DEVELOPING SYNTHETIC BIOLOGY ENABLED WHOLE
CELL BIOSENSORS
A THESIS SUBMITTED TO
THE GRADUATE SCHOOL OF ENGINEERING AND SCIENCE OF BILKENT UNIVERSITY
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
MASTER OFSCIENCE IN
MATERIALS SCIENCE AND NANOTECHNOLOGY
By
SİDE SELİN SU SARAYLI December2017
DEVELOPING SYNTHETIC BIOLOGY ENABLED WHOLE CELL BIOSENSORS
By Side Selin Su Saraylı December 2017
We certify that we have read this thesis and that in our opinion it is fully adequate, in scope and in quality, as a thesis for the degree of Master of Science.
Urartu Özgür Şafak Şeker (Advisor)
Adil Denizli
Çağlar Elbüken
Approved for the Graduate School of Engineering and Science
Ezhan Karaşan
ii ABSTRACT
DEVELOPING SYNTHETIC BIOLOGY ENABLED WHOLE CELL BIOSENSORS
Side Selin Su Saraylı
M.Sc. in Material Science and Nanotechnology
Advisor: Urartu Özgür Şafak Şeker December2017
Main causative agent for environmental pollution is human activities. Especially with accelerated industrial improvements, many inorganics such as heavy metals or hazardous organic toxic materials are released to the environments via inadequate disposal policies of chemical wastes or accidental spills. These pollutants such as heavy metals could not be degraded easily in the environment. Moreover, tracking heavy metals levels in environmental samples is significant not only for controlling accumulation level of heavy metals in environment but also for human health due to heavy metal accumulation in kidney and liver via food web. Therefore, continuous monitoring of heavy metal pollution is important. For this purpose different kinds of sensor systems including biosensors
iii
are widely used. Even these conventional analytic methods are used in a broad range, there are attempts to propose innovative systems with advanced capabilities. Bacterial cells are able to serve as a whole cell biosensor and an analytic device because they naturally have all three layers of conventional sensor systems which are recognition layer, transducer layer and output actuators. Cells can detect changes in the environment and internal conditions via receptors worked as recognition element and placed strictly into membrane or freely in the cytoplasm. Receptors transmit this information into signal transduction pathways which serves as a transducer in the cell. Results of signal transduction pathways such as gene expression, gene repression, differences in concentration of specific molecules are classified as a signal and measured by appropriate device.
Here we present our completed whole cell biosensors for urea, uric acid. Additionally we are presenting our results for cloning and expression of transcription factors for heavy metal sensing. As a future aim, we try to develop a fast, reliable and cost effective whole cell biosensor with the intention of sensing many important biomarkers in a conventional blood panel.
iv ÖZET
SENTETİK BİYOLOJİ YÖNTEMLERİ İLE OLUŞTURULMUŞ TÜM HÜCRE BİYOSENSÖRLERİNİN GELİŞTİRİLMESİ
Side Selin Su Saraylı
Malzeme bilimi ve Nanoteknoloji, YüksekLisans
Tez Danışmanı:Urartu Özgür Şafak Şeker Aralık, 2017
Çevre kirliliği için başlıca etken madde insan faaliyetidir. Özellikle hızlanan endüstriyel gelişmelerle, ağır metaller veya tehlikeli organik toksik maddeler gibi birçok inorganik madde, kimyasal atıkların yetersiz atılma politikaları veya kazara dökülmeler yoluyla doğaya salınmaktadır. Ağır metaller gibi bu kirleticilerin çevre içinde kolaylıkla bozunması mümkün değildir. Bazı ağır metaller, çeşitli organizmaların metabolik aktivitelerinde, ancak çok az miktarda rol oynayabilir. Dahası, çevresel numunelerde ağır metal seviyelerinin izlenmesi, yalnızca ağır metallerin birikim seviyesinin kontrolünde değil, besin ağı vasıtasıyla böbrek ve karaciğerde ağır metal birikimi nedeniyle insan sağlığı için de önemlidir.Bu nedenle, ağır metal kirliliğinin sürekli izlenmesi önemlidir. Bu amaçla
v
biyosensörler de dahil olmak üzere farklı çeşit sensör sistemleri yaygın olarak kullanılmaktadır. Bu geleneksel analitik yöntemler geniş bir aralıkta kullanılmasına rağmen gelişmiş yeteneklere sahip yenilikçi sistemler önerme çabaları bulunmaktadır.Bakteriyel hücreler, bir bütün hücre biyosensörü ve analitik bir cihaz görevi görebilirler çünkü doğal olarak, tanıma katmanı, dönüştürücü katman ve çıkış aktüatörleri olan geleneksel sensör sistemlerinin üç katmanına da sahiptirler.Hücreler, tanıma elemanı olarak çalışan ve membrana veya sitoplazmada serbestçe yerleştirilen reseptörler vasıtasıyla çevre ve iç koşullardaki değişimleri tespit edebilir.Reseptörler, bu bilgiyi hücredeki bir transdüktör görevi gören sinyal iletim yollarına iletirler. Gen ifadesi, gen baskılaması, özgül moleküllerin derişimlerindeki farklılıklar, gibi sinyal iletim yollarının sonuçları bir sinyal olarak sınıflandırılır ve uygun cihazla ölçülür.
Burada üre, ürik asit için tamamlanmış tüm hücre biyosensörlerimizi sunuyoruz. Ek olarak, ağır metal algılama için traskripsiyon faktörlerinin klonlanması ve ekspresyonu için sonuçlarımızı sunuyoruz. Gelecekteki amaç olarak, geleneksel bir kan panelinde birçok önemli biyolojik belirteç algılama niyetiyle hızlı, güvenilir ve düşük maliyetli bir tüm hücre biyosensörünün geliştirilmesi hedeflenmektedir.
vi
ACKNOWLEDGEMENT
First and foremost, I would like to thank to my advisor Assist.Prof.Dr.Urartu Özgür Şafak Şeker. I’d like to thank Prof. Dr.Adil Denizli and Assist.Prof.Dr.Çağlar Elbüken for their support whole time with their invaluable comments.
I would like to specially thank to Dr. Esra Yuca, our postdoctoral researcher because she is an angel for me. She never let me down and answered all my questions. I feel very lucky to know her. Any moment that I shared with her was amazing for me because I learned many things from her like work ethics, communication, kindness and inspiration. I’d like to acknowledge my all dear colleagues in Synthetic Biosystems Lab; especially Elif Ergül for her guidance for statistical analysis and her endless support, Ebru Şahin Kehribar for great times in our coffee breaks, Musa Efe Işılak for his help in protein expression analysis and our group alumni Onur Apaydın and undergraduate researcher Aysel Gurbanova for their help in my experiments. Also, I’d like to thank Recep Erdem Ahan and Sıla Köse for sharing their experimental results with me and guiding me with their protocols.
I want to thank Funda Büyükbaş not only for her help in my experiments but also for creating a breathing space with her beauty, sense of humor and imagination in this world.
vii
I would also like to thank my amazing friends Ayşegül Dede, Eren, Egemen Deniz Eren, Latif Önen, Seda Kizir for their support, belief, inspiration and always reminding me what it means to be a friend, in good times and bad times.
I’d like to convey my sincere thanks for my friends Ezgi Ergenç, Zeynep Seda Soylu, Özge Uyar, Biran Ceren Yetiş, Eray Yetiş and their lovely child Deniz Yetiş, for everything since we met first time. Our hearts and souls are always together.
I ‘d like to specially thank Burcu Tefon (also Uzay) ,Volkan Yıldırım, Eser Ünsaldı, Ayça Hatıl, Erkan Hatıl, Çiğdem Yılmaz, Elif Tekin, Zeynep Eran Ak , İsmail Cem Yılmaz and Mustafa Demir to improve my scientific skills and sharing really good times.
