferenceAlessandriniandFacci(2005).Pyramidaltip
attheendofcantilevercanbesufficientlysharptoachieve
res-olutionoflessthan1nm.AFMhasseveraladvantagesoverother
microscopytechniques.Firstofall,samplepreparationforAFMis
easier,sinceitdoesnotneedanypretreatmentofspecimensuchas
staining,labelingorcoating(Allisonetal.,2010).Moreover,images
obtainedwithAFMcanbedemonstratedinathree-dimensional
format.Anotheradvantageisthatcellsandbiomoleculescanbe
imagedinaphysiologicallyrelevantenvironmentwithoutfreezing
oranyothertreatment(Allisonetal.,2010).
ForAFM imagingof peptidenanostructures,silicon waferor
freshlycleavedmicacanbeusedassubstrate.Bulletal.prepared
thesamplebydropcasting0.05wt%diluteaqueoussolutionof
pep-tidemoleculesontofreshlycleavedmica(Stoneetal.,2009).AFM
imagesshowedthatthesemoleculesformone-dimensional
nanos-tructureswith5–10nmwidthandheight.Similarityofwidthand
heighthasledauthorstosuggestthatnanostructureshave
cylindri-calshape.Guleretal.(2005)used10L0.1wt%solutionofPAand
dropcastedthesampleontosiliconwaferwhichwaspre-cleaned
byultrasonicationinwaterandisopropanol.Aggregatedviewof
nanofiberbundlescouldbeobservedfromthisimage.Inanother
study,sampleswerepreparedfrom2wt%peptideamphiphile
solu-tion,initiallygelled,andthendilutedto0.1wt%(Hsuetal.,2008).
These sampleswere later drop casted onto pre-cleaned silicon
waferand air dried. AFM wasperformedin tapping modeand
peptidenanofiberswith5nmdiameterwereclearlyobservable.
Zhouetal.visualizedFmoc-basedpeptidehydrogelsbydiluting
anddroppingontomicasurface.Waterwasremovedbycapillary
action,andsampleswerewashedandimagedwithtappingmode
AFM(Zhouetal.,2009).Interwovennetwork ofnanofibersand
bundlesobservedinthis studysuggested thatFmoc-based
pep-tidehydrogelspossessathree-dimensionalnanofibrousstructure.
Sargeantetal.coatedNiTi(nickel-titanium)surfaceswith
bioac-tivePAnanofibersforbiofunctionalizationofimplantsurfaces.AFM
imagingwasperformedbyusingtappingmodeandshowedthat
PAnanofiberscoatedtheNiTisurfaceshomogenously(Sargeant
etal.,2008b).Bulletal.(2008)usedmicasurfaceinanotherstudy
where0.1wt%solutionwasdropcastedandrestofthesolutionwas
removedandairdried.Peptidenanofibersandnanoparticleswith
nearly5nmheightwereobservedbythismethod.Inanotherstudy,
-amyloidpeptidesweredepositedontofreshlycleavedmica
sur-face andwashed withwater after 1.5min(Cohen etal., 2006).
Formationofseveralmicronslongandafewnanometerswide
amy-loidfibrilsafterpolymerizationphasewasobservedbythisway.
Moreover,inhibitionofpolymerizationbyindolederivativeswas
alsodeducedfromAFMimagestaken(Cohenetal.,2006).Toimage
reassemblyofbolaamphiphilicpeptidesformingnanofibersafter
theirbreakdownwithsonication,tappingmodeAFMwasused(Qiu
etal.,2008).Sampleswerewashedawayfrommicasurfaceafter
afewsecondsofincubationandairdried.AFMimagingshowed
thatlengthofpeptidenanofiberswereincreasedfrom∼300nm
to∼800nmafter25days,whichindicatesreassemblyofbroken
nanofibers.
AFM images can also provide information about secondary
structuresformedbypeptides.Itispossibletodeterminerightor
nanostruc-Fig.6.DifferentusageareasofmodernSEMtechniquesincharacterizationofpeptidenanomaterials.(A)Cryo-SEMimageofnanofibrousgelformedbyself-assemblyof Fmocdipeptide.(B)Imageofnanofibrousnetworkofpeptideamphiphiles(whitearrows)inliposome.SamplewaspreparedbyQFDEmethod.(C)Mesenchymalstemcells inPAnanofibergel.Samplewaspreparedbyfixationandcriticalpointdrying.(D)ESEMimagingofchondrocytecells(whitearrows)inFmoc-dipeptidegel.
FigureA,DwerereprintedwithpermissionfromJayawarnaetal.(2006).Copyright©2006,JohnWiley&Sons,Inc.FigureBwasreprintedwithpermissionfromLeeetal. (2008).Copyright©(2008)TheRoyalSocietyofChemistry.FigureCwasreprintedwithpermissionfromShahetal.(2010).Copyright©(2010),NationalAcademyofSciences, U.S.A..
Fig.7. Differentsupramolecularaggregatesformedbytripeptideamphiphilesdependingonthenatureofendgroup(A)determinedbyAFMimaging.Reprintedwith permissionfromLietal.(2007).Copyright©2007,JohnWiley&Sons,Inc.Straightcylindricalnanofibersformedwhenendgroupisacetate(B),howeverhelicalstructures withregularpitchformedwhenbulkierendgroupisusedsuchasdimethylacetate(CandD).
turesandmeasurepitchvaluesbetweenhelices(Lietal.,2007; Muraokaetal.,2008).Tripeptideamphiphilesarefoundtoform
helicalstructuresinorganicsolventswhenthebulkierendgroup
isused,mostlikelycausingtwistingofcylindricalnanostructures
(Fig.7A–C)(Lietal.,2007).Delicateobservationofheightprofiles
obtainedbyAFMindicatedlefthandednessofhelicalstructures
(Fig.7D).Moreover,pitchvaluesobtainedfordifferenthelical
struc-turesshowedcorrelationwithincreasedbulkinessofendgroup,
suggestingtorsionalstrainmightbethemechanismforformation
ofthesehelices(Lietal.,2007).
