ContentslistsavailableatScienceDirect
Journal
of
Pharmaceutical
and
Biomedical
Analysis
jou rn al h om e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
The
relationship
between
serum
clozapine
concentrations
and
hematological
parameters
by
a
validated
mass
spectrometric
method
Karam
Mazin
Kamil
Gharab
a,
Duygu
Eryavuz
Onmaz
a,∗,
Sedat
Abusoglu
a,
Memduha
Aydin
b,
Abdullah
Sivrikaya
a,
Oguzhan
Tok
a,
Gulsum
Abusoglu
c,
Ali
Unlu
a aDepartmentofBiochemistry,SelcukUniversity,FacultyofMedicine,Konya,TurkeybDepartmentofPsychiatry,SelcukUniversity,FacultyofMedicine,Konya,Turkey
cDepartmentofMedicalLaboratoryTechniques,SelcukUniversity,VocationalSchoolofHealth,Konya,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received24September2019 Receivedinrevisedform 10December2019 Accepted18December2019 Availableonline20December2019 Keywords:
Therapeuticdrugmonitoring Clozapine
Tandemmassspectrometry Sideeffects
a
b
s
t
r
a
c
t
Objective:Clozapineisoneofthemosteffectivedrugsforresistantschizophrenia,butitsseveremetabolic andhematologicalsideeffectslimittheuseofclozapine.Ithasbeenreportedthatclozapineblood concen-trationsshouldbemaintainedbetween350−600ng/mL.Ouraimwastodevelopadeterminationmethod forclozapineanditsmainmetabolitesnorclozapineandclozapine-N-oxide,toperformvalidationstudies andtoinvestigatethechangeofvariousbiochemicalparametersinpatientsusingclozapine.
Methods:Aliquidchromatography-tandemmassspectrometry (LC–MS/MS)method wasdeveloped andvalidatedforclozapinemeasurement.Thus,bloodsampleswerecollectedfrom38patientswith schizophreniaand32healthyvolunteers.Biochemicalandhematologicalparametersweremeasuredby Beckman-CoulterAU5800(BeckmanCoulter,Brea,USA)andBeckmanCoulterLH780analyzer(Beckman Coulter,Miami,FL,USA),respectively.HormonelevelswereanalyzedusingCobas6000analyzer(Roche Diagnostics,Germany).
Results:TheLC MS/MSmethodwaslinearbetween1.22−2500ng/mL(r2=0.9971)forclozapine.The retentiontimesofclozapine,norclozapineandclozapine-N-oxidewere0.92,0.89and0.95,respectively. Bloodglucose(GLU)(p=0.025),lowdensitylipoprotein(LDL-cholesterol)(p=0.015),triglyseride(TG) (p=0.042)andtotalcholesterol(TC)(p=0.024)levelswerehigher;hemoglobin(HGB)(0.015),mean corpuscularhemoglobin(MCH)(0.036),redbloodcellcount(RBC)(0.020),neutrophil(NEU)(0.034),and platelet(PLT)(P=0.005)levelswerelowerintheclozapinegroup.
Conclusions:ThisLC–MS/MSmethodwasrapid,simple,cost-effectiveandsuitablefortheroutine cloza-pinemonitoring.Furthermore,norclozapineandclozapine-N-oxidewerealsodetermined.Monitoring ofmetabolicandhematologicalparameterswithclozapinelevelsisveryimportant.However,the limi-tationsofthestudywerethatthemethodwasnotvalidatedfornorclozapineandclozapine-N-oxide,so thevalidationparameterswerenotevaluatedforthesetwometabolites.
©2019ElsevierB.V.Allrightsreserved.
Abbreviations:ALT,alanineaminotransferase;AST,aspartateaminotransferase;CLSI,TheClinical&LaboratoryStandardsInstitute;CRE,creatinine;ELISA,enzyme-linked immunosorbentassay;FDA,foodandDrugAdministration;fT3,freeT3;fT4,freeT4;GC–MS,gaschromatography-massspectrometry;GC–MS/MS,gas chromatography-tandemmassspectrometry;GLU,glucose;HDL-cholesterol,highdensitylipoprotein;HGB,hemoglobin;HPLC,high-performanceliquidchromatography;K,potasium; K2-EDTA,dipotassiumethylenediaminetetraaceticacid;LC–MS,liquidchromatography-massspectrometry;LC–MS/MS,liquidchromatography-tandemmassspectrometry; LDL-cholesterol,lowdensitylipoprotein;LYM,lymphocyte;MCH,meancorpuscularhemoglobin;MCV,meancorpuscularvolume;MPV,meanplateletvolume;Na,sodium; NEU,neutrophil;PRL,prolactin;RBC,redbloodcellcount;TC,totalcholesterol;TG,triglyseride;TSH,thyroidstimulatinghormone;WBC,whitebloodcellcount.
∗ Correspondingauthorat:BiochemistryDepartment,SelcukUniversity,FacultyofMedicine,AlaaddinKeykubatCampus,42075,Selcuklu,Konya,Turkey. E-mailaddress:duygueryavuz@hotmail.com(D.E.Onmaz).