I’d like to express my sincere appreciation to my family but I don’t know enough words. First I’d like to thank my mother Esin Yirmibeşoğlu, most amazing woman in the world and my idol, for everything she did in my whole life including these hard times, to my father Huzur Sait Yirmibeşoğlu for being my hero all the time. I’d like to thank my grandmother Rahime Özköylü for her efforts during my life. Also I want to thank Dilek Saraylı and Amir Saraylı for their support. Moreover, I’d like to thank Korhan Vardarsuyu for all times that we have fun and my lovely cousin Heather Vardarsuyu for all time including talking nerd stuff. Lastly, I want to thank my lovely husband Emir Akay Saraylı for his endless support in my whole life and patience
viii
while I cried during my graduate adventure. Without their support, love and encouragement, I could not finish this thesis because they believe me more than myself during this time. I’d like to dedicate my thesis to my whole family whom I want to be the most proud of me.
ix
TABLE OF CONTENTS
ABSTRACT ... ii
ÖZET ... iv
ACKNOWLEDGEMENT ... vi
LIST OFFIGURES ... xiii
LIST OFTABLES ... xxii
ABBREVIATIONS ... xxiii
CHAPTER 1 ... 1
1.INTRODUCTION ... 1
1.1.Synthetic Biology Enabled Whole Cell Biosensors ... 1
1.2.Biomedical markers for kidney failure ... 4
1.3.Environmental Monitoring ... 7
1.4.Aim of the study ... 10
CHAPTER 2 ... 13
2.MATERIALS AND METHODS ... 13
2.1.Preparation of Growth Medium and Buffers ... 13
x
2.2.1.Plasmid Purification... 20
2.2.2.Chromosomal DNA Isolation ... 20
2.2.3.Polymerase Chain Reaction (PCR) ... 20
2.2.4.Restriction Enzyme Digestion ... 21
2.2.5.Agarose Gel Electrophoresis ... 21
2.2.6.Gel Extraction ... 22
2.2.7.Ligation of Double Digested DNA Fragments ... 22
2.2.8.Gibson Assembly ... 23
2.3.Transformation ... 24
2.3.1.Escherichia coli DH5 alpha and BL21 Competent Cell Preparation . 24 2.3.2.Transformation of Recombinant Plasmids to E. coli Cells... 24
2.4.Sequence alignments of constructs ... 25
2.5.Protein Expression, Purification and Characterization ... 25
2.5.1.Overexpression of Proteins and Total Protein isolation ... 25
2.5.2.Protein Purification ... 26
2.5.3.Western Blot analysis ... 27
xi
CHAPTER 3 ... 30
3.RESULTS ... 30
3.1.Kidney Failure Sensors ... 30
3.1.1.Experimental designs for uric acid sensor ... 31
3.1.1.1.Cloning and sequence verification of uric acid sensor ... 33
3.1.1.2.Characterization of uric acid sensor ... 41
3.1.1.2.1.Protein expression analysis of uric acid sensor ... 42
3.1.1.2.2.Fluorescence analysis of uric acid sensor ... 43
3.1.2.Experimental designs for urea sensor ... 43
3.1.2.1.Cloning and sequence verification of urea sensor ... 47
3.1.2.2.Characterization of urea sensor ... 49
3.1.2.2.1.Protein expression analysis of urea sensor ... 49
3.1.2.2.2.Fluorescence analysis of urea sensor ... 50
3.2.Cloning and preparation of Heavy metal sensors ... 53
3.2.1.Experimental designs for arsenic sensor... 54
3.2.1.1.Cloning and sequence verification of arsenic sensor ... 56
3.2.2.Experimental designs for cadmium sensor ... 60
xii
3.2.2.2.Characterization of cadmium sensor ... 64
3.2.2.2.1.Fluorescence analysis of cadmium sensor ... 64
3.2.2.2.2.Protein expression analysis of cadmium sensor ... 65
3.2.3.Experimental designs for lead sensor ... 66
3.2.3.1.Cloning and sequence verification of lead sensor... 68
3.2.4.Experimental designs for copper sensor ... 69
3.2.4.1.Cloning and sequence verification of copper sensor ... 72
3.2.4.2.Characterization of copper sensor ... 73
3.2.4.2.1.Protein expression analysis ... 73
CHAPTER 4 ... 75
4.DISCUSSION ... 75
CHAPTER 5 ... 77
5.CONCLUSION AND FUTURE PERSPECTIVE ... 77
BIBLIOGRAPHY ... 78
xiii
LIST OF FIGURES
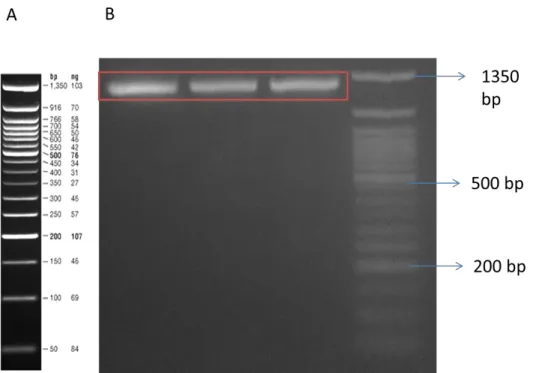
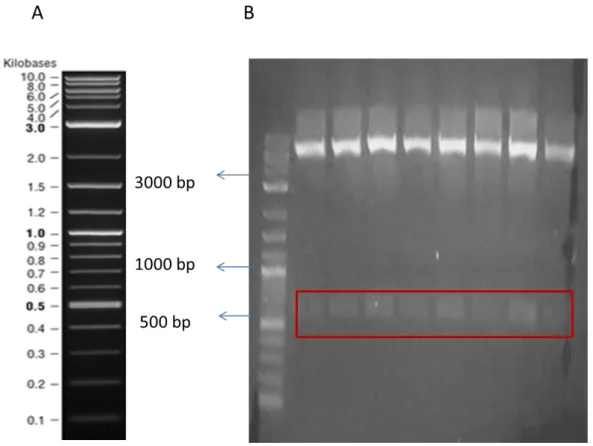
Figure 1: General working mechanism of biosensors. A. Processing circuits are activated via signals such as small metabolites, chemicals, ions, temperature shift or light which the cell obtained from its environment. Signal is created based on the process inside the cell ( i.e. Transcriptional regulation ,artificially introduced logic operation ). The cell’s response such as chemical secretion, motility changes or reporter expression depends on the process in the cell. B. Theranostic application of synthetic gene circuits. Working principle of whole cell biosensors used for medical purposes. In this system disease biomarkers are recognized and logic gate operations are used for generating signal [61] ... 11 Figure 2: Working mechanism of uric acid sensor: HucR is a transcriptional regulator serves as a repressor and it is constitutively expressed in both uric acid present and absent conditions. In uric acid absent conditions (A) HucR proteins forms dimers and blocks the transcription from the PhucO promoter. In uric acid present conditions (B) HucR proteins binds to uric acid molecules, this prevent the interaction with DNA and this situation allows transcription of reporter protein (GFP) from Phuco promoter as an output. In both cases uric acid transporter uacT is constituvely expressed and sent to membrane ... 33 Figure 3: First PCR result of amplification of Phuco sfGFP part. Expected length is 1112 bp. 50 bp Ladder is shown A... 34 Figure 4: Second PCR result of amplification of Phuco sfGFP part. Expected length is 1168 bp. 2 log Ladder is shown A. ... 35 Figure 5: Third PCR result of amplification of Phuco sfGFP part. Expected length is 1219 bp. 2 log Ladder is shown A. ... 35
xiv
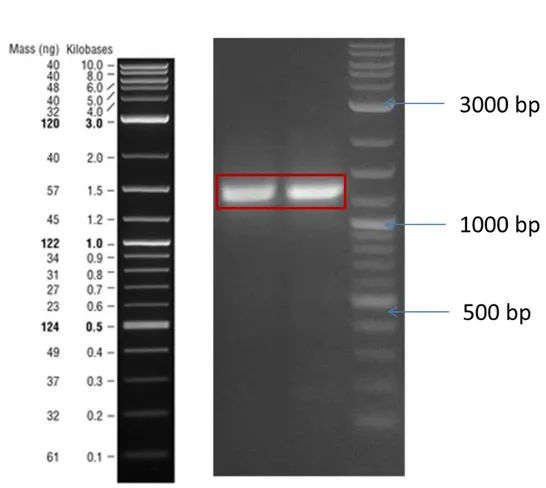
Figure 6: Fourth PCR result of amplification of Phuco sfGFP part. Expected length is 1246 bp. 2 log Ladder is shown A. ... 36 Figure 7: PCR result of amplification of pZa backbone part. Expected length is 1246 bp. 2 log Ladder is shown A. ... 37 Figure 8: PCR results of HucR and ProD parts . Expected size of HucR is 618 bp, Expected size of ProD is 174 bp. 2 log Ladder is shown A... 38 Figure 9: PCR results of pZa Phuco sfGFP backbone amplification (B) and digest result of pZa Phuco sfGFP backbone PCR product (C) Expected size of PCR product is 3690 bp, Expected size of digest product is 3274 bp. 2 log Ladder is shown A. ... 38 Figure 10: Digest result of pZs mProD plasmid. Expected length is 3573 bp and 892 bp. 2 log Ladder is shown A. ... 39 Figure 11: PCR amplification of UacT gene. Expected length is 1485 bp. ... 39 Figure 12: Agarose gel electrophoresis image of amplified samples. NEB 2 log marker is shown (A). Expected length of desired part is 626 bp.. (B)... 40 Figure 13: Agarose gel electrophoresis image of digested samples. NEB 2 log marker is shown (A), . Expected length of desired part is 626 bp.. (B)... 41 Figure 14: SDS PAGE analysis of HucR overexpression M indicates Pageruler ladder (A). SDS PAGE result is shown in B. Numbers indicate the following samples: Total protein isolation from induced plasmid free E. coli cells (1), Total protein isolation from induced pET22 T7 HucR his plasmid bearing E. coli cells selected colony 1. Expected size of HucR protein is 19 kDa. ... 42 Figure 15: Fluorescence measurement of uric acid sensor. Double transformed cells and plasmid free E. coli are induced with uric acid as indicated before. For