ContactmodeAFMisanotherchoiceforimagingpeptide
nanos-tructures.SincecontinualcontactofAFMtipwithsurfacemight
damagefeaturesonthesurface,researchersusenon-contact or
tappingmodeAFMforthispurpose.However,byusingsoftcontact
mode,itispossibletogethighresolutionimageswithout
damag-ingorganicsonthesurface.Toachievethis,itiscrucialtouseAFM
tipswithlowspringconstant(0.2N/morbelow),whichare
appro-priateforsoftcontactmodepurposes.Wehaveusedcontactmode
tovisualizedifferentpeptideamphiphilenanofibersandwereable
toobtainhighresolutionimages(Toksozetal.,2011).
Peptidenanostructurescanalsobevisualizedinsolution,which
isbettertoobservetheirnativeformbyeliminatingeffectscaused
bysampledrying. Imagingbiologicalsamplesinnativeaqueous
environmentis a keyadvantage ofAFM over othermicroscopy
techniques.One ofthe main issues in wetimaging is
immobi-lizationofspecimentothesurface. Whiletappingmodeallows
visualizingbiologicalprocesses andmolecules weaklyadsorbed
tothesurface,duetominimizationoflateralforcesinthismode,
immobilizationiscriticalforcontactmodeimaging,wherelateral
forcesofAFMtipmaydriftspecimen(Engeland Muller,2000).
Twodifferenttypesofimmobilizationarenoncovalentand
cova-lentmethods. Noncovalent approach ismore simple and based
onphysicaladsorptionofspecimenontosurface throughforces
likevanderWaalsforces,electrostaticdouble-layer(EDL)forces,
hydrationforcesandhydrophobiceffects(Wagner,1998).
Chem-icalmodificationofsurfacecanincreaseadsorptionofmolecules
ontosurface.Forexample,silanizationofsurfacecanhelp
adhe-sionofbiopolymers(Wagner,1998).Covalentlylinkingspecimen
tothesurfacebecomesimportantwhendisplacementofmolecule
onthesurfaceisacriticalproblem.Limitingissueof
immobiliza-tiontechniqueisinactivationofthebiologicalstructureorprocess
tobeobserved(EngelandMuller,2000;Wagner,1998).Surface
energy,surfacechargesandhydrophobicityarepropertiestobe
consideredforpreservationofstructuresimmobilizedtosurface.
Forexample,hydrophobicsurfacesarenotrecommendedforwet
imaging.Because,theyinterferewithAFMimagingduetoincreased
adhesionanddenaturationofproteins(Wagner,1998).
Horiietal.(2007)imagedpeptidenanofibersinaqueous
solu-tionbyusingtappingmodeAFM,whereobservedsizeofnanofibers
were greater than expected. It is acceptable to assume that
nanofibersbecame hydratedin solutionand observed sizewas
greaterthanexpectedsize.
RadiusoftheAFMtipisanimportantparameterforresolution,
sincetipconvolution(alsocalledastipimaging)shouldbeavoided
inordertounderstandexactsizeandshape ofobservedfeature
onthesurface.Forthispurpose,tipwidthshouldbesmallerthan
widthofstructuresonthesurface.Otherwise,observedimagewill
beaffectedbytipshape.Itisbettertousetipswithradiismaller
than10nmtoobservefinenanostructuresonthesurface.Genovéet
al.usedsuchAFMtipsinordertovisualizenanostructuresonthe
surface.Sampleswerepreparedwith0.01wt%peptidesolutions
andobservedbyusingtappingmodeAFM(Genovéetal.,2005).
Itisalsopossibletosubtractconvolutioneffects,byusing
mathe-maticalmodelsdevelopedforsample–tipinteraction.Byassuming
geometricshapeofstructuresonthesurface,itispossibletoconvert
observedwidthtorealwidth(Hongetal.,2003).
Atomic force microscopy imaging can yield high resolution
images that can be used to study various conformations of
biomolecules and their secondary structures. Scheuring et al.
(2003) have carried out an extensive high-resolution study on
Rhodobactersphaeroideslightharvestingcomplex2,demonstrating
the capability of force microscopy for topographic
character-ization of biomolecules with higher than 0.1nm resolution.
High-resolutionimaging of biomolecules can bedoneby using
dynamicAFM.SanPauloandGarcía(2000)haveshownthe
impor-tanceofchoosingcorrectimagingparametersinobtainingartifact
freeimagesofantibodieswithminimalsampledamage.
Surface properties of substrate used in sample preparation
mightsignificantly affectquality ofimagesobtainedwithAFM.
Jiangetal.(2007)preparedthesamplesbydippingsiliconwaferor
goldsurfaceinto0.1wt%solutionofPAandair-dryingafterslowly
withdrawingthem.Interestingly,theyobservedhigh-aspect-ratio
cylindricalnanofibersonsiliconsurface,whilesignificantlyless
lin-earfeaturesongoldsurface,withlessclearimage(Fig.8).Authors
explainedthisbysuggestingthatsurfaceroughnessofgoldsurface
probablyinterfereswithAFMimaging.Surfacepropertiesmight
affect formation of nanostructures on the surface, especially if
samplepreparationmethodisbasedonairdrying.Toeliminate
effectsofsurfaceonnanostructureformation,solutionsshouldbe
coatedontodifferentsurfaceswithvaryingchemicalproperties.