https://doi.org/10.1016/j.jpba.2019.113056
Clozapine, a derivative of tricyclic dibenzodiazepine, is first
atypicalantipsychoticdrugintroducedtoclinicalpsychiatry[1].It
isasecondgenerationagentusedinthetreatmentofschizophrenia
orschizoaffectivedisordersresistanttootherantypsycoticsaswell
asschizophreniapatientswithsuicidaltendency[2].Itissuperior
fromconventionalantipsychoticsbecauseitdoesn’traiseprolactin
(PRL)levels,andleadstolessextrapyramidalsymptoms,especially
tardivedyskinesia[3].Furthermore,inthestudynamedastheCost
UtilityoftheLatestAntipsychoticDrugs(CUtLASS2),itwasstated
thattreatmentefficacyofclozapinewashigherthanotheratypic
antypsycotics[4]. Despitealltheseadvantages,there are
differ-entsideeffectsthatlimittheuseofclozapine.Itcancauserare,
serioushematologicalsideeffectssuchasagranulocytosis,
throm-bocytopeniaandanemia[5].Agranulocytosis,aserioussideeffect
in1–2%ofpatientstreatedwithclozapine,limitedtheuseofthe
drugandrequiredthefollow-upofleukocytesandabsolute
neu-trophilscountsbyroutinebloodtests[6].Clozapinehasvarious
metabolicadverse effectssuchasincreaseofblood glucoseand
lipidelevels[7].Atthesametime,sideeffectssuchas
cardiotoxi-city,hepatotoxicity,hypersalivationlimittheuseofclozapine[8].
Consideringitspharmacodynamicproperties,ithasawiderange
ofreceptorbindingcapacity.Clozapinehasahigherantagonistic
effectforD1andD4receptorsthanD2andalsoantagonizes
sero-tonin5-HT2,muscarinic,histamineand␣-adrenergicreceptors[9].
Itisalmostcompletelyabsorbedwhentakenorallyandits
bioavail-abilityvariesbetween60–70%dependingonthefirstpasseffect.
Theeliminationhalf-lifeisabout14hatsteady-stateconditions.
ItismetabolizedbythehepaticcytochromeP450enzymesystem
totwomajormetabolitesknownasnorclozapine,active
metabo-lite,andclozapine-N-oxide[10].Clozapineismetabolizedmainly
byCYP1A2attherapeuticconcentrations,whereasitis
metabo-lizedmainlybyCYP3A4athighconcentrations[11].80%ofthe
doseiseliminatedasa metaboliteinurine(50%)andfeces(30
%)[12].Itwasdemonstratedthatthepharmacokineticsof
cloza-pinevaryconsiderably intra-and inter-individualduringrutine
theurapeuticdrugmonitoring[13].GeneticpolymorphismsinCYP
enzymesmayexplainsomedifferencesinclozapinemetabolism.
However,non-geneticfactors(hormones,diseases, age,
medica-tion,smoking) canalso modifyCYP activity and thereby affect
drugmetabolism,leadingtovariationsinserumlevelsofclozapine
[14].Consequently,serumclozapineconcentrationsvarywidely
among individuals. However, clinical response is highly
corre-latedwithserum clozapineconcentrations. Recentstudieshave
reportedthat clozapineserum concentrations should be
main-tainedbetween350and600ng/mlinordertoachieveaneffective
clinicalresponseandavoidseriousadverseeffects[15].Clozapine
drugmonitoringisveryimportanttopreventdrug-relatedadverse
effects,tooptimizedosage,toevaluatepatients’compliancewith
treatment,and topreventdrug-druganddrug-diet interactions
[16].
Up to date, clozapine levels were measured with various
methods such as HPLC-UV, HPLC-DAD, gas
chromatography-mass spectrometry (GC–MS), gas chromatography-tandem
mass spectrometry (GC–MS/MS), capillary
electrophore-sis however, these methods are very laborious, require
long pretreatment steps, are time consuming and have
low selectivity, specificity and accuracy. LC–MS/MS
has high sensitivity, specificity, selectivity and
cost-effective.
OuraimofthisstudybydevelopingLC–MS/MSdetermination
methodforclozapineanditsmainmetabolites, wasperforming
validationstudiesforclozapinemeasurementandtoinvestigate
thechangeof variousbiochemicalparameters inpatientsusing
clozapine.
2.1. Patients
ThisstudywascarriedoutinKonyaSelcukUniversityFacultyof
MedicinebetweenFebruaryandDecember2018.38patients
diag-nosedwithschizophreniaorschizoaffectivedisorderaccordingto
DSM-IVcriteriaand32controlswereenrolledtothestudy.Patients
includedinthestudyweretreatedwith100(n=15),300(n=4),500
(n=3),600(n=3)and900(n=13)mgclozapinedailyforatleast4
months.Patientswereexcludedfromthisstudyiftheyhad1)no
responsetoclozapinetreatment;2)intoleranttoclozapine
treat-ment;3)usedadifferentantipsychoticinadditiontoclozapine;4)
usedlipid-loweringagent,antidiabetic,betablockers;5)reported
disease suchas cardiovascular, thyroid, hypertension, diabetes,
adrenal,hepatic.Thecontrolgroupconsistedof32healthy
vol-unteerswithoutanychronicorpsychiatricdisease.Themeanages
ofclozapineandcontrolgroupwere40.94±10.15,40.09±1.67,
respectively.Thisstudywasapprovedbytheethicscommitteeof
SelcukUniversity.(Number:2017/308,Date:01/11/2017).
2.2. Laboratorymeasurements
Bloodsamplesweretakenintotubeswithgelandtubes
contain-ingdipotassiumethylenediaminetetraaceticacid(K2-EDTA)after
12hoffasting.Hematologicalparameters includinghemoglobin
(HGB),meancorpuscularhemoglobin(MCH),redbloodcellcount
(RBC), mean corpuscular volume (MCV), mean platelet volume
(MPV),whitebloodcellcount(WBC),neutrophil(NEU)and
lym-phocyte (LYM)counts weremeasured fromcomplete blood by
Beckman Coulter LH 780 analyzer (Beckman Coulter, Miami,
FL, USA). Serum total cholesterol (TC), low density
lipopro-tein(LDL-cholesterol),highdensitylipoprotein(HDL-cholesterol),
triglyseride(TG),creatinine(CRE),glucose(GLU),aspartate
amino-transferase(AST), alanineaminotransferase (ALT), sodium(Na),
potasium(K),urealevelswereanalyzedwithBeckman-CoulterAU
5800(BeckmanCoulter,Brea,USA)accordingtothemanufacturer’s
instructions.Thyroidstimulatinghormone(TSH),freeT4(fT4),free
T3(fT3), vitamin B12and PRLlevelsweremesured using
elec-trochemiluminescence method (Roche Diagnostics, Cobas 6000
analyzere601module,Germany).