xv
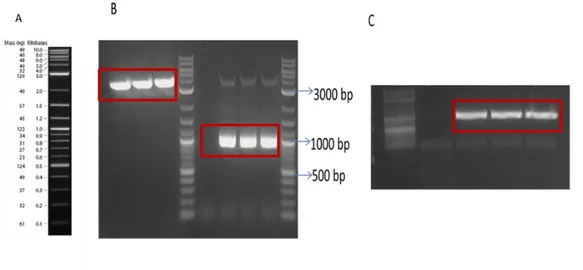
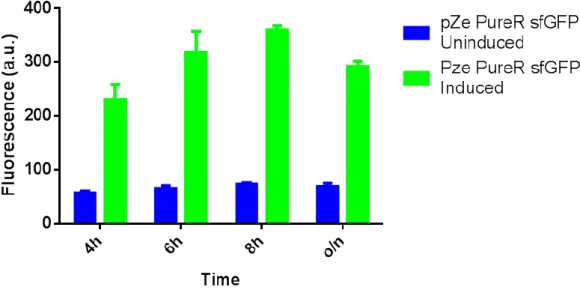
uninduced samples of sensor, no uric acid is added. ... 43 Figure 16: Working mechanims of constructed three different urea sensor design. UreR is a transcriptional regulator serves as an activator and it is contstitutively expressed in both urea present and absent conditions. In urea absent conditions UreR proteins forms dimers and H-NS blocks the transcription from the PureR promoter. In urea present conditions UreR dimers binds to urea ions, this enhances the interaction with DNA and interaction of urea and H-NS results in removal of H-NS blockage in DNA sequence. This situation allows transcription of reporter protein (sfGFP) from PureR promoter as an output. In both cases H-NS expressed from genome. ... 46 Figure 17:Agarose gel electrophoresis images of PCR. NEB 2 log ladder (A). Amplicons of pZs vector backbone, UreR from ordered plasmid and control reaction. Second PCR reaction (C) results for addition of overhangs to UreR obtained from first PCR reaction shown in B, control reaction. Expected results for pZs vector amplicon is3375 bp, UreR PCR product shown in B is 882 bp, in C is 922 bp. ... 47 Figure 18: Agarose gel images. 2 log ladder (A).. PCR Product of PureR and Digest of pZa vector (B). PCR Product of sfGFP (C) Expected results of PureR 164 bp. Expected results of digest result of pZa vector 2000 bp Expected result of sfGFP PCR product is 714 bp... 48 Figure 19: Agarose gel electrophoresis images of digested products M indicates the 2 log ladder (A). Numbers are indicated digested pZa vector (1) and digested pZe vector (2) that used in origin of replication change. Expected results are 789 bp for pZa vector, 814 bp for pZe vector. ... 49
xvi
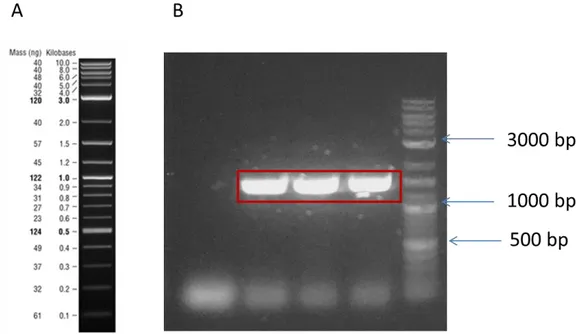
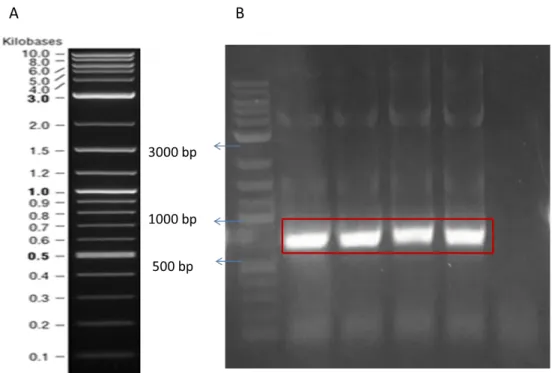
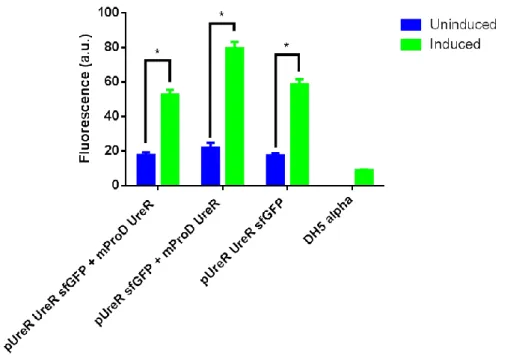
Figure 20: SDS PAGE analysis of pZs mProD UreR containing cells. Expected size of UreR protein is 33kDa ... 50 Figure 21: Fluorescence measurement of constructed three urea sensor plasmid containing cells and plasmid free E. coli cells after 2 hours induction... 51 Figure 22: Fluorescence measurement of constructed three urea sensor plasmid containing cells and plasmid free E. coli cells after 4 hours induction... 51 Figure 23: Fluorescence measurement of constructed three urea sensor plasmid containing cells and plasmid free E. coli cells after overnight induction ... 52 Figure 24: Fluorescence measurement of induced and uninduced pZe PureR sfGFP plasmid containing cells. ... 52 Figure 25: Working mechanism of arsenic sensor design ArsR is a transcriptional regulator serves as a repressor and it is constitutively expressed in both arsenic present and absent conditions. In arsenic absent conditions (A) ArsR forms dimers and blocks the transcription from the Pars promoter. In arsenic present conditions (B) ArsR binds to arsenic ions, this prevents the formation of dimers and this situation allows transcription of reporter protein (YFP) from Pars promoter as an output. ... 56 Figure 26: Agarose gel electrophoresis image of digested samples. NEB 2 log marker (A), Expected length of desired Pars YFP part is approximately 908 bp.. (B). ... 57 Figure 27: Agarose gel electrophoresis image of PCR samples. NEB 2 log marker (A) Expected length of desired ProD ArsR part is 673 bp (B). ... 58 Figure 28: Agarose gel electrophoresis image of PCR samples.NEB 2 log marker (A)..Expected length of desired ArsR part is approximately 479 bp.(B) ... 59
xvii
Figure 29: Protein expression of induced samples of pET22b T7 ArsR his plasmid containing cells ... 60 Figure 30: Working mechanism of cadmium sensor design CadC is a transcriptional regulator serves as a repressor and it is contstitutively expressed in both cadmium present and absent conditions. In cadmium absent conditions (A) CadC proteins forms dimers and blocks the transcription from the PcadA promoter. In cadmium present conditions (B) CadC binds to cadmium ions, this prevent the formation of dimers and this situation allows transcription of reporter protein (CFP) from PcadA promoter as an output. ... 61 Figure 31: Agarose gel electrophoresis image of PCR samples. NEB 2 log marker (A). PCR result of CadC amplification Expected length of desired CadC part is 539 bp . (B) PCR result of CFP amplification. Expected length of desired CFP part is 808 bp . (C) PCR result of PcadA amplification Expected length of desired PcadA part is 539 bp PcadA is approximately 186 bp (D). ... 63 Figure 32: Plasmid map of designed pET22b T7 CadC plasmid. NEB 2 log marker(A). .Expected length of desired CadC part is approximately 492 bp (B). ... 64 Figure 33: Fluorescence analysis of cadmium sensor ... 65 Figure 34: Protein expression analysis of cadmium sensor M indicates the Pageruler marker (A). SDS PAGE analysis of total protein isolation samples of uninduced cells containing pZa ProD CadC PcadA CFP plasmid (1), induced cells containing pZa ProD CadC PcadA CFP plasmid (2), uninduced E. coli cells without any designed plasmid (3), induced E. coli cells without any designed plasmid (4) Expected size of CadC protein is 13 kDa and expected size
xviii
of CFP protein is 26 kDa. ... 66 Figure 35: Working mechanism of lead sensor design PbrR is a transcriptional regulator serves as an activator and it is constitutively expressed in both lead present and absent conditions. In lead absent conditions (A) PbrR proteins forms dimers and blocks binding the transcription from the PpbrA promoter. In lead present conditions (B) PbrR proteins binds to lead ions; this enhances the transcription of reporter protein (sfGFP) from PpbrA promoter as an output. ... 68 Figure 36: Agarose gel electrophoresis image of PCR samples. NEB 2 log marker (A). Expected length of desired PbrR part is approximately 478 bp (B). ... 69 Figure 37: Working mechanism of copper sensor design CueR is a transcriptional regulator serves as an activator and it is contstitutively expressed in both copper present and absent conditions. In copper absent conditions (A) CueR proteins forms dimers and blocks binding the transcription from the PcopA promoter. In copper present conditions (B) CueR proteins binds to copper ions, this enhances the transcription of reporter protein (mCherry) from PcopA promoter as an output... 71 Figure 38: Agarose gel electrophoresis image of PCR samples. NEB 2 log marker (A), NEB 50 bp Ladder (C). PCR results of CueR amplification from genomic DNA. Expected length of desired CueR part is approximately 503 bp(B). PCR results of addition of PcopA sequence to previously obtained CueR sequence Expected length of desired CueR part is approximately 553 bp(D). .. 72 Figure 39: Agarose gel electrophoresis image of PCR samples. NEB 50 bp marker (A), Expected length of desired CueR part is approximately 488 bp. (B).