Forthis purpose,Ashkenasyet al.(2006)usedmica,highly
ori-entedpyrolyticgraphite(HOPG)andhydroxylatedsiliconoxidefor
visualizingnanotubesformedbycyclicpeptidesandimagedwith
tappingmodeAFM.Asauthorsobservedsimilarnanotube
struc-turesonallmaterials,theyconcludedthatnanotubesformedin
solution,andnotbyinteractionwithmaterialsurface.
Besidesgivingtopographical informationabouttheanalyzed
surface,AFMcanalsoprovideinformationaboutthestiffnessof
surfacenanostructuresandtheadhesionpropertiesbetween
pep-tidesandabareorfunctionalizedAFMtip.Suchnanomechanical
mappingproducesanelasticmodulusmapofthesurface,
includ-ingquantitativevaluesforeachfeatureonthesurface(Sahinand
Erina,2008).Severalestablishedtechniquesexistfor
nanomechan-icalmapping(Heubergeretal.,1995;Maivaldetal.,1991;Miyatani
etal.,1997;Radmacheretal.,1993;RosaZeiseretal.,1997).
How-ever,organicmolecules are typicallyfragile and measurements
mustbeperformedinawaythatdoesnotapplylargeforcesand
pressuresduringmapping(SanPauloandGarcía,2000).Recently,
noveltechniques that can produce nanomechanical maps with
highresolutionandminimalsampledamagehavebeenintroduced
(Dong etal.,2009;Husaleetal.,2009).While,noveltechniques
continuetogainpopularity,conventionalforcespectroscopyhas
been instrumental in nanomechanical characterization. Dagdas
etal.(2011)usedAFMtocarryoutforce-distancemeasurements
for identifying stiffness values of PA nanofibers. Authors used
surfaceswithknownstiffnessvalues(siliconandPMMA)to
esti-mate and compare elasticmoduli of PA nanofiber films made
withcalciumionsorHCl.Elasticmoduliofbothnanofilmswere
0.1±0.05GPa,whileonemadewithcalciumhadslightlyhigher
stiffness(0.2±0.1GPa).Helenetal.(2011)alsoinvestigatedthe
effect of gelation conditions onadhesion and stiffness of
self-assembledorganicmaterialsandtheyusedforce-spectroscopyin
liquidtocorrelateadhesionandstiffnesspropertiestomacroscopic
mechanicalproperties.Smithetal.(2006)hasappliedforce
spec-troscopyonamyloidfibrilsself-assembledfrominsulin,byprobing
freestandingfibrilsongoldsurfacespatternedwithgrooves.Such
geometriesareparticularlysuitableforquantitativeanalysisdue
towelldefined boundaryconditions,andSmith etal.extracted
anelasticmodulusof3.3±0.4GPaandstrengthof0.6±0.4GPa.
Adhesionis also an importantparameter in understandingthe
mechanical andchemical properties ofpeptidic nanostructures.
inves-Fig.8.AFMimagingofPAnanofibersformedongold(A)andsilicon(B)surface. ReprintedwithpermissionfromJiangetal.(2007).Copyright©(2007)TheRoyalSocietyofChemistry.
tigateadsorptionmechanismofpeptidesonhydrophobicsurfaces,
tofindout that multiplemechanismsare responsiblein
deter-miningtheadsorptionstrength.Themechanicalmappingcanbe
performedbothinairandinliquid,enablingstudyofvarious
envi-ronmentsonmechanicalandadhesionproperties.Dongetal.has
studiedforce-extensiononself-assembledfibrils formedby
29-residueamphiphaticpeptidehormoneglucagoninbufferwithapH
of2.0(Mingdongetal.,2008).Kimetal.hasusedafunctionalized
tiptomeasurethebindingpropertiesoflipopolysaccharides(LPS)
onimmuneproteins(lipopolysaccharidebindingprotein[LBP]and
CD14).Intheirstudy,Kimetal.(2007)havedirectlyobservedthe
concentrationdependentinhibitoryeffectofantimicrobialpeptide,
polymyxinB(PMB)onbindingofLBP-LPSandCD14.In
function-alized tipstudies, typically a linker(or spacer)is used toaffix
biomoleculesonto thetip. Thisallows freemotionand
confor-mationofthemoleculeonthetip.Theeffectoflinkerproperties
onadhesionpropertieshavebeenstudiedpreviously(Craigetal.,
2008),anditwasfoundthatoptimalspacerlengthsyieldedhighest
adhesion,underlyingthecomplex natureof suchdirect
quanti-tativemeasurements.Innanomechanicalmappingandadhesion
studies,typicallyalargenumberofmeasurementsareperformed
anddataisanalyzedusinghistogramstoobtainstatistically
sig-nificantinformation about the sample. During nanomechanical
measurements,forforce-extensionexperimentstypicallysoft
can-tileverswithspringconstantsontheorderof 0.01–0.1N/mare
used,whileforelasticmodulusmeasurementsstiffercantilevers
(1–10N/m)arepreferred.