2.3. LC–MS/MSanalysis
2.3.1. Chemicalsandreagents
Clozapine(CASNumber:5786-21-0),acetonitrile(CASNumber:
75-05-8),high-performanceliquidchromatography(HPLC)grade
water(CASNumber:7732)-18-5),formicacid(CASNumber:
64-18-6),sodiumhydroxide(CASNumber:1310-73-2),ethylacetate
(CASNumber:141-78-6),Acetaminophen(CASNumber:103-90-2)
wereobtainedfromSigmaAldrich(St.Louis,MO,USA).
2.3.2. Instrumentationandconditions
ShimadzuHPLCsystem(Kyoto,Japan)consistedofapump
(LC-20AD),anautomaticsampler(SIL-20ACHT)andaunitforonline
degasser(DGU-20A3).API3200triplequadrupolemass
spectrom-eterequippedwithanelectrosprayionizationinterfacewasused
(AppliedBiosystems/MDSSciex)asdetector.
SeparationwascarriedoutusingaPhenomenexC18HPLC
col-umn(50mmx4.6mm,partno:00B-4041-E0).Themobilephase
Awascontaining0.1%formicacidandHPLCgradewaterandthe
mobilephaseBwascontaining0.1%formicacidandacetonitrile.
Theflowratewas1mL/minandgradientconditionswere0.1min
50%B,1min50%B,2min100%B,3min100%B,4min50%
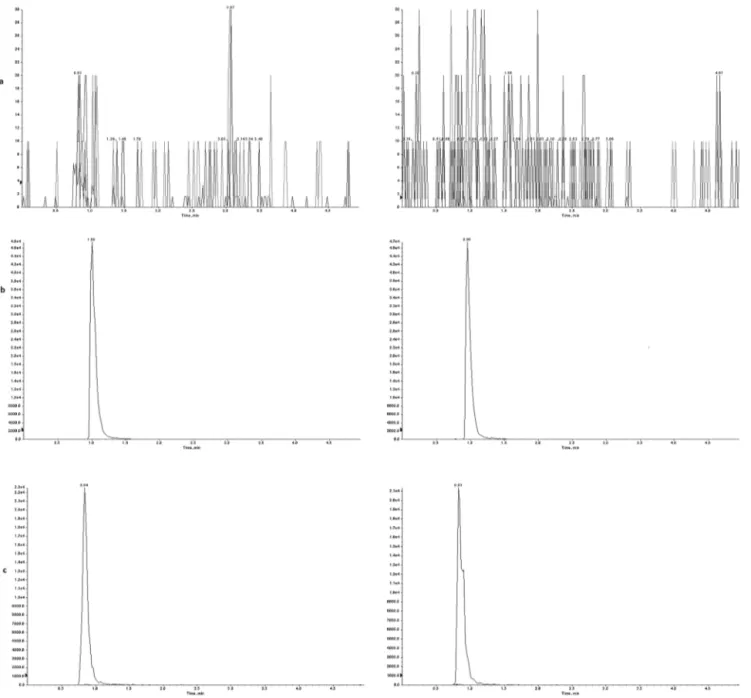
Fig.1. ThechromatogramoftheblankserumsamplespikedwiththeanalytesattheLLOQconcentrations. Representativechromatogramsofblankserumsamplespikedwith1.22ng/mLclozapine.
Table1
Precisionandaccuracyresultsofclozapine.
Intra-day Inter-day
QC Concentration(ng/mL) Mean(ng/mL) Accuracy% Precision(CV%) Mean(ng/mL) Accuracy% Precision(CV%)
LLOQ 1.22 1.3 106.39 6.48 1.38 113.11 7.75 LQC 3.66 3.62 98.96 5.16 3.55 96.99 6 MQC1 1250 1272.4 101.79 3.53 1256 100.48 5.78 MQC2 1875 1930 102.93 3.1 1920 102.4 5.56 HQC 2500 2374 94.96 2.24 2464 98.56 2.96 Table2
Clozapinerecovery%andmatrixeffectresults.
Recovery Matrixeffect
Concentration(ng/mL) 625 312 156 625 312 156
Results(%) 97.76 97.11 102.10 4.24 −6.1 −8.5
Table3
Clozapinestabilitystudybias%results.
Frozen(-20◦C)for45day Freeze-thawstability
Concentrations(ng/mL) 15.Day(%) 30.Day(%) 45.Day(%) 2.(%) 3.(%) 4.(%)
625 −1.5 −5.3 −10.4 −0.7 −1.7 −4.4
250 −2.3 −5.7 −11.2 −1.5 −9.2 −15.9
andtheinjectionvolumewasadjusted40l.Totalruntimewas
determined5min.
TheQ1toQ3iontransitionsweredeterminedas327.1/270.1,
152.1/65.1, 313/192.2 and 343/192.2 for clozapine, internal
standard (acetaminophen),norclozapine and clozapine-N-oxide,
respectively.Declustering, entrance, collisioncellexit potential,
collisionenergy,ionsprayvoltage,sourcetemperature,curtain,ion
source(GS1)andionsource(GS2)gasvalueswereadjustedto50,
10,4,35,5000V,350OC,20,40,60psi,respectively.