xix
... 73 Figure 40: Protein analysis of pET22 T7 CueR plasmid . Ladder is a Spectra Low Range protein marker (A). SDS PAGE analysis of total protein isolation from uninduced and induced samples of pET22 T7 CueR his plasmid containing cells (B). Western Blot analysis of unbound proteins (UBP) and purified proteins (P) collected at purification of CueR protein from pET22 T7 CueR his plasmid containing cells. Expected size of CueR is 14 kDa. ... 74
Figure A. 1: Plasmid map of pZa Phuco sfGFP plasmid ... 96 Figure A. 2: Plasmid map of designed pZa ProD HucR Phuco sfGFP plasmid . 96 Figure A. 3: Plasmid map of designed pZs mProD UacT plasmid ... 97 Figure A. 4: Alignment result of pZa Phuco sfGFP plasmid sequence in alignment (A) and text view (B). ... 98 Figure A. 5: Alignment result of pZa ProD HucR Phuco sfGFP plasmid sequence in alignment (A) and text view (B). ... 99 Figure A. 6: Alignment result of pZs mProD UacT plasmid sequence in alignment (A) and text view (B). ... 100 Figure A. 7: Plasmid map of designed pET22b T7 HucR his plasmid ... 101 Figure A. 8: Alignment result of pET22 T7 HucR plasmid sequence in alignment (A) and text view (B) ... 102 Figure A. 9: Plasmid map of pZs mProD UreR plasmid ... 103 Figure A. 10: Alignment result of pZs mProD UreR plasmid sequence in alignment (A) and text view (B) ... 104 Figure A. 11: Plasmid map of designed pZe PureR UreR sfGFP plasmid ... 105
xx
Figure A. 12: Alignment result of pZe PureR UreR sfGFP plasmid sequence in alignment (A) and text view (B). ... 106 Figure A. 13: Plasmid map of pZa ProD ArsR Pars YFP plasmid ... 107 Figure A. 14: Alignment result of pZa ProD ArsR Pars in alignment (A) and text view (B). ... 108 Figure A. 15: Plasmid map of designed pZa Pars YFP plasmid ... 109 Figure A. 16: Alignment result of pZa Pars YFP plasmid sequence in alignment (A) and text view (B). ... 110 Figure A. 17: Plasmid map of designed pET22b ProD ArsR plasmid ... 111 Figure A. 18: Alignment result of pET22b ProD ArsR plasmid sequence in alignment (A) and text view (B). ... 112 Figure A. 19: Plasmid map of pET22b T7 ArsR His Plasmid ... 113 Figure A. 20: Alignment result of pET22b T7 ArsR his plasmid sequence in alignment (A) and text view (B). ... 114 Figure A. 21: Plasmid map of designed pZa ProD CadC PcadA CFP plasmid 115 Figure A. 22: Alignment result of pZa ProD CadC Pcada plasmid sequence alignment (A) and text view (B) ... 116 Figure A. 23: Plasmid map of designed pET22b T7 CadC His plasmid ... 117 Figure A. 24: Alignment result of pET22b T7 CadC His plasmid sequence in alignment (A) and text view (B). ... 118 Figure A. 25: Plasmid map of designed pZa ProD PbrR Ppbr sfGFP plasmid 119 Figure A. 26: Alignment result of pZa ProD PbrR Ppbr plasmid sequence in alignment (A) and text view (B). ... 120 Figure A. 27: Plasmid map of designed pET22b T7 PbrR plasmid ... 121
xxi
Figure A. 28: Alignment result of pET22b T7 PbrR plasmid sequence in alignment (A) and text view (B). ... 122 Figure A. 29: Plasmid map of pZa PcopA CueR plasmid design ... 123 Figure A. 30: Alignment result of pZa PcopA CueR plasmid sequence in alignment (A) and text view (B). ... 124 Figure A. 31: Plasmid map of designed pET22b T7 CueR his plasmid ... 125 Figure A. 32: Alignment result of pET22b T7 CueR his plasmid sequence in alignment (A) and text view (B). ... 126
xxii LIST OF TABLES
Table 1:Stock and Working solution concentrations of used antibiotics ... 13 Table 2:Components and their required amounts of micronutrient solution. For preparation of micronutrient solution all these components are mixed in 40 ml autoclaved water, and then total volume is adjusted to 50 ml. This mixture is stored at room temperature. ... 17 Table 3: Components and their required amounts for 10X MOPS mixture For preparation of 10X MOPS mixture, each component should prepared separately, then amount indicated into the specified volume should mixed. This mixture is stored at room temperature. ... 18 Table 4: Components of 10X MOPS mixture Amount and order of solutions required to add MOPS/tricine/FeSO4 solution. ... 19 Table 5: Restriction Enzyme Digestion mix reaction components and their amounts in the mix ... 21 Table 6: Ligation reaction components and their amounts in the mix ... 23 Table 7: Inducers and final concentrations of inducers that used in this study. . 26
Table A. 1: Primers used in this study ... 85 Table A. 2: Reaction components and conditions of Q5 Polymerase... 92 Table A. 3: Reaction components and conditions of Phusion Polymerase ... 93 Table A. 4: Reaction components and conditions of Taq Polymerase ... 94 Table A. 5: Reaction components and conditions of Pfu Polymerase ... 95
xxi ii ABBREVIATIONS
ORF Open Reading Frame
WHO World Health Organization
EPA US Environmental Protection Agency
EMA European Medical Agency
ICP/MS Inductively Coupled Plasma/ Mass Spectrometry
AAS Atomic Absorption Spectrometry
MS Mass Spectrometry
R-FS X-ray fluorescence spectroscopy
FIAAS Flow Injection Atomic Absorption Spectrometry
ISE Ion Selective Electrode
MNPs Metal Nanoparticles
QDs Quantum Dots
2-DE Two Dimensional Gel Electrophoresis
LC-MS Liquid Chromatographic Mass Spectrometry
SELDITOF-MS Surface-Enhanced Laser Desorption/ Ionization
Time of Flight Mass spectrometry
AKD Acute Kidney Disease
CKD Chronic Kidney Disease
GFR Glomerular Filtration Rate
ACR Albumin : Creatinine Ratio
SCr Serum Creatinine
E.coli Escherichia coli
xxi v
R.metalidurans Ralstonia metallidurans
D.radiodurans Deinococcus radiodurans
P.mirabilis Proteus mirabilis
LB Luria-Bertani
TSS Transformation & Storage Solution
PEG Polyethylene Glycol
DMSO Dimethyl Sulfoxide
TAE Tris-Acetic acid-Ethylenediaminetetraacetic acid
EDTA Ethylenediaminetetraacetic acid
SDS Sodium Dodecyl Sulfate
SDS-PAGE Sodium Dodecyl Sulfate-Polyacrylamide Gel
Electrophoresis
APS Ammonium Persulfate
TEMED Tetramethylethylene-diamine
CBB Commassie Brilliant Blue
TBS Tris buffered saline
TBS-T Tris buffered saline with Tween 20
PBS Phosphate Buffer Saline
IDT Integrated DNA Technologies
PCR Polymerase Chain Reaction
NEB New English Biolabs
IPTG Isopropyl-b-D-thiogalactopyranoside
UBP Unbound proteins
DNA Deoxyribonucleic acid
xx v
YFP Yellow Fluorescent Protein
CFP Cyan Fluorescent Protein
sfGFP Super Folder Green Fluorescent Protein
GFP Green Fluorescent Protein
HTH Helix-turn-helix
1
CHAPTER 1
1.
