Cagatay Nuhoglu,1 Evrim Hepkaya,1 Zehra Esra Onal,1 Narin Akici,1 Tamay Gurbuz,1 Vildan Atasayan,1
Omer Ceran2
1Department of Pediatrics, Haydarpasa Numune Training and Research Hospital, Istanbul, Turkey 2Department of Pediatrics, Istanbul Medipol University Faculty of Medicine, Istanbul, Turkey
ABSTRACT
OBJECTIVE: We aimed to evaluate the sensitivity of tuberculin skintest (purified protein derivative-PPD) by topical zinc application on test site to improve diagnostic reliability.
METHODS: We performed this study in 100 children aged 6–14 years, and plasma zinc levels were analyzed after 10–12 hours fasting. After PPD,we applied 40% zinc oxide cream on one forearm and placebo on the other forearm. PPD indurations were measured 72 hours later.
RESULTS: In this study, 26% of the children showed increases in PPD induration following local zinc applications. There was no correlation between indurations size and serum zinc levels.
CONCLUSION: We concluded that topical zinc cream application can enhance sensitivity of tuberculin reactivityin the diag-nosis of tuberculosis.
Keywords: Purified protein derivative; tuberculin skin test; zinc.
Received: August 15, 2017 Accepted:August 21, 2017 Online: January 11, 2018
Correspondence: Dr. Cagatay NUHOGLU. Haydarpasa Numune Egitim ve Arastirma Hastanesi, Cocuk Klinigi, Istanbul, Turkey. Tel: +90 216 542 32 32 e-mail: cnuhoglu@hotmail.com
© Copyright 2018 by Istanbul Provincial Directorate of Health - Available online at www.northclinist.com North Clin Istanb 2018;5(1):37-40
doi: 10.14744/nci.2017.88896
Immunomodulator effect of topical zinc oxide
application in tuberculin skin test
Orıgınal Article
CHILD HEALTH & DISEASEST
uberculosis, is a serious illness in developingcoun-tries, particularly in asymptomatic, immunosup-pressed children. It is estimated annual risk of tuber-culosis infection in children in developing countries is 2–5% [1].The estimated risk of developing tuberculosis diseases for a young child infected with mycobacterium tuberculosis as indicated by positive tuberculin test is ap-proximately 10% [2]. Nearly 8–20% of the deaths caused by tuberculosis occur in children [3]. We can diagnose the latent infection by means of tuberculin skin test (pu-rified protein derivative-PPD). However, the reliability of this test is limited by false-negative results, particu-larly in children who have poor nutritional status. Defi-ciency of zinc, a micronutrient that modulates immune
response and supports antibacterial immunity, can cause false-negative results in PPD skin test [4-6]. Therefore, we investigated whether the application of topical zinc application would enhance the sensitivity of PPD for the diagnosis of the disease.
MATERIALS AND METHODS
This study was performed in 100 children aged 3–14 years (mean age, 8.98±2.75 years) and hospitalized in Haydarpaşa Numune Training and Research Hospital Pediatric Department. After receiving ethical approval and informed written consent, venous blood of the chil-dren after 10–12 hours fasting were collected. Plasma
zinc levels were analyzed using atomic absorption spec-troscopy. The patients who had severe infections that could affect immune system anergic viral infections, steroid administration, and immune suppressive status were excluded.
PPD was performed and evaluated by the same doc-tor during the study. The docdoc-tor injected 0.1 ml tuber-culin (5U in 0.1 ml Aventis-Pasteur) in the volar surfaces of both the forearms. After implementations, he covered skin-test side on the left arm with zinc oxide dissolved in aqueous cream of 40% elemental zinc, whereas he cov-ered skin-test sides on the right arm with 1 ml of placebo creams. Both creams had the same color and odor and were provided in a similar looking cup. After 72 hours, he measured the sizes of indurations in both arms by ‘’ball-point-pen.”
Number Cruncher Statistical System (NCSS) 2007 and PASS (Power Analysis and Sample Size) 2008 Sta-tistical Software (Utah, USA) were used. While evalu-ating the data of the study, we used defining statistical methods such as mean value, standard deviation, median, and rate. We performed student’s t test order to demon-strate serum zinc levels according to genders. We used Mann–Whitney U test and Wilcoxon Signed Rank test in evaluating PPD indurations with placebo and zinc supplementation. For evaluating the associations of the parameters with each other, we used Spearman’s corre-lation analysis. Statistical significance was accepted as p<0.05.