2.3.3. Samplepreparation
250Loftheinternalstandardwasaddedto250Lstandard
solutionorserumsamples.1mLofNaOH(pH11–12)wasadded
toprovidealkaliconditions.Serumsampleswereextractedwith
1.5mLofethylacetate(30svortexed).Themixturewascentrifuged
at3500×rpmfor5min.Aftercentrifugation,theorganiclayerwas
taken into glasstubes and evaporated undernitrogen gas.The
residuewasdissolvedin200Lacetonitrile-water(50:50;v:v%)
Fig.2.Representativechromatogramsof(a)blankserum,(b)internalstandardand(c)theclozapineat625ng/mLinserumwithlow(leftpanel)andhigh(rightpanel) olanzapinelevels.
2.3.4. Methodvalidation
ThevalidationstudywascarriedoutinaccordancewithCLSI
(ClinicalandLaboratoryStandardsInstitute)andFDA(Food and
DrugAdministration)protocols[17,18].Linearity,accuracy,
selec-tivity,precision,recovery,parameterswereevaluatedatthisstudy.
2.4. Statisticalanalysis
StatisticalanalysiswascarriedoutbyusingSPSSstatistical
soft-warepackageversion21.0,EPEvaluatorRelease8versionandExcel
(2010),p<0.05wasconsideredasstatisticallysignificant.
3. Results
3.1. LC–MS/MSanalysis
3.1.1. Methodvalidationofclozapine
Thecalibrationstandardswerepreparedbyspikingworking
solution(10% of total serumvolume) toblankserum and
lin-earity study was performed with these spiked samples in the
concentration range of 0.076–2500ng/mL and each
concentra-tion level was analyzed in duplicate. Results were evaluated
by linear regression analysis. The LC–MS/MS method was
lin-earbetween1.22−2500ng/mL.Correlationcoefficient,slopeand
interceptparametersweredeterminedas0.9971,0.9969and0,
respectively.
Limitofdetectionwascalculatedbasedonasignaltonoiseratio
of3.Limitofdetectionwasdeterminedas0.076ng/mL.Thelowest
concentrationlevelatwhichthesignaltonoiseratiowas
approxi-mately10withacceptableaccuracyandprecisionwasdetermined
asthelowerlimitofquantification(LLOQ).TheCLSIguideline
rec-ommendstheuseofdatafrom20replicatesofaspikedsample
fromatleast5differentrunsovera5-daytimeperiodforLLOQ.
LLOQhaveaprecisionoflessthanorequaltoacoefficientof
vari-ation(CV)of20 %and anaccuracyof80–120%ofthenominal
analyteconcentration.LLOQwasdeterminedas1.22ng/mL.The
intraandinter-dayprecisionandaccuracyatLLOQconcentration
Table4
Clozapineinterferencestudybias%results.
D1 D2 D3 D4 D5
625ng/mLbias% −2.14 −2.70 −2.72 −3.98 −4.16
312.5ng/mLbias% −1.24 −1.80 −1.12 −2.56 −4.56
D1:0%,D2:5%,D3:50%,D4:75%,D5:100%interferant(olanzapine)level.
valuewasacceptedtobe1.22ng/mL,sincetheconcentrationlevel
wherethesignal-to-noiseratiowas≥10withacceptableaccuracy
andaccuracywas1.22ng /mL.Thechromatogramoftheblank
serumsamplespikedwiththeanalytesattheLLOQconcentrations
demonstratedinFig.1.
Accuracyandprecisionweredeterminedanalyzingsixsamples
perlevelattheLLOQ,low,medium1,medium2andhighQC
sam-ples(1.22,3.66,1250,1875,2500ng/mL)for5consecutivedays.
Accuracyiscalculatedasapercentageofthemeasuredvalueto
theexpectedvalue,andtheprecisionresultsareexpressedasCV%.
ResultsofprecisionandaccuracystudieswereexpressedinTable1.
Theacceptabilitycriterionforaccuracyand precisionexceptfor
LLOQisthatthedeviationvalueislessthan15%.Theacceptability
criteriaforaccuracyandprecisionatLLOQisupto20%.The
accu-racyandprecisiondeviationlevelsarelowerthan15%foralllow,
medium1,medium2andhighqualitycontrollevels,andlessthan
20%forLLOQ.Theresultsshowedverygoodaccuracyandaccuracy.
Toevaluatetheefficiencyoftheextractionprocess,samples
werepreparedat threedifferentconcentrationlevels(156, 312
and625ng/mL)andrecoverystudywasperformed.Recoverystudy
wascarriedoutbycomparingthemeasurementresultsofextracted
andunextractedsamples.Thematrixeffectstudywasperformed
according to the proceduredescribed by Chambers et al. [19].
Resultsofrecoveryand matrixeffectstudieswerepresentedin
Table2.
Stability studies were performed at two different (250 and
625ng/mL)concentrationlevelstoassessthestabilityof
clozap-ineinserumat-20◦Candafterfreeze-thawcycles.Resultswere
presentedinTable3.
Carry-overstudywasperformedbyanalyzingconsecutivelow
andhighlevelstandards.Themeanandstandarddeviationvalues
ofgroupswerecalculatedwithEPEvaluatorRelease8program.The
carry-overvaluewasdeterminedas-21.4ng/mL.
Theselectivitystudywasperformedbyanalyzingsixdifferent
humanblankserum samplesandinvestigate potential
interfer-encesofolanzapin.Thechromatogramsofblankserum,internal
spikedserumandanalytespikedserumwerecomparedatlowand
higholanzapinelevels(Fig.2).