INTRODUCTION
1.1. Synthetic Biology Enabled Whole Cell Biosensors
Biosensors are analytical devices including biological components for monitoring a parameter of an analyte or target molecule[1]. History of whole cell biosensor development starts with a glucose biosensor development by Clark and Lyon in 1962[2].In general biosensor approach, target compound is sensed by recognition element and biological response is converted to a detectable signal that can be measured in a different ways such as electrochemical, optic, acoustic, mechanic, calorimetric or electronic in the analyte concentration correlated manner [3, 4]. In biosensor technology many different biologically element like enzymes, proteins, nucleic acids, antibodies, antigens, biofilms and whole cells are used in recognition layer and this measurement converted via transducer to create a signal as an output [2]. Although among these biological components enzymes are widely used in biosensor development due to their high selective capacity, purification of enzymes is costly and time consuming[5]. Furthermore, for specific cases, enzyme based biosensors require cofactors and highly developed and expensive electronic equipment to generate detectable signal[6, 7].For not only enzyme based biosensors but also other biological component containing biosensors , there are some disadvantages such as the specificity of the molecules used in recognition layer is not always high, problems related with the renewal of this layer, low sensitivity of transducer layer and electronics dependence of signal recognition. As a novel approach, whole cell biosensors offer advantages about aforementioned drawbacks. Whole cell biosensors are defined as an analytical
2
devices created via programming microorganisms in order to obtain measurable signal usually proportional to the concentration of analytes [2]. Using whole cell as a sensor is a cost effective and more stable option to using enzymes or other biological molecules because they are easy to handle and grow, not required to expensive purification step and contains all components of biosensors which eliminates the requirement external transducer or signal amplifier equipment. Whole cell biosensors mainly based on microorganisms due to their ability to continuously sense their environment to seek any changes. Therefore, they equipped with specific genetically encoded sensor systems combined with signaling pathways which continuously detect and respond the environmental signals because adaptation of the environment is the vital skill for microorganisms to survive. [8]. Bacterial cells could be classified as sensing factory which detect molecules with very high specificity and sensitivity and generate complex signal depends on the input [9]. Whole cell biosensors are versatile tools and they can easily adapt to microfluidics [10] or micropatterning [11]. Whole cell biosensors are provisioned a wide range of applicability for biomedical and environmental monitoring [12, 13].
Another important feature of whole cell biosensors is their capability of modification. In this perspective, synthetic biology helps for development of all elements of a biosensor by generation of novel circuits from pre-defined parts or modification of existing pathways [14]. Synthetic biology aims extension or modification of organisms’ behavior and application of engineering principles to them for performance of novel tasks [15]. According to group of high level expert researchers chosen from European Commission, synthetic biology defined as engineering and studying for biological systems and apply this approach to
3
understand life processes, create and combine modular components , improve novel applications or processes [16]. In principle, synthetic biology is a method used for making the biological system design rational and systematic [17]. Synthetic biology puts this into practice via adapting existing engineering principles such as, abstraction, standardization, modularity, predictability, reliability and uniformity [15]. Abstraction levels in synthetic biology listed as DNA, Parts, Devices, and Systems [18]. A biological part defined as a genetically encoded, discrete components such as promoter, open reading frame (ORF), terminator etc. show biological function [19]. Devices are formed via assembly of biological parts to perform human defined functions such as biological, logic or information process related [20]. Systems are defined as any combination of devices in appropriate chassis [18]. Although in other engineering disciplines, abstraction in synthetic biology increases the speed of the circuit and design tractability [15]. Furthermore, standardization of all members in abstraction levels has great importance to combine them for developing larger systems as in the case of other engineering disciplines. For this purpose, BioBrick standards are applied for parts level standardization [21]. Moreover, with refinements of these standards characterization combined with standardization [22]. At that point, this combination results in modularity. Modularity means ability separation and recombination of parts [23]. Due to standardization, characterization and modularity of parts, predictability, reliability and uniformity are directly obtained in higher levels of abstraction hierarchy.
In the light of these, development of synthetic biology enabled whole cell biosensors is powerful solution to overcome the aforementioned drawbacks of conventional methods. The power of this approach is rooted from combination
4
properties of synthetic biology and benefits of whole cell biosensors in real life applications.
1.2. Biomedical markers for kidney failure
Besides the economic and ecological importance of tracking heavy metal levels in environmental samples such as soil and water, detection of heavy metals is significant for human health. Health is defined as “a state of complete physical, mental and social well-being, not merely the absence of disease or infirmity” [24]. In order to identify disease, conventional methodology is based on comparison of healthy and diseased conditions. This requires monitoring disease specific markers from body fluids such as blood, saliva, urine etc. Conventional methods are usually rooted from displaying differential protein expression [25]. Two dimensional gel electrophoresis (2-DE) [26], one- or two-dimensional liquid chromatographic (LC-MS) [27], or surface-enhanced laser desorption/ ionization time of flight mass spectrometry (SELDITOF-MS) [28] are most powerful techniques for investigating the protein expression profiles. Yet, these techniques have some drawbacks as in the case of environmental monitoring. They require expensive equipment, specialized personnel to use the equipment, costly and time consuming preparation of samples etc. Therefore, researchers keep seeking novel biomarkers for diseases with high mortality and morbidity rates globally and requirement of continuous monitoring. In this perspective both acute kidney disease (AKD) and chronic kidney disease (CKD) are investigated due to their high morbidity and mortality rates [29]. According to World Health Organization (WHO), CKD is responsible for 12.2 deaths per 100.000 people [30]. According to Global Health Observatory, this number will be increased to 14 deaths per 100 000 people until 2030 while even deaths related with HIV and complications will
5
have a decreased trend which have increased trend than deaths from CKD since 1990 [31].
To identify structural and functional reduction in kidney, glomerular filtration rate (GFR) is used as a marker for renal disease patients [32]. GFR is an indirect method because it is calculated as equals the total amount of fluid filtered through all of the functioning nephrons per unit of time and compares renal clearance of exogenous markers with a reference marker [33]. The important property of these reference markers are preferably not coupling with a plasma protein, not involving in a metabolic processes, secretion or reabsorption from tubular pathways which results in extraction via urine by glomerular filtration [34]. In order to be classified a patient with CKD , GFR results should be less than 60 mL/min per 1.73 m² for at least three months and test results should be positive at least one of the kidney failure markers like albuminuria (albumin : creatinine ratio [ACR] ≥30mg/g), urinary sediment abnormality, histological abnormalities [31]. Although these results require some calculations and normalization, there is a risk of mistakes such as false positives. On the other hand serum creatinine (SCr) is used as a predictor of AKD together with GFR for AKD patients [35]. In order to identified a patient with AKD, SCr levels should increase more than 0.3 mg/dl in 48 hours or decrease more than 1,5 fold from a known or assumed baseline depend on the patient’s conditions, together with reduction of urinary output to less than 0.5 ml/kg/h for 6 hours [36]. Yet, in real life these measurements are complicated issue in clinics because unavailable conditions of serum creatinine for serial measurements , difficulties to differentiate AKD with CKD depends on the values and non-informative nature of these tests about adverse outcomes [31]. Moreover, chronic or acute renal failure can cause increase the serum urea levels
6
as 50–70 mM and 120–150 mM, respectively [37] because in protein metabolism, urea is an organic end product. Also, it is main nitrogen source of urine and due to urine metabolism urea levels give clue about liver and kidney status.