RESULTS
This study included 41% males and 59% females in a total of 100 children (mean age, 8.98±2.75 years). All patients had scars. The mean serum zinc level in females was 89.86 mcg/dl, while it was 90.15 mcg/dl in males. Serum zinc levels of 12 patients was lower than normal (<70 mcg/dl). The mean serum zinc levels of the chil-dren were 89.98±24.61 mcg/dl. There was no significant correlation among plasma zinc levels between the PPD induration sizes with placebo and with zinc application (r=0.139; p=0.167). The mean age of the children was 8.98±2.75 (min: 3, max: 14) years. When the mean PPD indurations size of the children with placebo ointment was 2.53±4.44 (min: 0, max: 20) the mean PPD indura-tions size with zinc application was 3.55±5.67 (min 0, max: 20) mm (p=0.001). The mean PPD indurations size with zinc application was significantly higher than that with placebo (p<0.001) (Table 1).
North Clin Istanb 38
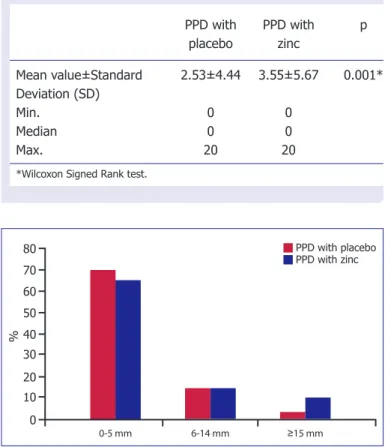
Seventy six percent induration of the responses to PPD with placebo cream were smaller than 5 mm, while 22% were 6–14 mm and 2% were larger than 15 mm. Induration responses to PPD with zinc ointment were as follows: 71% were smaller than 5 mm, 21% were 6–14 mm, and 8% were larger than 15 mm (Fig. 1).
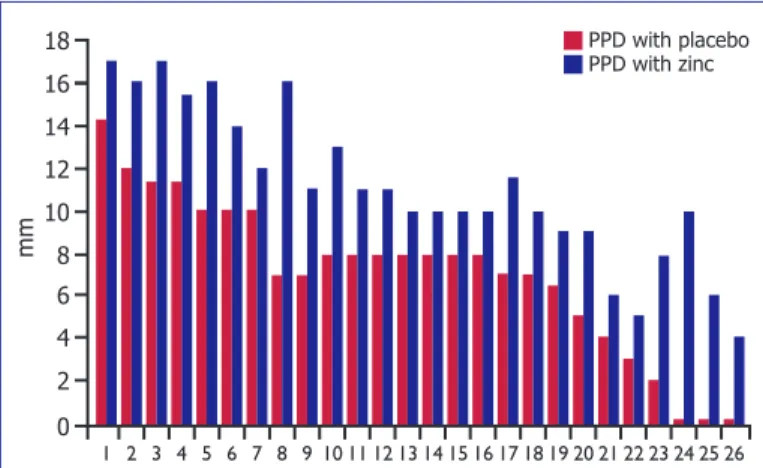
Of 100 children, 74 of did not show induration with placebo or zinc cream application following PPD. Topi-cal zinc cream application did not have an effect in non-reactive tuberculin test. The remaining 26 children had indurations larger than 0 mm. In this study, 26 of the patients showed increases in PPD indurations, following local zinc application. When 5 of them had a negative PPD response in the placebo sites, PPD response was evaluated as suspiciously positive following topical zinc application. When 6 of them had indurations of6–14 mm in the placebo cream sites, they were measured as positive (>15 mm) in the local zinc cream sites. Of 26 patients, 11 had significantly higher PPD induration sizes on zinc cream sites than placebo cream sites.
Apply-Table 1. Distribution of mean purified protein derivative-PPD indurations size with zinc and placebo
PPD with PPD with p placebo zinc Mean value±Standard 2.53±4.44 3.55±5.67 0.001* Deviation (SD) Min. 0 0 Median 0 0 Max. 20 20
*Wilcoxon Signed Rank test.
Figure 1. Distribution of PPD indurations size with placebo
and zinc supplementation.
80 % 70 30 60 50 10 40 20 0 0-5 mm 6-14 mm ≥15 mm PPD with placebo PPD with zinc
ing zinc creams modulated response of PPD by increas-ing induration sizes (Fig. 2).