Thesechromatogramsdemonstratethatthismethodwas
selec-tive for clozapine. Furthermore, samples containing 312.5 and
625ng/mL clozapine in six different individual sources of the
appropriateblankserummatrixforeachconcentrationlevelwere
preparedthen0%,25%,50%,75%,100%olanzapinewasaddedto
eachsample.Theresultsoftheinterferencestudieswereexpressed
asbias%inTable4.
3.1.2. LC–MS/MSmeasurementofclozapinelevels
Serum samples of 38 patients using clozapine were
ana-lyzed by LC–MS/MS. Clozapine serum levels altered between
42.90–1785ng/mL. Serum clozapine and norclozapine levels
were measured as 594.90±492.90 and 220.33±182.55ng/mL,
respectively. The median (min-max) serum concentrations of
patientsusing clozapineat dosesof100, 300,500, 600,900mg
dailyweredeterminedas137.85(42.90–760.5),336(186–292.5),
451.5(426–549), 844.5(784.5–944.5), 1078.5(603–1785) ng/mL,
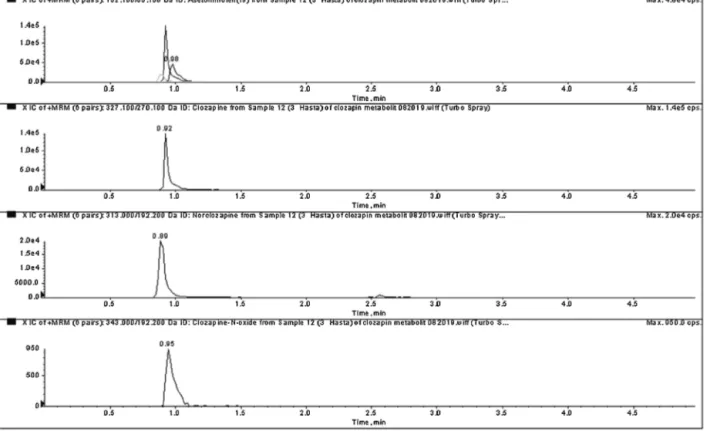
respectively.Achromatogramofclozapineanditsmain
metabo-litesisshowninFig.3.
Table5
.Demographicandlaboratoryparametersofallgroups.
Clozapinegroup Controlgroup p
Age 40.94±10.16 40.10±8.67 0.640
Gender(F/M) 21/17 18/14
ALT 17(5–77)U/L 17(8–28)U/L 0.339
AST 21(13–40)U/L 18.5(12–34)U/L 0.136
GLU 93(76–343)mg/dL 89(75–98)mg/dL 0.025 5.2(4.2–19)mmol/L 4.94(4.2–5.4)mmol/L Urea 24.26±8.48mg/dL 27.81±7.43mg/dL 0.060 4.0±1.41mmol/L 4.63±1.24mmol/L CRE 0.76±0.16mg/dL 0.77±0.15mg/dL 0.880 0.07±0.01mmol/L 0.06±0.01mmol/L TG 147.5(40–493)mg/dL 97.5(75–98)mg/dL 0.042 1.7(0.5–5.6)mmol/L 1.1(0.8–1.1)mmol/L HDL 42(30–71)mg/dL 45(27–67)mg/dL 0.065 1.08(0.8–1.8)mmol/L 1.2(0.7–1.7)mmol/L LDL 142(57–187)mg/dL 122(94–157)mg/dL 0.015 3.7(1.5–4.8)mmol/L 3.2(2.4–4.1)mmol/L TC 218.63±36.24mg/dL 200.56±27.45mg/dL 0.024 5.7±0.94mmol/L 5.2±0.91mmol/L Na 139(136–143)mmol/L 138.5(134–143)mmol/L 0.226 K 4.30(3.86–14.11)mmol/L 4.34(3.82–5.53)mmol/L 0.641 PRL 14.67(4.39–138)g/L 12.31(4.74–80.46)g/L 0.869 B12 344.1(207–686)ng/L 311.3(176–601)pg/mL 0.596 TSH 1.55(0.28–13.71)mU/L 1.8(0.59–4.88)mU/L 0.612 fT3 2.95±0.46ng/L 3.08±0.38pg/mL 0.19 4.53±0.71pmol/L 4.73±0.58pmol/L fT4 1.27(0.61–1.8)ng/dL 1.26(0.83–1.93)ng/dL 0.804 16.7(7.85–23.16)pmol/L 16.21(10.6–24.8)pmol/L RBC 4.76±0.45106/L 5.02±0.45106/L 0.020 4.76±0.451012/L 5.02±0.451012/L MCV 89.24±4.71fL 86.10±3.34fL 0.002 MCH 21.95±1.79pg 30.80±1.48pg 0.036 1.36±0.11fmol 1.91±0.09fmol PLT 212.59±84.55k/L 265.47±62.34k/L 0.005 212.59±84.55109/L 265.47±62.34109/L MPV 8.2(6.7–12.5)fL 8.8(8.1–10.5)fL 0.003 WBC 7.25(3.6–108)k/L 7.1(4.3–11.6)103/L 0.581 7.25(3.6–108)109/L 7.1(4.3–11.6)109/L HGB 14.12±1.16g/dL 14.75±0.89g/dL 0.015 141.2±11.6g/L 147.5±8.9g/L NEU 3.84(2.22–7.5)k/L 4.79(2.46–7.0)103/L 0.034 3.84(2.22–7.5)109/L 4.79(2.46–7.0)109/L LYM 1.99±0.54k/L 2.24±0.81103/L 0.140 1.99±0.54109/L 2.24±0.81109/L
3.2. Biochemicalmeasurementresults
38 patients used clozapine and 32 heathy volunteers were
includedthisstudy.Themeanageofclozapineandcontrolgroups
was40.94±10.15,40.09±1.67,andtherewasnostatistically
sig-nificant difference between themean ages of the two groups.