Although there are traditional markers for both AKD and CKD, novel marker investigations for these diseases are continue to increase the efficiency of disease identification, understanding disease mechanism and relationship with other mechanisms in the organism. With the improvements in genetics, genes and several loci related with CKD are identified via genetic polymorphisms, single nucleotide polymorphisms [38]. Moreover, results about investigation of AKD progress shows hyperuricemia , which means presence of uric acid in serum more than 6.5 mg/dL in women and 7 mg/dL in men, and because of this reason uric acid is novel marker as well as injuries related with surgeries especially cardiovascular surgery and heat stress such as contrast agents administration [39]. Although disease markers are preferred from inert molecules, uric acid is a biologically active molecule in different mechanisms such as inducer in innate immune response, pro- and anti-oxidant and a neurostimulant [39]. Therefore, it is claimed that uric acid is a potential causative agent of other diseases such as hypertension, diabetes, metabolic syndrome and coronary artery diseases [39]. Despite the fact that whole cell biosensors are promising candidates for clinic usage due to their advantages, they have not been used for medically important markers in the clinic. The main reason behind this situation is necessity of whole cell biosensors abilities adjustment to the clinic such as increasing the to-noise ratio from heterologous clinical samples, improving limited signal-processing capacity etc. [40]. At that point synthetic biology enabled whole cell biosensors contain logic gates offers a solution to overcome these disadvantages
7
[41]. Wiring different systems to each other via logic gates result in improving the specificity of the sensor and accuracy of biological control as well as recognition of multiple conditions in one cell [42]. Other important aspects of logic gates are orthogonally. For this purpose sensor reporter systems based on transcriptional regulator- inducible promoter pairs are usually used. In that case, depend on the target molecules concentration, alterations in the reporter gene expression due to target molecule and transcriptional regulator interaction is measured [42]. In the light of these, synthetic biology enabled whole cell biosensors could satisfy the requirements of the clinical usage.
1.3. Environmental Monitoring
Accelerated industrial activities of humanity are main causative agent of environmental pollution. Pollutants such as heavy metals or hazardous organic compounds could be released to environment because insufficient disposal policies or accidental spills [13]. Major drawback of these pollutants is being highly stable in the environment. Moreover, natural ecological systems require different amount of heavy metals in some specific processes, yet the amount of pollutants are usually much higher than required amount. Therefore, heavy metals show low biodegradability due to their stable nature [6]. Since these pollutants could not remove from the environment, they start to accumulate in both living organisms and environment. Also, this situation generates a broad range of problems including ecological toxicity and many different human diseases [8]. In the case of human diseases, carcinogenic, mutagenic and toxicological effects of different heavy metals on different organs are investigated by many research groups. For instance, most frequent pollutants Hg2+, Pb2+ and As3+ affect central
8
nervous system, Ni2+, Cu2+, Cd2+, and Cr 3+/6 affect skin, bones and teeth, Cu2+, Cd2+, Hg2+ and Pb2+ damage kidney and liver [6]. In ecological perspective, soil quality is decreased; crop yield is reduced via heavy metal pollution in soil [43]. Moreover, contamination in soil could spread aquatic environments such as ground waters, rivers; seas etc. and heavy metals are accumulated in members of food web from microorganisms to fish in an increasing manner [44]. Due to aforementioned effects of heavy metal accumulation both human health and environment, environmental agencies like World Health Organization (WHO), US Environmental Protection Agency (EPA) and European Medical Agency (EMA) recommends a threshold value ranging from ppt to ppm for different heavy metal pollutants based on the findings in the literature [45, 46] According to these facts, there is a requirement for regular analysis to detect and quantify pollutants for environmental quality control protection policies, human health hazard potential and regulations of industrial operations etc. [47]. In order to analyze the samples, conventional analytical methods are used in last few decades [48]. For example, heavy metal ions could be detected at nanomolar concentrations via inductively coupled plasma/ mass spectrometry (ICP/MS) [49] , atomic absorption spectrometry (AAS) [50], mass spectrometry (MS) [51], X-ray fluorescence spectroscopy (R-FS) [52] , potentiometric methods [53], flow injection atomic absorption (FIAAS), optical, electrochemical, piezoelectric or ion selective electrode (ISE) sensors are reported for heavy metal detection [54]. Most of these conventional analytic methods require strong acids to digest the samples for detection of heavy metals without making discrimination between bioavailable and non-bio-available portion of the pollutants [55]. Although these methods could detect extremely low concentrations of heavy metals, innovative approaches
9
have been developed to propose new modes f detection [56]. Novel approaches such as noble metal nanoparticles (MNPs), quantum dots (QDs), and magnetic nanoparticles or nanotubes are combined with sensing systems in past decades. This combination not only increased selectivity, sensibility, and reproducibility of sensor systems but also improves detection limits and allows miniaturization of devices [54]. Another benefit of using nanomaterials is their modification ability. With the help of surface modifications, heavy metal sensors could be combined another analytes such as organic pollutants from small organic ligands to large bio macromolecules [57]. Synthetic biology enabled whole cell biosensors have been also proposed as an innovative approach to build a new class of biosensors [3]. As discussed earlier, developing microbial biosensors for heavy metal detection is studied for a long time in diverse dimensions such as coupling with micro/nanotechnologies. Although, most efforts of these studies for developing a sensor system applicable to use in the field, there are many limitations for this such as regulations of genetically modified organisms usage, variations in environmental conditions, limited genetic stability of the system and slow diffusion rates of the analytes from the external environment into the cells [58]. The improvements in the technology combined with whole cell biosensors which leads the novel techniques for environmental monitoring. In that sense, Hurdebise et al (2015) design a plasmid contains ZntA promoter followed by a reporter gene GFP. Moreover, they combine these sensors with flow cytometry in order to obtain relative fluorescence and quantifying fluorescence intensity in a single cell level. They aim to detect presence of three heavy metal ions Zn, Pb and Cd as well as bioavailable portion of these heavy metals in the contaminated soil samples. They claimed that their biosensor is suitable for both monitoring and
10
bioremediation relevance of contaminated soil [59]. In another study, Yagur-Kroll et al (2014) reported that they develop an online continuous water monitoring system via recombinant reporter bacteria integrated flow-cell. For this purpose, they develop a porous aluminum oxide chips. They first placed recombinant bacteria on these chips and investigate the response. Then they placed solid agar with different concentrations of target analyte and gather dose-dependent fluorescence. After reaching this goal, they add special designed flow system and check for suitability of online water quality monitoring with six-member bacterial reporter array. Then demonstration is done in both laboratory media and wastewater samples. According to their results, this chip is a good application which is a disposable, reusable because of high diffuse rate, ready-to-use platform combined with freeze-dried bacterial reporters which does not require trained personnel [60].
1.4. Aim of the study
In this study, we aim to develop synthetic biology enabled whole cell biosensors based on transcription regulatory proteins and their promoter couples for most pollutant heavy metals arsenic, cadmium, lead, copper and kidney failure markers urea and uric acid. For this purpose, we aim to design genetic circuits to develop whole cell biosensors which can work as a sensor against arsenic, cadmium, lead, copper, uric acid and urea that have most toxic effects and generate rapid output (Figure 1).
11
Figure 1: General working mechanism of biosensors. A. Processing circuits are activated via signals such as small metabolites, chemicals, ions, temperature shift or light which the cell obtained from its environment. Signal is created based on the process inside the cell ( i.e. Transcriptional regulation ,artificially introduced logic operation ). The cell’s response such as chemical secretion, motility changes or reporter expression depends on the process in the cell. B. Theranostic application of synthetic gene circuits. Working principle of whole cell biosensors used for medical purposes. In this system disease biomarkers are recognized and logic gate operations are used for generating signal [61]
With the help of synthetic biology, these repressors and their promoter couples could be used to create sensor systems. In our designs we aim to sense arsenic SmtB/ArsR family member transcriptional regulatory ArsR protein and its promoter couple Pars from Escherichia coli by mimicking the naturally occurring system [62], cadmium with SmtB/ArsR family member transcription regulatory
12
CadC protein from the naturally occurring system of Staphylococcus aureus and its promoter pair PcadA [63], lead with MerR family member transcriptional regulatory PbrR protein and its promoter couple Ppbr from Ralstonia
metallidurans CH34 [64], copper with MerR family member transcriptional
regulatory CueR protein and PcopA promoter from Escherichia coli [65].In order to detect uric acid, uric acid sensitive MarR family, transcription regulator and its responsive promoter couple Phuco from Deinococcus radiodurans R1is used [66]. In our urea sensor system, AraC family member transcriptional activator UreR from Proteus mirabilis and its responsive promoter PureR is used [67].
13
CHAPTER 2
2. MATERIALS AND METHODS
2.1. Preparation of Growth Medium and Buffers
For 1 L of Luria-Bertani (LB) broth media preparation , 10 g Tyrpton (Sigma-Aldrich), 5 g NaCl (Merck) and 5 g Yeast Extract (Merck) are dissolved in deionized water. For 1 L of Luria-Bertani (LB) agar media preparation, 15 g agar (Sigma-Aldrich), was added to LB media.
