Are
the
leading
drugs
against
Staphylococcus
aureus
really
toxic
to
cartilage?
夽
Mustafa
Dogan
a,
Mehmet
Isyar
b,∗,
Ibrahim
Yilmaz
c,
Bulent
Bilir
d,
Duygu
Y.
Sirin
e,
Selami
Cakmak
f,
Mahir
Mahirogullari
baNamikKemalUniversitySchoolofMedicine,DepartmentofInfectiousDiseases, 59100Tekirdag,Turkey
bIstanbulMedipolUniversitySchoolofMedicine,DepartmentofOrthopaedicsand Traumatology,34214Istanbul,Turkey
cRepublicofTurkeyMinistryofHealth,StatementofHospital, Pharmacovigilance&RationalDrugUseTeam,59100Tekirdag,Turkey
dNamikKemalUniversitySchoolofMedicine,DepartmentofInternalMedicine, 59100Tekirdag,Turkey
eNamikKemalUniversityFacultyofArts,DepartmentofMolecularBiologyand Genetics,59100Tekirdag,Turkey
fGulhaneMilitaryMedicalAcademy,HaydarpasaTrainingHospital, DepartmentofOrthopaedicandTraumatology,34668Istanbul,Turkey
Received26June2015 ;receivedinrevisedform22September2015;accepted5October2015
KEYWORDS Chondrocyte; Linezolid; Teicoplanin; Toxicity; Vancomycin
Summary Manystudieshaveshownthatthetoxiceffectsoflocalantibioticson bone and cartilage limit orthopedic surgeons. In this study,we evaluated three antibacterialagentsusedlocallytotreathighlymortalandmorbiddiseasesinthe fieldoforthopedics,suchassepticarthritis.Arevancomycin,teicoplanin,and line-zolid,whicharearchenemiesofStaphylococcusaureus,reallytoxictochondrocytes? Thepurposeofthestudywastoinvestigatetheeffectsofantibiotics,whichareused againstS.aureus,onhumanchondrocytesinvitro.
Primarycellculturesobtainedfromgonarthrosispatientsweredividedintotwo maingroups.Oneofthesegroupswasdesignatedasthecontrolchondrocyteculture. The other group wasdivided intothree subgroups,and each group wasexposed
夽 LevelofEvidence:LevelI,invitroclinicalresearchstudy.
∗Correspondingauthor.Tel.:+905337227971;fax:+902124607070.
E-mailaddresses:mustafadogaan@hotmail.com(M.Dogan),misyar2003@yahoo.com(M.Isyar),ibrahimyilmaz77@yahoo.com (I.Yilmaz),bulentbilir@yahoo.com(B.Bilir),dysirin@nku.edu.tr(D.Y.Sirin),selamicakmak@gmail.com(S.Cakmak),
mahirogullari@yahoo.com(M.Mahirogullari). http://dx.doi.org/10.1016/j.jiph.2015.10.004
tovancomycin,teicoplanin,orlinezolid.Cellculturesampleswerecharacterizedby immunophenotypingfollowingincubationwiththethreedifferentantibiotics.Before and afterthe agents wereadministered, the cultures were subjected toinverted andenvironmentalscanningelectron microscopy.The numberoflivecells andthe proliferationrateweremonitoredwiththeMTT-assay.
We found that vancomycin, teicoplanin, and linezolid do not have chondrotoxic effects.
Vancomycin,teicoplanin,andlinezolidhadnochondrotoxicactivityduringinvitro cul-ture,whichsupportstheargumentthattheseagentscansafelybeusedinorthopedic surgery,especiallyagainstmethicillin-resistantS.aureusagents.
©2015KingSaudBinAbdulazizUniversityforHealthSciences.PublishedbyElsevier Limited.Allrightsreserved.