Whenthetwogroupswerecomparedstatistically,itwasshown
thatGLU(p=0.025),TG(p=0.042),LDL-cholesterol(p=0.015),TC
(p=0.024),MCV(0.002)levelswerehigherintheclozapinegroup
comparedtothecontrolgroupandRBC(p=0.020),MCH(0.036),
HGB(0.015),PLT(0.005),NEU(0.034)levelswerelowerthanthe
control group.Furthermore, PLT(179.18±84.70, 239.45±76.03
k/L [179.18±84.70, 239.45±76.03 109/L]; p=0.027) and fT3
(2.74±0.36, 3.12±0.47ng/mL [4.21±0.55, 4.79±0.72pmol/L];
p=0.010)levelswerestatisticallysignificantlowerinpatientswith
serum clozapineconcentrations above 600ng/mL than patients
withserumclozapineconcentrationsbelow600ng/mL.All
demo-graphicfindingsand laboratorymeasurementsarepresentedin
Table5.
Furthermore,itwasshowedthatserumclozapinelevelswere
positively correlated with TC (r=0.335, p=0.040)
concentra-tion and negatively correlated withHGB (r=−0.342, p=0.025),
PLT (r=−0.362, p=0.025), NEU (r=−0.385, p=0.017) and fT3
Fig.3.Chromatogramforclozapine(1530ng/mL),norclozapine(310ng/mL)andclozapine-N-oxideintheserumsample.
4. Discussion
Primaryoutcomewastovalidateamassspectrometricmethod
forclinicaluseand tofindout themetabolicchanges and
rela-tionship with serum clozapine concentrations. Clozapine is an
atypicalantipsychoticthatiscommonlyusedinthetreatmentof
schizophreniaandiseffectiveagainstbothnegativeandpositive
symptomsofschizophrenia.Clozapineissuperiortoconventional
antipsychoticsbecauseit causesless extrapyramidal symptoms
anddoesn’taffectprolactinlevels,butitcancausesevere
hema-tological,metabolic sideeffects that limit the useofclozapine.
Atthesametime, theraupeuticdrugmonitoringisessential for
clozapine due to the fact that clozapine levels vary
consider-ablyamongindividualsandthefrequencyofseriousdrug-related
adverseeffects increaseswithincreasingdrugblood
concentra-tion.Variousstudies [20,21], haveshown that theincidence of
adverse effects increases when serum clozapine concentration
exceeds750ng/mL.Therefore,monitoringofclozapinelevelswith
metabolicandhematologicalparametersinpatientsusing
cloza-pineisextremelyimportant.DifferentLC–MS/MSmethodswere
developedforclozapinemeasurement.
Wohlfarthetal.[22]developedamethodforthedetermination
ofclozapineanditsmetabolitesbyQTraptandemmass
spectrom-eter,andthismethodperformedchromatographicseperationin
15min.Ourmethodcanperformseperationinamuchshortertime
(5min).AravagiriM.andMarderSR[23]performedtandemmass
analysisusing500Lofsampleinthepretreatmentsteps,250L
sampleissufficientforourmethod.Inthemethoddevelopedby
AravagiriM.andMarderSRwasusedahighvolume(7mL)mixture
oforganicsolventsconsistingofethylacetate,methylenechloride
andpentaneinthepretreatmentsteps.Itwasusedonlyoneorganic
solvent(ethylacetate)inasmallervolume(1.5mL)during
extrac-tioninourmethod.Itsvortexingtimeduringextractionis10min
whileinourmethod30sissufficient.Inthemethoddevelopedby
AravagiriM.andMarderSR,CV%valuesforclozapinevarybetween
3.2%–12.5%andinourmethoditvariesbetween2.24%–7.75%.Its
extractionrecoveryis84%andourrecoveryisover97%.In
sum-mary,ourmethodishighlyadvantageousinthatitrequiresasmall
amountofsample,isaneconomical,practicalmethod,andhashigh
accuracyand recovery.However,theLLOQ value(1ng/mL)and
retentiontime(<3min)forclozapineweresimilartoourmethod
(1.22ng/mLand<1.5min,respectively).
Domingues et al. [24] reported LC–MS/MSmethods for the
determination of various drugs including clozapine and this
methodwas linear in the 1.5−1550ng/mL concentration range
andcorrelationcoefficientwashigherthan0.99forclozapine.Our
methodwaslinearbetween1.22−2500ng/mLandcorrelation
coef-ficientwashigherthan0.99.
Niederlaenderetal.[25]developedLC–MS/MSmethodbyusing
on-linesolidphaseextractiontodetermineclozapinelevels,
quan-titationanddetectionlimitswerereportedas0.15and50ng/mL,
respectively.Ourlimitofdetectionandquantitationvalueswere
0.076and1.22ng/mL,respectivelyfurthermoreourmethodis
sim-pleandcost-effective.
Raoetal.[26]determinedintra-dayprecisionCVsas4.6%and
for 325ng/mL and 2.1 %for 607ng/mL. Between-day CVswere
expressedas6.7 %for 325ng/mL and 4.1 %for607ng/mL. Our
resultsareconsistentwiththesevalues.
The extraction recovery was determined between
97.11%–101.13%,theextractionstepisverysimpleandsufficient.