To prepare Transformation & Storage Solution (TSS), 5 g polyethylene glycol (PEG) 800 mixed with 1,5 mL of 1 M MgCl2 (or 0.30g MgCl2*6H20) and 2,5 mL of dimethyl sulfoxide (DMSO). LB was added to reach 50 mL. Then, solution was filtered with 0,22 µm filter and aliquoted to store -20ºC.
Antibiotics are prepared as in stock solution and diluted into working solution while using according to Table 1.
Table 1:Stock and Working solution concentrations of used antibiotics
Name Stock Solution Working Solution Ampicillin 100 mg/ml 100 µg/ml Kanamycin 34 mg/ml 34 µg/ml Chloramphenicol 100 mg/ml 100 µg/ml
In order to prepare glycerol stocks for long term storage Glycerol (Sigma-Aldrich) diluted in double distilled water with 50% (v/v) final concentration is mixed with overnight culture in 1:1 ratio and stocks are stored at -80ºC.
14
For agarose gel electrophoresis applications, 50X Tris-Acetic acid-Ethylenediaminetetraacetic acid (TAE) buffer was prepared. 242 g Tris (Merck) was dissolved in deionized water and 100 mL of 0,5 M Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich) solution was added. Finally, 57,1 mL of 100% acetic acid was added. Deionized water was added to reach 1 L.
For Gibson mix preparation, first step is preparation of 5X Isothermal buffer which contains 3 ml of 1 M Tris-HCl pH 7.5, 150 μl of 2 M MgCl2, 60 μl of 100 mM dGTP, 60 μl of 100 mM dATP, 60 μl of 100 mM dTTP, 60 μl of 100 mM dCTP, 300 μl of 1 M DTT, 1.5 g PEG-8000, 300 μl of 100 mM NAD and water in the final volume of 6 ml. Gibson mix was prepared with 320 μl 5X Isothermal buffer, 0.64 μl of 10 U/μl T5 exonuclease, 20 μl of 2 U/μl Phusion polymerase, 160 μl of 40 U/μl Taq ligase and water with final volume 1.2 ml. Mix is aliquoted for 1 reaction and store -20ºC.
To analyze protein expression, Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) is used. Deionized water, Acrylamide/Bis-acrylamide (VWR), Tris (Merck), Sodium Dodecyl Sulfate (SDS) (Amresco), ammonium persulfate (APS) (Biorad) and tetramethylethylene-diamine (TEMED) (Biorad) is used. 15% resolving gel and 4% stacking gel is prepared.
The running buffer for SDS PAGE was prepared as 10X Stock solution with 30 g Tris(Merck), 10 g SDS (Amresco) and 144 g glycine (Amresco) dissolved in
15
deionized water and final volume was adjusted to 1 L. Then it was diluted to 1X working solution before use in SDS-PAGE.
6X Laemmli sample buffer was prepared by mixing 3 ml 1 M Tris-HCl pH:6,8 , 1,2 gr SDS, 6 ml 100% glycerol, 0,6 ml of 14.7 M β-mercaptoethanol, 1,5 ml 0,5 M EDTA and 0,012 gr bromophenol blue according to final concentrations for 1X as 50 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol , 1% β-mercaptoethanol , 12.5 mM EDTA 0.02 % bromophenol blue.
Commassie Brilliant Blue (CBB) staining protocol was used for staining SDS Gels. For this purpose, 1 g of Coomassie Brilliant Blue (Bio-Rad) is dissolved in 1 L of solution contains 50% [v/v] Methanol (Merck), 10% [v/v] Glacial acetic acid (Merck) and 40% [v/v] deionized water. This solution was stirred for 3-4 hours and then filter through Whatman filter paper. SDS gels puts on this solution after electrophoresis was finished and staining was applied via overnight incubation or heating up for 1 minutes at highest level of microwave and leave to chill. For destaining, gels were moved another holder and washed with running water to eliminate excess amount of dye. Then Destaining solution , which is composed of 30% [v/v] Methanol (Merck), 10% [v/v] Glacial acetic acid (Merck) and 60% [v/v] deionized water, was applied to gels until all the dye is removed.
For purification of his tagged proteins HisPur™ Cobalt Resin (Thermo Scientific) is used. In this protocol a lysis buffer which is composed of 50mM sodium phosphate, 300mM sodium chloride, 10mM imidazole; pH 7.4 , an elution buffer composed of 50 mM sodium phosphate, 300 mM sodium chloride, 150 mM imidazole; pH 7.4 was used. For regeneration of resin, MES Buffer composed of
16
20 mM 2-(N-morpholine)-ethanesulfonic acid, 0.1M sodium chloride; pH 5.0 and 20% ethanol was used.
Tris buffered saline (TBS) was used for western blot analysis. 20 mM Tris and 150 mM sodium chloride was dissolved in deionized water. Tris buffered saline with Tween 20 (TBS-T) solution was prepared by adding 0,1% Tween 20 to TBS solution. Primary and secondary antibiodies were diluted into 5% (w/v) non-fat milk powder dissolved in TBS-T solution.
10X phosphate buffer saline (PBS) was prepared via dissolving 80 g sodium chloride, 1 g potassium chloride, 14,4 g sodium dihydrogen phosphate and 2,4 g potassium dihydrogen phosphate in deionized water with 1L final volume Stock solution was diluted into 1X before using.
MOPS media was used in some of the fluorescence measurements. To prepare MOPS media, first step is preparing micronutrient stock and components of 10X MOPS mixture according to Table 2 and Table 3 respectively.
17
Table 2:Components and their required amounts of micronutrient solution. For preparation of micronutrient solution all these components are mixed in 40 ml autoclaved water, and then total volume is adjusted to 50 ml. This mixture is stored at room temperature.
Component Formula Molecular
Weight Grams for 50 ml Ammonium molybdate (NH4)6Mo7O24•4H2O 1235.9 0.009 boric acid H3BO3 61.83 0.062 cobalt chloride CoCl2 237.9 0.018 cupric sulfate CuSO4 249.7 0.006 manganese chloride MnCl2 197.9 0.040
18
Table 3:Components and their required amounts for 10X MOPS mixture For preparation of 10X MOPS mixture, each component should prepared separately, then amount indicated into the specified volume should mixed. This mixture is stored at room temperature.
Component Molecular Weight Stock concentration (M) Required amount (Grams) Volume (ml) NH4Cl 53.49 1.9 50.82 500 K2SO4 174.3 0.276 4.8 100 CaCl2•2H2O 147 0.02 0.294 100 MgCl2 203.3 2.5 50.75 100 NaCl 58.44 5 292.2 1000
10X MOPS mixture was prepared via starting 83.72 gr MOPS ad 7.17 gr Tricine in approximately 300 ml deionized water. After pH is adjusted to 7.4 with the help of 10 M KOH, total volume was adjusted to 440 ml. 10 ml freshly prepared 0,01 M FeSO4 solution was added to MOPS/Tricine solution. Then solutions of each
component were added to MOPS/tricine/FeSO4 solution according to the order in
Table 4 and final solution was filter sterilized, aliquoted. Aliquots were stored in -20ºC.
19
Table 4:Components of 10X MOPS mixture Amount and order of solutions required to add MOPS/tricine/FeSO4 solution.
Component Volume 1.9 M NH4Cl 50 ml 0.276 M K2SO4 10 ml 0.02 M CaCl2•2H2O 0.25 ml 2.5 M MgCl2 2.1 ml 5 M NaCl 100 ml Micronutrient stock 0.2 ml Deionized water 387 ml TOTAL 1000 ml
Finally, MOPS Minimal Medium was prepared via combination of 100 ml of 10X MOPS mixture, 10 ml 0.132 M K2HPO4 , 880 ml of deionized water and 0.1 ml
1mg/ml thiamine with total volume 990 ml. pH is adjusted to 7.2 with 10 M NaOH. Media was filter sterilized and carbon source 0.2% glycerol was added before the use.
M63 minimal medium (Amresco) was also used. M63 is purchased and prepared as manufacturer instructed.
2.2. Cloning of Gene Fragments
Transcription factor and their promoter pairs for arsenic and cadmium sensors are ordered from Integrated DNA Technologies (IDT) Company as a gBlock.
20
Required transcription factor and promoter for lead sensor are purchased from GENEWIZ Company as a fragment gene.
2.2.1. Plasmid Purification
Plasmid isolation was performed by using MN Plasmid Isolation Kit according to manufacturer’s instructions. Plasmid DNA was eluted with preheated water (65°C). Plasmid concentrations were determined with Nanodrop 2000 Spectrophotometer. They were stored at -20°C.