AFMisahighlysophisticatedtechnique,whichcanbringout
highresolutionimagesnotonlyaboutthree-dimensional
topogra-phyofsurface,butalsomechanicalpropertiesofmoleculesonthe
surface.Besidesthis,laborioussamplepreparationtechniquesare
notrequiredinthismethod.AllthesemakeAFMveryattractivefor
researchersworkingwithpeptidenanomaterials.
5. Opticalmicroscopy-basedtechniques
Despitehavinglowerresolutionandmagnification,several
opti-cal microscopy techniquessuchas polarizing,fluorescence and
confocalmicroscopy provideinvaluableinformation for
charac-terizationofpeptidenanostructures.Immunostainingofsamples
provideexcellentcontrastforfinestructuresandallowsobtaining
imageswhichcannotbeobtainedwithTEM,SEMorAFM.
Inpolarizingmicroscopy,thesampleisilluminatedby
polar-izedlight,whichinteractswithanisotropicdomainsandgenerates
contrastbetweenanisotropicdomainsand otherpartsof
mate-rial.Self-assemblyofpeptidemoleculesproducedifferentliquid
crystallinedomainsin gels. Polarizinglight microscopyallowed
identificationofauniqueopticalproperty–birefringency–ofthese
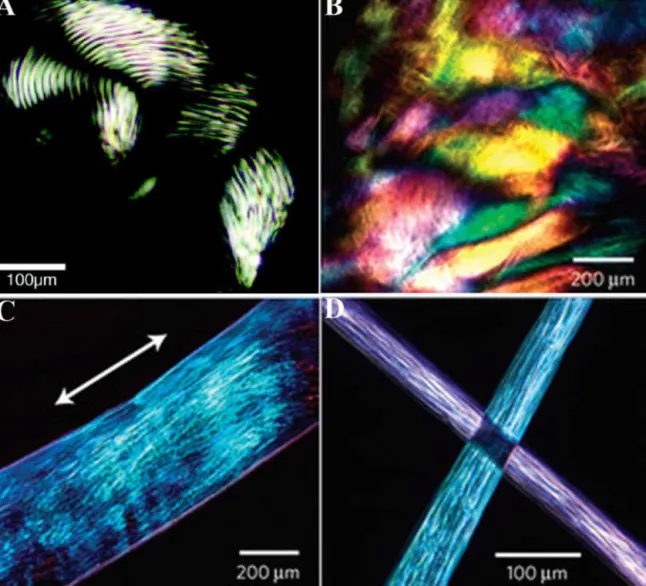
anisotropicdomains.Hartgerinketal.(2002)showedorientation
ofliquidcrystallinephaseofpeptideamphiphilegelsintherange
oftensofmicrons(Fig.9A).Concentrationdependentvariationof
birefringencepropertyofpeptideamphiphilegelshasalsobeen
demonstrated(HungandStupp,2007).Hexagonalliquidcrystalline
phasechangestonematicphasewithweakerbirefringentproperty
asPAconcentrationdecreases.Uniformandlarge(tensof
millime-ters)birefringentdomainswereobservedinpolarizingmicroscopy
imagesofalignedmonodomainPAgelfilms,whereuniformityof
birefringencyindicatedalignmentofnanofiberstoconstitutegel
film(Fig.9B–D) (Zhanget al.,2010).Stainingalsostands tobe
apowerfulmethodtodetectpeptidenanostructuresinsolution.
Congoreddye,whichbindsto-sheet-richregions,iswidelyused
todetectamyloid-likestructures.Congored-stainedamyloid-like
peptidecoatedsurfacesshowbirefringentyellow-greendomains
(GileadandGazit,2004).
Orderedassembly of nanostructurescan giveriseto
higher-orderstructures,whichcanbeobservedevenbylightmicroscopy.
Forexample,peptidenanotubes(basedondiphenylalanine
self-assembly)filmsformedondifferentsurfacessuchasgold,SiO2and
InPwereimagedwithopticalmicroscopyalongwithotherimaging
techniques(Hendler et al.,2007).Peptide nanotube
crystalliza-tiongaverisetowell-organizedspherulites,whichwereclearly
observedatthismagnification.
Immunostainingofnanostructuresisanothermethodfor
visu-alization under fluorescence microscope. Limiting step here is
thelackofspecificantibodiesagainstpeptidemoleculesforming
the nanostructures. Amyloid nanofibrils are appropriate
exam-plesforthiscasesincetheyareformedbyaggregationofnatural
polypeptides.Inonestudy,fibrilsformedindrosophilabrainby
-amyloidpolypeptide,whichplaysacriticalroleinthepathology
ofneurodegenerativediseasessuchasAlzheimer’sdisease,were
fluores-Fig.9. Polarizinglightmicroscopyimagesofpeptideamphiphilegels.(A)WellknownPAgel,formedbyshortanisotropicdomains.(B)PAgelfilm,formedbynoodle-likegels, showinglargeandsimilaranisotropicdomains.(C)Onenoodle-likegelstringshowsalignedmonodomainextendingovercentimeters.(D)Lightextinctionatcross-points oftwogelstringsshowsuniformalignmentineachstring.