DISCUSSION
Zinc is essential for normal development and function of cell-mediated immunity, neutrophils, and natural killer (NK) cells. It is needed for DNA synthesis, RNA tran-scription, cell division, and cell activation. Zinc deficiency adversely affects the secretion and functions of cytokines, which are the basic messengers of the immune system. Zinc is involved in maturation and differentiation of T cells. The gene expression of interleukin (IL)-2 and in-terferon (IFN)-γ are zinc dependent. IL-2 is involved in
the activation of NK and T cytolytic cells. IFN-γ and
IL-12 together play a major role in killing of parasites, viruses, and bacteria by macrophages–monocytes. Zinc deficiency is associated with impaired phagocyte func-tion, lymphocyte deplefunc-tion, decreased immunoglobulin production, reduced T4/T8 ratio, and decreased IL-2 production. Severe zinc deficiency is characterized by se-verely depressed immune function, frequent infections, bullous pustular dermatitis, diarrhea, and alopecia. Zinc circulates at a concentration of 70–120 mcg/dl, with 60% percent bound to albumin and 30%tightly bound to macroglobulin [7-13].
Low plasma zinc is usually defined as a value less than 60 mcg/dl. Some investigators argue that plasma zinc measurements are relatively insensitive and that zinc levels in neutrophils and lymphocytes may be more sensitive. The criteria for zinc deficiency is the decreased zinc level either in lymphocytes (<50 mcg/10 cells) or in granulocytes (<42 mcg/10cells) [14, 15].
Rao et al. performed a study on 50 volunteer healthy adults. They evaluated serum zinc levels. They placed PPD at the proximal sides of the palmar forearms and placed Candida antigens at distal sides ofthe forearms. They placed placebo cream in one forearm and zinc cream in the other one. The indurations sizes were measured in 24, 48, and 72 hours. The induration size of PPD was larger than 32% percentage of the zinc cream applied arm. Topical zinc caused greater tuberculin skin test aug-mentation in zinc deficient subjects. Zinc cream had no effect on subjects with normal zinc levels. Skin tests with zinc applications were significantly more likely to have positive results than the contra lateral control skin tests with placebo cream application (94% zinc, 76% placebo) [16]. The main difference of this study from ours was statistically significant difference in PPD responses of patients with lower zinc levels; however, there was no dif-ference in the patients within normal serum zinc levels. In our study, there was a significant difference in PPD in-duration sizes after local zinc application independently plasma zinc levels. The similarity of our study with this one was the evaluation of immunomodulatory effect of using local zinc application to enhance PPD response.
Kwok et al. investigated the effect of topical zinc ap-plication to increase the sensitivity of PPD. Of 58 el-derly patients, 38 (66%) had negative reactions with placebo ointment. Of these negative responders, 14 (37%) showed positive reactions with topical zinc oint-ment, 12 (32%) had weakly positive reactions, and 12 (32%) remained negative. They determined that 37%of patients with negative PPD had reacted as positive after applying zinc cream ointment. There was no significant difference in the plasma zinc levels between the differ-ent grades of topical zinc effect in the negative respon-ders [17]. Although this study was different because it included elderly people, it supported the findings of our study because it had shown that topical zinc application modulated the immune response.
Cuevas et al. investigated the differences of positive PPD responsiveness in patients younger than 15 years old. PPD was performed in patients at an interval of 2 years. The group receiving oral zinc application before applying the test showed increase in positive respon-siveness and indurations measurements compared with the placebo group [18]. In this study patients were given oral zinc supplements but, our patients were given topi-cal zinc application, differently from the previous study. However, the findings of both studies supported each other because they revealed that zinc modulates the
cell-Nuhoglu et al., Tuberculin skin test 39
Figure 2. Subjects showing increased PPD indurations
fol-lowing zinc supplementation.
16 18 mm 14 6 12 10 2 8 4 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 PPD with placebo PPD with zinc
mediated immune responsiveness.
Castillo-Duran et al. evaluated the immunity and de-velopment after adding zinc application in 32 marasmic infants. Half of the subjects were given 2 mg/kg oral zinc during 3 months, and the other half were given placebo. They evaluated PPD responses in the first and 90th day. Before applying zinc, 33% of the patients showed positive PPD results. After zinc application, 67% of the patients showed positive PPD results. There was no significant difference of PPD responsiveness in placebo group [19]. This study supported the immunomodulatory effect of the zinc application for enhancing the PPD reactivity.
Kramer TR et al. investigated T lymphocyte prolif-eration in 140 children aged 6–13 years by evaluating the PPD responsiveness after oral zinc application with 25 mg/day during 6 months. The group receiving zinc application showed significant increase in PPD respon-siveness compared with the one receiving placebo [20]. This study differed from ours by systemic use of zinc combined with vitamin A in a longer duration. However, it also supported our findings that zinc application in-creased the immunomodulatory effect.