2.2.2. Chromosomal DNA Isolation
Qiagen Genomic DNA Isolation Kit was used to purify E. coli genome according to manufacturer’s instructions to obtain transcription factor of copper sensor CueR.
2.2.3. Polymerase Chain Reaction (PCR)
Desired genes are amplified via PCR with proper forward and reverse primers to construct plasmids listed in Table A.1 in the appendix. Melting temperature is calculated depend on the primers that used in the reaction via online tool
(https://tmcalculator.neb.com/#!/) belongs to New English Biolabs (NEB). For
polymerase chain reactions, Q5 Fidelity DNA Polymerase, Phusion High-Fidelity DNA Polymerase, Taq DNA Polymerase and Pfu Polymerase produced in our lab is used according to the components and conditions given in the appendix Table A.2, Table A.3, Table A.4 and Table A.5 respectively.
21 2.2.4. Restriction Enzyme Digestion
For construction of sensor systems DNA sources such as ordered plasmids, PCR products or backbones were digested via restriction enzymes (NEB). Moreover, in order to control formed circuits single restriction enzyme digestion was applied. Used enzymes were chosen according to plasmids and buffers were chosen based on the activity of enzymes. Reaction mixes were set up according to components and amounts shown in Table 5. Mixes were incubated at 37 °C for an hour and loaded onto agarose gel
Table 5: Restriction Enzyme Digestion mix reaction components and their amounts in the mix
Component Amount DNA 500 ng Enzyme 1 0,5 µL Enzyme 2 0,5 µL 10x buffer 5 µL Deionized water Up to 20 µL Total 20 µL
2.2.5. Agarose Gel Electrophoresis
1% (w/v) agarose gel was used to observe DNA fragments larger than 200 base pairs (bp). For smaller DNA fragments than 200 bp 2% (w/v) agarose gel was used to observe digested DNA fragments or PCR products. In order to make
22
agarose gel, agarose (VWR) was dissolved in 1X TAE via heating up the solution. Agarose and 1X TAE amounts were depend on the volume of the tank and agarose percentage of the gel. Then the solution was cooled down, SYBR Safe (Invitrogen) was added and mixed with the solution. After that, solution was poured into the gel cast and waited for complete solidification. Horizontal electrophoresis apparatus was used to carry out experiments. For loading the gel, digested fragments or PCR products were combined with 6X Purple loading dye (NEB) and then this mixture was loaded to the agarose gel. 1X TAE buffer was used as a running buffer. Usually 120 Volts were applied for 45 minutes to carry out gel electrophoresis. 2-log DNA ladder (NEB) or 50 bp ladder (NEB) was used as a marker to determine molecular weights of the PCR products.
2.2.6. Gel Extraction
Extraction of DNA from the agarose gel is done via MN Gel Extraction Kit following manufacturer’s protocol. Nanodrop 2000 Spectrophotometer was used for measurement of final DNA concentration.
2.2.7. Ligation of Double Digested DNA Fragments
DNA fragments and vectors which digested with same restriction digestion enzymes were ligated to formation of desired plasmids. Components and their amounts of ligation reaction are shown in Table 6. Amounts of vector and insert was calculated from online ligation calculator with 1:1 insert vector ratio
(http://www.insilico.uni-duesseldorf.de/Lig_Input.html) from online ligation
23
Table 6: Ligation reaction components and their amounts in the mix
Component Amount
T4 DNA Ligase Buffer (10X) (NEB) 2 μl
Vector DNA (4 kb) 50 ng (0.020 pmol)
Insert DNA (1 kb) 37.5 ng (0.060 pmol)
Nuclease-free water to 20 μl
T4 DNA Ligase (NEB) 1 μl
Total 20 μl
2.2.8. Gibson Assembly
Gibson Assembly was used to construct plasmids. Gibson assembly mix was prepared in the laboratory as mentioned above. In order to obtain inserts PCR with primers specific for Gibson assembly to add flanking regions to insert, Agarose gel electrophoresis and Gel extraction were done. For backbones, restriction enzyme digestion or PCR, Agarose Gel electrophoresis and gel extraction were done. After gel extraction, according to the nanodrop results, vector and insert amounts were calculated as described in ligation section. Then required vector and insert amounts were combined with Gibson assembly master mix with a final volume as 10 µL. Final reaction mixes were incubated at 50ºC for an hour then transformation was applied.
24 2.3. Transformation
2.3.1. Escherichia coli DH5 alpha and BL21 Competent Cell Preparation
E.coli cells were grown in LB media on shaker 37 °C and 200 rpm for overnight.
Then, E.coli cells were diluted as 1/100 into new media and this culture were placed on shaker at 37 °C and 200 rpm until OD600 was reached approximately
0,5. Then, culture was incubated on ice for 10 minutes and centrifuged for 10 minutes at 3000 rpm at 4°C. Supernant was discarded; cell pellets were dissolved in 10% of culture volume of TSS buffer and aliquoted into 100 µL portions to store at -80°C.
2.3.2. Transformation of Recombinant Plasmids to E. coli Competent Cells
Competent cells were thawed on ice approximately 20 or 30 minutes. 1 to 5 μl of plasmid DNA (usually 10pg to 100ng), all reaction mix in ligation or Gibson assembly were mixed into 100 μL of competent cells in a microcentrifuge tube. This mixture was incubated on ice for 20-30 minutes. Heat shock was applied to samples at 42°C in water bath or heat block for 1 minute. Then tubes were incubated on ice for 2 minutes. 500 μl LB is added to samples then they were placed in shaker incubator at 37 °C and 200 rpm for at least 45 minutes. After incubation finished, centrifugation is applied at 3000 rpm for 10 minutes to the samples. Supernatant was discarded by decanting and cells were resuspended with residual LB at the bottom of the microcentrifuge tube which is approximately 100 µl. Cells were spread on to LB agar plates containing appropriate antibiotics and grown overnight at 37 ºC.
25 2.4. Sequence alignments of constructs
Geneious software was used to align the results of Sanger Sequencing.
2.5. Protein Expression, Purification and Characterization
2.5.1. Overexpression of Proteins and Total Protein isolation
Cells containing sensor constructed plasmids were cultured in LB media on shaker 37 °C and 200 rpm overnight as three replicates for both uninduced and induced samples. Not plasmid bearing cells as a control group were also cultured in the same conditions of sensor plasmid containing cells except proper antibiotic. Then cells were diluted as 1/100 into new media with appropriate antibiotic. These cultures were placed on shaker at 37 °C and 200 rpm until OD600 was
reached approximately 0,6. Then inducers were added with a final concentration decided based on the literature information as indicated in Table 7 for induced samples and same amount of deionized water was added to uninduced samples. During induction cells are incubated at 37ºC and 200 rpm in shaker for 6 hours or 30ºC and 200 rpm for overnight. After induction, 100 µL of culture was taken for total protein analysis. Cell density was determined according to OD600 value and
total protein analysis samples were prepared as in equivalent amounts. Remaining cells are collected by centrifugation and supernatant is discarded.
26
Table 7: Inducers and final concentrations of inducers that used in this study.
Inducer Chemical Formula Final concentration Reference Arsenic Na2HAsO4 · 7H2O 10 µg/mL [62] Cadmium Cd(CH3COO)2 30 µM [63] Uric Acid C5H4N4O3 7 mg/dL [39] Urea CH4N2O 100 mM [37] IPTG C9H18O5S 1 mM [68] 2.5.2. Protein Purification
For constructs contain his tag , pET22b T7 ArsR his, pET22b T7 CadC his, pET22b T7 PbrR his, pET22b T7 CueR his and pET22b T7 HucR his protein purification was applied. For this purpose, previously IPTG induced cell pellets were resuspended with aforementioned lysis buffer. Cells were burst via freeze-thaw approach. According to this approach cells were frozen in liquid nitrogen and then thawed in water bath. Freeze-thaw was applied 5 times for each sample. Then samples were centrifuged at maximum speed for 15 min at +4ºC. During this time, resin was prepared via centrifugation at 700 g for 2 minutes and washing with 10 times bed volume lysis buffer. After discarded supernatant, resin was waited for approximately 2 minutes on the bench for complete settle down. Then equilibrated resin and supernatant of centrifuged samples in previous step was mixed. This mixture was incubated at room temperature for 1 hour on a rotator. Centrifugation at 700 g for 2 minutes was applied after incubation finished and supernatant was collected as unbound proteins (UBP). Then resin was washed with 10 bed volume of lysis buffer twice and supernatants were collected as