FigureAwasreprintedwithpermissionfromHartgerinketal.(2002).Copyright©(2002)NationalAcademyofSciences,U.S.A.FigureB-Dwerereprintedwithpermission fromZhangetal.(2010).Copyright©(2010)MacmillanPublishersLtd.
centmicroscopewhereself-assembledfibrillarstructuresstained
with-amyloidantibody couldbe observed(Scherzer-Attali et
al.,2010).Filamentoustemperature-sensitiveproteinZ(FtsZ)isa
prokaryoticmonomericprotein(homologoustoeukaryoticprotein
tubulin)whichself-assemblesintofilamentousstructures
form-ing contractile ring or Z-ring. Formation of contractile ring is
criticalforprokaryoticcelldivision.Inspiredbyself-assemblyof
FtsZmolecules,OstrovandGazit(2010)synthesizednanowires,
withthesemolecules.Forthispurpose,FtsZproteinsexpressedin
bacteriaweretaggedwithgold-binding,silver-reducingor
biotiny-lation motives at gene level. Biotinylated FtsZ polymers were
stainedwithfluorescentavidinandimagedbyconfocalmicroscopy,
whichallowedobservingfluorescentlystainedfilamentous
struc-tures.
Peptide-basednanostructurescan alsobe labeledcovalently
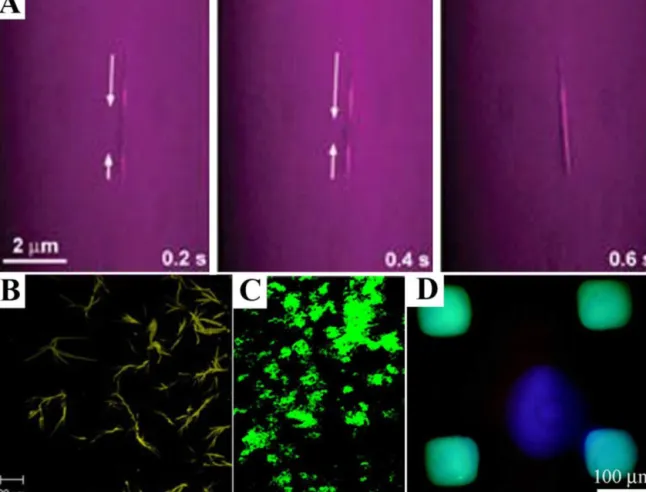
withfluorescentdyesforreal-timeimagingofdynamicprocesses.
Kametaet al. labeled bolaamphiphileswith Alexadye through
aminegroupsandobservedfluorescentnanotubesformedbythese
molecules.ToinvestigateencapsulationandtransportationofGFP
inAlexananotubes,time-lapsefluorescencemicroscopywasused
(Fig.10A).Excitationand absorptionfilters used inmirror unit
ofmicroscopealloweddetectionofFRET(fluorescenceresonance
energytransfer)fromGFPtoAlexa,which occursinnanotubes,
whilecuttingbulkGFPfluorescenceinsolutionand direct
exci-tationofAlexa(Kametaetal.,2008).
Staining -sheet-rich nanostructures with fluorescent dyes
suchasThioflavinT(ThT)isanotherpossibilityforfluorescence
imagingofpeptidenanostructures.ThTbindsto-sheetsin
amy-loidfibrilsandgivesacharacteristicshiftinitsemissionspectrum.
Tamamisetal.(2009)stainedtriphenylalanineassembliesbyusing
ThTandanalyzedthesesampleswithconfocalmicroscopywhich
showedelongatedfibrillarstructures(Fig.10B).
Fluorescent imaging alsoallows following each molecule in
bulk gel, when latter one is formed by mixing two or more
moleculessuchasheparin and heparin-bindingPA. Fluorescent
labelingofheparinallowedobservingheparininPAgelbyusing
confocalmicroscopy(Fig.10C)(Rajangametal.,2006).Sometimes,
chemicalstructuresofsomepeptidemoleculesallowdisplaying
photoluminescencepropertywithoutfurtherstainingprocedure.
Diphenylalanine(FF)peptidenanotubesshowphotoluminescence
in blue and UV regions of excitation origin caused by
Fig.10.Fluorescentstainingofpeptidenanostructures.(A)Time-lapsedimagingofencapsulationandtransportationofGFPinnanotubeslabeledwithAlexa.2brightlines, appearingattwoendsofnanotubeandextendingtocentralpartlaterareduetoFRETfromGFPtoAlexa.(B)Fluorescenceimageofself-assembledtriphenylalaninenanofibrils stainedwithThTdye,staining-sheetstructures.(C)ConfocalmicroscopyimageofgelformedbymixingPAandfluoresceinheparin.(D)Fluorescentmicroscopyimageof surfacepatternedwithdiphenylalaninepeptidenanotubes.Bluesquaresarephotoluminescencefromnanotubes,whilepurplecircleatthecenteriscausedbyreflectionsof excitationbeamfromsurface.(Forinterpretationofthereferencestocolorinthisfigurelegend,thereaderisreferredtothewebversionofthearticle.)
FigureAwasreprintedwithpermissionfromKametaetal.(2008).Copyright©(2008)JohnWileyandSons.FigureBwasreprintedwithpermissionfromTamamisetal. (2009).Copyright©(2009)Elsevier.FigureCwasreprintedwithpermissionfromRajangametal.(2006).Copyright©(2006)AmericanChemicalSociety.FigureDwas reprintedwithpermissionfromAmdurskyetal.(2009).Copyright©(2009)AmericanChemicalSociety.
et al., 2009). Patterned surface withFFnanotubes showed
flu-orescence (excitation at 340–380nm) from expected regions
(Fig.10D).
FRETcanbeusedtodetectspecificinteractionsin
biomolec-ularanddynamicsystemssuchaspeptideamphiphilegels.Ina
co-assembly systemcomprised of fluorophore attachedPA and
non-fluorescentsecondaryPA,FRETwasobservedbetweendonor
– fluorescent PA and fluorophore carrying heparin – acceptor,
which binds tonon-fluorescent PA (Behanna et al., 2006).The
energytransferwasverifiedbyacceptorphotobleaching
experi-ment,whichrecoveredemissionfromthedonor.