Anotherimportantaspectofourstudywastheinvestigation
ofthechangeofvariousbiochemicalparametersinpatientsusing
clozapine.Variousstudieshavereportedthatclozapinetreatment
isassociatedwithincreasedserumlipidandGLUlevelsandmay
causemetabolicdisorderssuchasweightgain,hyperlipidemiaand
hyperglycemia.Lindenmayeretal.[27]reportedincreasedserum
GLUandTClevelsinpatientsusingclozapine.Idonijeetal.[28]
reportedincreasedLDL-cholesterol,TC,TGlevelsandreduced
HDL-cholesterollevelsinclozapinegroupcomparedwithcontrolgroup.
lev-elswerehigherintheclozapinegroupthaninthecontrolgroupand
therewasapositivecorrelationbetweenclozapineblood
concen-trationandTClevels.
Alsoinourstudy,serumfT3levelswerenegativelycorrelated
withserumclozapinelevels.Clozapinemightaffectthyroid
func-tions. Thus metabolic disturbances in patientstaking this drug
might berelated withaltered thyroid function. Althoughthese
resultswereobservedwithlimitednumberofpatientsinthisstudy,
thisissuemustberesearchedbyanalyzingalargescaleparticipiant
study.
Althoughneutropeniaisawell-knownhematologicalsideeffect
ofclozapine,differenthematologicaladverseeffectssuchas
throm-bocytopenia andanemia due toclozapinetreatmenthavebeen
reportedinvariousstudies[29,30].WefoundthatNEU,HGB,RBC,
MCHlevelswerelowerinpatientsusingclozapinecomparedto
controlgroup,andtherewasanegativecorrelationbetween
cloza-pinebloodconcentrationandHGB,NEUandPLTlevels.
5. Conclusions
TheLC–MS/MSmethodisveryfast,simpleandcost-effective.
Ithashighaccuracy,sensitivityand specificitythusit hasideal
validationproperties.Itcanalsodetectnorclozapineand
clozapine-N-oxideinserum.Ourmethodissuitableforroutinedruglevel
monitoringofclozapine.Inaddition,ourstudyiscomprehensive
anduniqueinthatitevaluatestherelationshipbetween
clozap-inelevelsandvariousclinicalparameters.Regularmonitoringof
biochemicaland hematological parametersaswellas clozapine
druglevelisextremelyimportant..However,thelimitationsofthe
studywerethatthemethodwasnotvalidatedfornorclozapineand
clozapine-N-oxide,sothevalidationparameterswerenot
evalu-atedforthesetwometabolite,lackofCYPpolymorphismstudiesof
ourpopulationandthereisaneedforfurtherstudiestoclarifythe
variationinclozapinebloodconcentrationsbetweenindividuals.
CRediTauthorshipcontributionstatement
Karam MazinKamilGharab: Validation, Investigation,Data
curation,Software. DuyguEryavuzOnmaz: Methodology,
Vali-dation,Investigation,Writing-originaldraft,Writing-review&
editing. Sedat Abusoglu:Methodology, Investigation, Writing
-review&editing.MemduhaAydin:Investigation,Project
adminis-tration.AbdullahSivrikaya:Writing-review&editing.Oguzhan
Tok:Validation,Software.GulsumAbusoglu:Validation,Software.
AliUnlu:Conceptualization,Projectadministration.
DeclarationofCompetingInterest
None.
Acknowledgment
This work was supported by Selcuk University Scientific
Research Projects Coordinator (grant number 17202068).The
authorsthanktheSelcukUniversityforfundingthisproject.
References
[1]N.P.Maric,S.P.Nikolic,I.Buzadzic,M.Jovicic,S.Andric,M.Mihaljevic,Z. Pavlovic,»Treatmentresistance«enigmaresolvedbypharmacogenomics-a casestudyofclozapinetherapyinschizophrenia,J.Med.Biochem.34(2015) 223–227.
[2]J.Kasckow,K.Felmet,S.Zisook,Managingsuicideriskinpatientswith schizophrenia,CNSDrugs25(2011)129–143.
[3]M.C.Mauri,S.Paletta,M.Maffini,A.Colasanti,F.Dragogna,C.diPace,A.C. Altamura,Clinicalpharmacologyofatypicalantipsychotics:anupdate,ExclıJ. 13(2014)1163–1191.
[4]S.Warnez,S.Alessi-Severini,Clozapine:areviewofclinicalpractice guidelinesandprescribingtrends,BMCPsychiatry14(2014)102.
[5]R.J.Flanagan,L.Dunk,Haematologicaltoxicityofdrugsusedinpsychiatry, Hum.Psychopharmacol.1(2008)27–41.
[6]F.C.Nucifora,M.Mihaljevic,B.J.Lee,A.Sawa,Clozapineasamodelfor antipsychoticdevelopment,Neurotherapeutics14(2017)750–761.
[7]M.Kumar,A.Sidana,Clozapine-inducedacutehypertriglyceridemia,IndianJ. Psychol.Med.39(2017)682–684.
[8]D.D.Tian,W.Wang,H.N.Wang,S.C.Sze,Z.J.Zhang,Pharmacokinetic evaluationofclozapineinconcomitantuseofradixrehmanniae,fructus schisandrae,radixbupleuri,orfructusgardeniaeinrats,Molecules21(2016) 696.
[9]M.M.Iqbal,A.Rahman,Z.Husain,S.Z.Mahmud,W.G.Ryan,J.M.Feldman, Clozapine:aclinicalreviewofadverseeffectsandmanagement,Ann.Clin. Psychiatry15(2003)33–48.
[10]I.Olmos,M.Ibarra,M.Vazquez,C.Maldonado,P.Fagiolino,G.Giachetto, Populationpharmacokineticsofclozapineandnorclozapineandswitchability assessmentbetweenbrandsinuruguayanpatientswithschizophrenia, BiomedRes.Int.2019(2019),3163502.