Although PPD plays a major role in the diagnosis of latent tuberculosis, nutritional status, immunity, chronic illness of the patients, and environmental factors can decrease its sensitivity. The incidence of tuberculosis in-creases not only in adulthood but also in childhood. As a result, the effect of zinc on PPD responsiveness gains more importance in diagnostic approach. Although sys-temic use of zinc application had been preferred in the previous studies, we aimed to benefit from local zinc cream application providing simple and inexpensive ef-fect on enhancing the sensitivity of PPD for the diagno-sis of the diseases.
We believe that future studies including more sub-jects can support the sensitivity of PPD responses in the cases with lower plasma zinc levels following topical zinc applications. We recommend using local zinc ointments without screening plasma zinc levels after performing PPD, to enhance the sensitivity of the test so that we can decrease the false-negative results. If we use the booster effect of topical zinc after performing PPD, we can en-hance tuberculin reactivity, causing optimal diagnostic approach.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has re-ceived no financial support.
Authorship contributions: Concept – C.N.; Design – C.N.; Super-vision – C.N.; Materials – E.H., N.A.; Data collection &/or processing – E.H., V.A.; Analysis and/or interpretation – C.N., Z.E.O., T.G.; Writ-ing – C.N., Z.E.O., T.G.; Critical review – C.N., O.C.
REFERENCES
1. Chugh S. Paediatric tuberculosis and DOTS strategy under RNTCP. J Indian Med Assoc 2008;106:799–802.
2. Enarson DA. The International Union Against Tuberculosis and Lung Disease model National Tuberculosis Programmes. Tuber Lung Dis 1995;76:95–9. [CrossRef ]
3. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuber-culin reaction in childhood and adolescence. Am J Epidemiol 1974;99:131–8. 4. Golden MH, Harland PS, Golden BE, Jackson AA. Zinc and
immuno-competence in protein-energy malnutrition. Lancet 1978;1:1226–8. 5. Lin RY, Busher J, Bogden GJ, Schwartz RA. Topical zinc sulfate
augmentation of human delayed type skin test response. Acta Derm Venereol 1985;65:190–3.
6. Karyadi E, West CE, Schultink W, Nelwan RH, Gross R, Amin Z, et al. A double-blind, placebo-controlled study of vitamin A and zinc sup-plementation in persons with tuberculosis in Indonesia: effects on clin-ical response and nutritional status. Am J Clin Nutr 2002;75:720–7. 7. Shankar AH, Prasad AS. Zinc and immune function: the biological basis
of altered resistance to infection. Am J Clin Nutr 1998;68:447S–63S. 8. Prasad AS. Zinc Deficiency. In: Trace Element in Human Disease.
1995;573-86.
9. Prasad AS. Diagnostic approaches to Trace Element Deficiencies. In: Chandra RK, editor. Trace Elements in Nutrition of Children. New York: 1995. p. 17–40.
10. Prasad AS. Zinc in growth and development and spectrum of human zinc deficiency. J Am Coll Nutr 1988;7:377–84. [CrossRef ]
11. Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol 2008;43:370–7. [CrossRef ]
12. Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp (Warsz) 2008;56:15–30. [CrossRef ]
13. Fraker PJ, Haas SM, Luecke RW. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr 1977;107:1889–95. 14. Hambidge KM, Casey CE, Krebs NF. In: Mertz W, editor. Trace elements
in human and animal nutrition. Orlando: Academic Press; 1986. p. 1. 15. Wood RJ. Assessment of marginal zinc status in humans. J Nutr
2000;130:1350S–4S. [CrossRef ]
16. Rao VB, Pelly TF, Gilman RH, Cabrera L, Delgado J, Soto G, et al. Zinc cream and reliability of tuberculosis skin testing. Emerg Infect Dis 2007;13:1101–4. [CrossRef ]
17. Kwok T, Fotherby MD, Cookson J, Potter JF, Castleden CM. Can topical zinc accentuate tuberculin reactivity in the elderly? Respir Med 1994;88:47–8. [CrossRef ]
18. Cuevas LE, Almeida LM, Mazunder P, Paixão AC, Silva AM, Ma-ciel L, et al. Effect of zinc on the tuberculin response of children ex-posed to adults with smear-positive tuberculosis. Ann Trop Paediatr 2002;22:313–9. [CrossRef ]
19. Castillo-Duran C, Heresi G, Fisberg M, Uauy R. Controlled trial of zinc supplementation during recovery from malnutrition: effects on growth and immune function. Am J Clin Nutr 1987;45:602–8. [CrossRef ]
20. Kramer TR, Udomkesmalee E, Dhanamitta S, Sirisinha S, Charoenki-atkul S, Tuntipopipat S, et al. Lymphocyte responsiveness of children sup-plemented with vitamin A and zinc. Am J Clin Nutr 1993;58:566–70.
North Clin Istanb 40