Ramanspectroscopyisanotherpowerfultechniqueto
inves-tigate the presence of various groups and their binding
prop-erties. Raman microscopy provides diffraction limited imaging
of biomolecular structures and conventionally cannot provide
single molecule resolution. Typically, the imaging volume is
about a micrometer cube. Even though individual fibers
can-not be resolved with confocal Raman microscopy, Matsui and
Douberly (2001) demonstrated that Raman signatures can be
usedtoidentifybundlesandindividualnanotubesself-assembled
out of the bolaamphiphilic peptide monomer
bis(N-R-amido-glycylglycine)-1,7-heptanedicarboxylate.Theyhaveshown that
Ramansignatures can be usedto discriminate peptidic
nanos-tructures bound to the bundles and the peptide nanotubes
disassembledfromthebundle.
TipandsurfaceenhancedRamanspectroscopy(TERSandSERS)
havebeenextensivelyusedtocharacterizebiomoleculesaswell.
TheversatilityoftheTERSmethodisdemonstratedbyNeugebauer
etal.(2006)whocollectedlocationdependentRamandataon
bac-terialsurfaces(S.epidermidiscells),showingpeptidicRamanbands.
Yeoetal.(2008)performedTERSonCytochromec(Cc),and
demon-stratedthesuperiorperformanceofTERSmethodonresolvingboth
thehemeandaminoacidvibrationalbands.Deckert-Gaudigand
Deckert(2010) demonstrated extremely highspatial resolution
(nanometer)ofTERSoninsulinfibrils.TERStechniqueistypically
appliedwithacombinedRamanandAFM/STMsystem,whereas
SERScanbeperformedusingasimplerRamanspectrometer.Aliaga
etal.(2011)havedemonstratedthatalargenumberofbandscan
beobservedandidentifiedusingSERSwithsilvernanoparticles,on
syntheticcarboxyterminalpeptideofhumanchorionic
gonadat-ropinb-subunit.ReproducibleSERSpectrawereobtainedbyadding
thecolloidalAgNPsolutionontothedriedanalytesample.
Simplic-ityoftheSERStechniqueallowswideapplicabilitycomparedto
TERS,inapplicationsnotrequiringspatialresolution.
Optical microscopy techniques take a snapshot of peptide
nanostructuresfromadifferentaspect.Thesetechniquesshould
beusedtoidentifyspecificdomainsorstructuresbylabelingfor
fluorescencemicroscopyandFRETtechniqueorbyusing
special-izedtechniquessuchaspolarizingmicroscopyandRaman-based
6. Conclusion
Peptidenanomaterialsarepromisingcandidatestosolvemany
issues regarding health, energy and information technology.
Progressin this field benefitsimmensely from characterization
studiesperformedbyhighlyadvanced microscopes.Capabilities
ofeachmicroscopytechniqueallowinvestigationof
nanomateri-alsfromdifferentaspects.TEMimaging,whichreliesonelectrons
travellinginsidethesample,allowsidentifyingultrafinepatterns
onnanostructures.Ontheotherhand,SEMimaginggets
informa-tionfromelectronsscatteredonthesurfaceofthesample,soitis
advantageousforimagingthickerand bulkiersamples.AFMcan
providetopographicalandmechanicalviewofthesurface,with
aneasiersamplepreparationprotocol.Opticalmicroscopy
tech-niques,inspiteoflowerresolution,provideveryusefulinformation
suchasidentificationofanisotropicdomainsinpeptidegelwith
polarizinglight microscopy.It isimportantfor researcherswho
workinthisareatounderstandlimitationsandadvantagesofeach
microscopytechniqueandchoosetherightoneforcharacterization
studies.
References
Acar,H.,Garifullin,R.,Guler,M.O.,2011.Self-assembledtemplate-directedsynthesis ofone-dimensionalsilicaandtitaniananostructures.Langmuir27,1079–1084. Adler-Abramovich,L.,Badihi-Mossberg,M.,Gazit,E.,Rishpon,J.,2010. Characteri-zationofpeptide-nanostructure-modifiedelectrodesandtheirapplicationfor ultrasensitiveenvironmentalmonitoring.Small6,825–831.
Adler-Abramovich,L.,Reches,M.,Sedman,V.L.,Allen,S.,Tendler,S.J.B.,Gazit,E., 2006.Thermalandchemicalstabilityofdiphenylalaninepeptidenanotubes: implicationsfornanotechnologicalapplications.Langmuir22,1313–1320. Alessandrini,A.,Facci,P.,2005.AFM:aversatiletoolinbiophysics.Meas.Sci.Technol.
16,R65–R92.
Aliaga,A.E.,Aguayo,T.,Garrido,C.,Clavijo,E.,Hevia,E.,Gómez-Jeria,J.S.,Leyton, P.,Campos-Vallette,M.M.,Sanchez-Cortes,S.,2011.Surface-enhancedRaman scatteringandtheoreticalstudiesoftheC-terminalpeptideofthe-subunit humanchorionicgonadotropinwithoutlinkedcarbohydrates.Biopolymers95, 135–143.
Allison, D.P., Mortensen, N.P., Sullivan, C.J., Doktycz, M.J., 2010. Atomic forcemicroscopyofbiological samples.WileyInterdiscip. Rev.:Nanomed. Nanobiotechnol.2,618–634.