[11]M.Grundmann,I.Kacirova,R.Urinovska,Therapeuticdrugmonitoringof atypicalantipsychoticdrugs,ActaPharm.64(2014)387–401.
[12]N.Kar,S.Barreto,R.Chandavarkar,Clozapinemonitoringinclinicalpractice: beyondthemandatoryrequirement,Clin.Psychopharmacol.Neurosci.14 (2016)323–329.
[13]L.J.Li,D.W.Shang,W.B.Li,W.Guo,X.P.Wang,Y.P.Ren,etal.,Population pharmacokineticsofclozapineanditsprimarymetabolitenorclozapinein chinesepatientswithschizophrenia,ActaPharmacol.Sin.33(2012) 1409–1416.
[14]K.Toth,G.Csukly,D.Sirok,A.Belic,A.Kiss,E.Hafra,etal.,Potentialroleof patients’cyp3a-statusinclozapinepharmacokinetics,Int.J.Neuropsychoph. 20(2017)529–537.
[15]G.Cadeddu,A.Deidda,M.E.Stochino,N.Velluti,C.Burrai,M.delZompo, Clozapinetoxicityduetoamultipledruginteraction:acasereport,J.Med. CaseRep.9(2015)77.
[16]C.J.Wenthur,C.W.Lindsley,Classicsinchemicalneuroscience:clozapine,ACS Chem.Neurosci.4(2013)1018–1025.
[17]Clinical&LaboratoryStandardsInstitute:CLSIGuidelines,2018(Accessed15 July2018)http://www.clsi.org/standards/.
[18]GuidanceforIndustry,BionanalyticalMethodValidation,USDepartmentof HealthandHumanServices/FoodandDrugAdministrationCentrefor DrugEvaluationandResearch(CDER)/CentreforVeterinaryMedicine(CVM), 2001,May.
[19]E.Chambers,D.M.Wagrowski-Diehl,Z.Lu,J.R.Mazzeo,Systematicand comprehensivestrategyforreducingmatrixeffectsinlc/ms/msanalyses,J. Chromatogr.BAnalyt.Technol.Biomed.LifeSci.852(2007)22–34.
[20]S.Ulrich,B.Baumann,R.Wolf,D.Lehmann,B.Peters,B.Bogerts,F.P.Meyer, Therapeuticdrugmonitoringofclozapineandrelapse–aretrospectivestudy ofroutineclinicaldata,Int.J.Clin.Pharm.Th.41(2003)3–13.
[21]A.P.Rajkumar,B.Poonkuzhali,A.Kuruvilla,M.Jacob,K.S.Jacob,Clinical predictorsofserumclozapinelevelsinpatientswithtreatment-resistant schizophrenia,Int.Clin.Psychopharm.28(2013)50–56.
[22]A.Wohlfarth,N.Toepfner,M.Hermanns-Clausen,V.Auwarter,Sensitive quantificationofclozapineanditsmainmetabolitesnorclozapineand clozapine-N-oxideinserumandurineusingLC-MS/MSaftersimple liquid-liquidextractionwork-up,Anal.Bioanal.Chem.400(2011)737–746.
[23]M.Aravagiri,S.R.Marder,Simultaneousdeterminationofclozapineandits N-desmethylandN-oxidemetabolitesinplasmabyliquid
chromatography/electrospraytandemmassspectrometryanditsapplication toplasmalevelmonitoringinschizophrenicpatients,J.Pharm.Biomed.Anal. 26(2001)301–311.
[24]D.S.Domingues,M.A.Pinto,I.D.Souza,J.E.Hallak,J.A.Crippa,M.E.Queiroz, Determinationofdrugsinplasmasamplesbyhigh-performanceliquid chromatography-tandemmassspectrometryfortherapeuticdrugmonitoring ofschizophrenicpatients,J.Anal.Toxicol.40(2016)28–36.
[25]H.A.Niederlander,E.H.Koster,M.J.Hilhorst,H.J.Metting,M.Eilders,B.Ooms, G.J.deJong,Highthroughputtherapeuticdrugmonitoringofclozapineand metabolitesinserumbyon-linecouplingofsolidphaseextractionwithliquid chromatography-massspectrometry,J.Chromatogr.BAnalyt.Technol. Biomed.LifeSci.834(2006)98–107.
[26]L.V.Rao,M.L.Snyder,G.M.Vallaro,Rapidliquidchromatography/tandem massspectrometer(LCMS)methodforclozapineanditsmetabolite N-desmethylclozapine(norclozapine)inhumanserum,J.Clin.Lab.Anal.23 (2009)394–398.
[27]J.P.Lindenmayer,P.Czobor,J.Volavka,L.Citrome,B.Sheitman,J.P.McEvoy, etal.,Changesinglucoseandcholesterollevelsinpatientswithschizophrenia treatedwithtypicaloratypicalantipsychotics,Am.J.Psychiat.160(2003) 290–296.
[28]O.B.Idonije,O.O.Festus,U.Akpamu,O.Okhiai,O.I.Iribhogbe,G.B.S.Iyalomhe, Acomparativestudyoftheeffectsofclozapineandrisperidonemonotherapy onlipidprofileinnigerianpatientswithschizophrenia,Int.J.Pharmacol.8 (2012)169–176.
[29]N.Kate,S.Grover,M.Aggarwal,P.Malhotra,M.S.Sachdeva,Clozapine associatedthrombocytopenia,J.Pharmacol.Pharmacother.4(2013)149–151.
[30]J.Lee,R.Bies,A.Bhaloo,V.Powell,G.Remington,Clozapineandanemia:a 2-yearfollow-upstudy,J.Clin.Psychiat.76(2015)1642–1647.