Amdursky,N.,Molotskii,M.,Aronov,D.,Adler-Abramovich,L.,Gazit,E.,Rosenman, G.,2009.Blueluminescencebasedonquantumconfinementatpeptide nano-tubes.NanoLett.9,3111–3115.
Ashkenasy,N.,Horne,W.S.,Ghadiri,M.R.,2006.Designofself-assemblingpeptide nanotubeswithdelocalizedelectronicstates.Small2,99–102.
Aulisa,L.,Forraz,N.,McGuckin,C.,Hartgerink,J.D.,2009.Inhibitionofcancercell proliferationbydesignedpeptideamphiphiles.ActaBiomater.5,842–853. Banerjee,I.A.,Yu,L.,Matsui,H.,2003.Cunanocrystalgrowthonpeptide
nano-tubesbybiomineralization:sizecontrolofCunanocrystalsbytuningpeptide conformation.Proc.Natl.Acad.Sci.U.S.A.100,14678–14682.
Behanna,H.A.,Rajangam,K.,Stupp,S.I.,2006.Modulationoffluorescencethrough coassemblyofmoleculesinorganicnanostructures.J. Am.Chem.Soc.129, 321–327.
Beniash, E., Hartgerink, J.D., Storrie, H., Stendahl, J.C., Stupp, S.I., 2005. Self-assemblingpeptideamphiphilenanofibermatricesforcellentrapment.Acta Biomater.1,387–397.
Bull,S.R.,Guler,M.O.,Bras,R.E.,Meade,T.J.,Stupp,S.I.,2005.Self-assembledpeptide amphiphilenanofibersconjugatedtoMRIcontrastagents.NanoLett.5,1–4. Bull,S.R.,Palmer,L.C.,Fry,N.J.,Greenfield,M.A.,Messmore,B.W.,Meade,T.J.,Stupp,
S.I.,2008.Atemplating approachformonodisperseself-assembledorganic nanostructures.J.Am.Chem.Soc.130,2742–2743.
Capito,R.M.,Azevedo,H.S.,Velichko,Y.S.,Mata,A.,Stupp,S.I.,2008.Self-assembly oflargeandsmallmoleculesintohierarchicallyorderedsacsandmembranes. Science319,1812–1816.
Carny,O.,Shalev,D.E.,Gazit,E.,2006.Fabricationofcoaxialmetalnanocablesusing aself-assembledpeptidenanotubescaffold.NanoLett.6,1594–1597. Chow,L.W.,Bitton,R.,Webber,M.J.,Carvajal,D.,Shull,K.R.,Sharma,A.K.,Stupp,S.I.,
2011.Abioactiveself-assembledmembranetopromoteangiogenesis. Bioma-terials32,1574–1582.
Chow,L.W.,Wang,L.J.,Kaufman,D.B.,Stupp,S.I.,2010.Self-assembling nanos-tructurestodeliverangiogenicfactorstopancreaticislets.Biomaterials31, 6154–6161.
Claussen,R.C.,Rabatic,B.M.,Stupp,S.I.,2003.Aqueousself-assemblyofunsymmetric peptidebolaamphiphilesintonanofiberswithhydrophiliccoresandsurfaces.J. Am.Chem.Soc.125,12680–12681.
Cohen,T.,Frydman-Marom,A.,Rechter,M.,Gazit,E.,2006.Inhibitionofamyloid fibrilformationandcytotoxicitybyhydroxyindolederivatives.Biochemistry45, 4727–4735.
Craig,J.A.,Rexeisen,E.L.,Mardilovich,A.,Shroff,K.,Kokkoli,E.,2008.Effectoflinker andspaceronthedesignofafibronectin-mimeticpeptideevaluatedviacell studiesandAFMadhesionforces.Langmuir24,10282–10292.
Cui,H.,Muraoka,T.,Cheetham,A.G.,Stupp,S.I.,2009.Self-assemblyofgiantpeptide nanobelts.NanoLett.9,945–951.
Cui,H.,Webber,M.J.,Stupp,S.I.,2010.Self-assemblyofpeptideamphiphiles:from moleculestonanostructurestobiomaterials.PeptideSci.94,1–18.
Dagdas,Y.S.,Tombuloglu,A.,Tekinay,A.B.,Dana,A.,Guler,M.O.,2011.Interfiber interactionsalterthestiffnessofgelsformedbysupramolecularself-assembled nanofibers.SoftMatter7,3524–3532.
Deckert-Gaudig,T.,Deckert,V.,2010.Tip-enhancedRamanscattering(TERS)and high-resolutionbionano-analysis—acomparison.Phys.Chem.Chem.Phys.12, 12040–12049.
Dolphin,G.T.,Dumy,P.,Garcia,J.,2006.Controlofamyloidbeta-peptide protofib-rilformationby adesignedtemplateassembly.Angew.Chem.Int. Ed.45, 2699–2702.
Dong,M.D.,Husale,S.,Sahin,O.,2009.Determinationofproteinstructuralflexibility bymicrosecondforcespectroscopy.Nat.Nanotechnol.4,514–517.
Dvir,T.,Timko,B.P.,Kohane,D.S.,Langer,R.,2011.Nanotechnologicalstrategiesfor engineeringcomplextissues.Nat.Nanotechnol.6,13–22.
Egerton,R.F.,2005.PhysicalPrinciplesofElectronMicroscopy:AnIntroductionto TEM,SEM,andAEM.Springer,NewYork,NY.
Engel,A.,Lyubchenko,Y.,Müller,D.,1999.Atomicforcemicroscopy:apowerfultool toobservebiomoleculesatwork.TrendsCellBiol.9,77–80.
Engel,A.,Muller,D.J.,2000.Observingsinglebiomoleculesatworkwiththeatomic forcemicroscope.Nat.Struct.Biol.7,715–718.
Farokhzad,O.C.,Langer,R.,2009.Impactofnanotechnologyondrugdelivery.ACS Nano3,16–20.
Fotiadis,D.,Scheuring,S.,Müller,S.A.,Engel,A.,Müller,D.J.,2002.Imagingand manipulationofbiologicalstructureswiththeAFM.Micron33,385–397. Gazit,E., 2007.Self-assembled peptidenanostructures:thedesignof
molecu-larbuildingblocksandtheirtechnological utilization.Chem.Soc.Rev.36, 1263–1269.
Gelain,F.,Bottai,D.,Vescovi,A.,Zhang,S.,2006.Designerself-assemblingpeptide nanofiberscaffoldsforadultmouseneuralstemcell3-dimensionalcultures. PLoSOne1,e119.
Genové,E.,Shen,C.,Zhang,S.,Semino,C.E.,2005.Theeffectoffunctionalized self-assemblingpeptidescaffoldsonhumanaorticendothelialcellfunction. Biomaterials26,3341–3351.
Gilead,S.,Gazit,E.,2004.Inhibitionofamyloidfibrilformationbypeptide ana-logues modified with ␣-aminoisobutyric acid. Angew. Chem. Int. Ed. 43, 4041–4044.
Guler,M.O.,Hsu,L.,Soukasene,S.,Harrington,D.A.,Hulvat,J.F.,Stupp,S.I.,2006. Pre-sentationofRGDSepitopesonself-assemblednanofibersofbranchedpeptide amphiphiles.Biomacromolecules7,1855–1863.
Guler,M.O.,Soukasene,S.,Hulvat,J.F.,Stupp,S.I.,2005.Presentationandrecognition ofbiotinonnanofibersformedbybranchedpeptideamphiphiles.NanoLett.5, 249–252.
Guler,M.O.,Stupp,S.I.,2007.Aself-assemblednanofibercatalystforesterhydrolysis. J.Am.Chem.Soc.129,12082–12083.
Hartgerink,J.D.,Beniash,E.,Stupp,S.I.,2001.Self-assemblyandmineralizationof peptide-amphiphilenanofibers.Science294,1684–1688.
Hartgerink,J.D.,Beniash,E.,Stupp,S.I.,2002.Peptide-amphiphilenanofibers:a ver-satilescaffoldforthepreparationofself-assemblingmaterials.Proc.Natl.Acad. Sci.U.S.A.99,5133–5138.
Helen,W.,deLeonardis,P.,Ulijn,R.V.,Gough,J.,Tirelli,N.,2011.Mechanosensitive peptidegelation:modeofagitationcontrolsmechanicalpropertiesand nano-scalemorphology.SoftMatter7,1732–1740.
Hendler,N.,Sidelman,N.,Reches,M.,Gazit,E.,Rosenberg,Y.,Richter,S.,2007. For-mationofwell-organizedself-assembledfilmsfrompeptidenanotubes.Adv. Mater.19,1485–1488.
Heuberger,M.,Dietler,G.,Schlapbach,L.,1995.Mappingthelocalyoungsmodulus byanalysisoftheelasticdeformationsoccurringinatomic-forcemicroscopy. Nanotechnology6,12–23.
Hong,Y.,Legge,R.L.,Zhang,S.,Chen,P.,2003.Effectofaminoacidsequenceand phonnanofiberformationofself-assemblingpeptidesEAK16-IIandEAK16-IV. Biomacromolecules4,1433–1442.
Horii,A.,Wang,X.,Gelain,F.,Zhang,S.,2007.Biologicaldesignerself-assembling peptidenanofiberscaffoldssignificantlyenhanceosteoblastproliferation, dif-ferentiationand3-Dmigration.PLoSOne2,e190.
Horinek,D.,Serr,A.,Geisler,M.,Pirzer,T.,Slotta,U.,Lud,S.Q.,Garrido,J.A.,Scheibel, T.,Hugel,T.,Netz,R.R.,2008.Peptideadsorptiononahydrophobicsurfaceresults fromaninterplayofsolvation,surface,andintrapeptideforces.Proc.Natl.Acad. Sci.U.S.A.105,2842–2847.
Hsu,L.,Cvetanovich,G.L.,Stupp,S.I.,2008.Peptideamphiphilenanofiberswith conjugatedpolydiacetylenebackbonesintheircore.J.Am.Chem.Soc.130, 3892–3899.
Huang,Z.,Sargeant,T.D.,Hulvat,J.F.,Mata,A.,Bringas,P.,Koh,C.-Y.,Stupp,S.I.,Snead, M.L.,2008.Bioactivenanofibersinstructcellstoproliferateanddifferentiate duringenamelregeneration.J.BoneMiner.Res.23,1995–2006.
Hung,A.M.,Stupp,S.I.,2007.Simultaneousself-assembly,orientation,and pat-terningofpeptide-amphiphilenanofibersbysoftlithography.NanoLett.7, 1165–1171.
Husale,S.,Persson, H.H.J.,Sahin, O.,2009. DNAnanomechanics allows direct digitaldetectionofcomplementaryDNAandmicroRNAtargets.Nature462, 1075–1138.