Introduction
Sideeffectsandadversereactionsoccurfrequently duringsystemicantibiotictherapies[1,2].The pro-longedand high-doseadministration ofantibiotics required to treat septic arthritis and osteomyeli-tis results in side-effect profiles that also involve toxicity problems [1,3—5]. To avoid such delete-rious effects, local antibiotics are often utilized, especially intra-operatively [6—11]. In orthope-dics,placingantibiotic-impregnatedSpongostanor gels/grafts in various forms into infected regions has beenfound useful to fight infection or to act as aprophylactic[2,3,7—9]. However,the toxicity oftheselocalantibioticshasbeendiscussedinthe literature[1].Additionally,reportsindicatinglocal osteotoxic and chondrotoxiceffectshave imposed some limitations on orthopedic surgeons [1,10], especiallywithregardtocertainprocedures involv-ingopensurgeries,suchasintra-articularfracture surgeryandmultipleligamentkneeinjuries.Insuch cases, both prophylaxis and the use of antibiotic therapy may be helpful [12,13]. In the present study,vancomycin(VCM)[6],knowntobe chondro-toxic, and its alternatives teicoplanin (TEIC) and linezolid (LZD), commonly used against Staphylo-coccus aureus, the most common cause of septic arthritis,werespecificallychosenduetotheir com-mon use in orthopedics. The chondrotoxicity of these three antibiotics are investigated in human primarychondrocytecultures.
Materials
and
methods
Prior to the present research, approval from the Namik Kemal University Medical Faculty Ethics Committee (Date:25/12/2014; Number: 127) and the participant written informed consents were obtained. Primary chondrocyte cultures consisted
ofsurgically harvestedosteochondral tissuesfrom gonarthrosispatientsundergoingroutinetotalknee arthroplasty, (n=6); VCM, TEIC, and LZD agents wereadded, andcellviability,toxicity,and prolif-erationanalyseswereconductedatbaseline(0h), beforetheapplicationofantibiotics,andafter24h. Surfacemorphologiesand theextracellularmatrix were examined using inverted and environmental scanning electron microscopes (ESEM). To mini-mize measurement and analysis errors, the same researcher performed the analyses both during and after the primary chondrocyte culturing. The researchers conducting the analyses were blinded to the type of drug used and the experimen-tal setup. All experiments were conducted three times.
Materials
Collagenase type II enzyme (1mg/mL, Invitrogen Corporation);Hank’sBalancedSaltSolution (HBSS-1X, 14025, Gibco); penicillin-streptomycin with fetal bovine serum (FBS); and Dulbecco’s Modi-fied Eagle’s Medium (DMEM) (1000mgglucose/L) were obtained from Sigma Chemical, St. Louis, USA. Sodium dodecyl sulfate (SDS) (10% L4522), insulin—transferrin—selenium (ITS) premix, and RPMI-1640 were obtained from Sigma—Aldrich GmbH, Germany. TheMTT Kit wasa Vybrant MTT Cell Proliferation Assay (Catalog /= V-13154) by CellBiolabs Inc.,USA. Alaminarflow cabinet(Air Flow-NUVE/NF-800 R) and an incubator (NUVE, 06750)wereobtainedin Ankara,Turkey. An Olym-pus CKX41inverted microscope was used and the images were recorded using an Olympus cell soft imaging systemprogram. A Mindray MR 96A (PRC) enzyme-linkedimmunosorbentassay(ELISA)device measured viability, cytotoxicity, and proliferation withacommercialkit.ThisstudyusedaQuanta250 FEG(FeiCompany,Hillsboro,Oregon,USA)ESEM.
Methods
Patientselectioncriteria
Osteochondral tissues to be used in the primary chondrocyte culture were obtained from patients who were not allergic to VCM, LZD, or TEIC. Additionally, the study excluded patients with Parkinson’s disease who had been treated with monoamine oxidase inhibitors, such as rasagiline, selegiline,ormoclobemide,intheprevious14days, aswellaspatientsreceivingantidepressantdrugs, to prevent drug interaction. Linezolid is also one a monoamine oxidase inhibitor itself. Therefore, it cancausean increasein the adrenergicpressor response.
Preparationofthedrugs
Mainstocksolutions werefreshly preparedby dis-solving VCM and TEIC in 1000 and 400mg DMEM culture media, respectively, in a flow cabin. The LZDwaspreparedusingareadymade300mL solu-tionwithaconcentrationof2mg/mL.Next,these main stock solutions were coded to keep the researchersblindedtothetreatmentbeforebeing transferredtodark-brownbottles.
Finalconcentrationsofthedrugsadministeredto thechondrocyteculturesweredeterminedin rela-tion to their minimal bactericidal concentrations (MBC). The practiced MBC reference values were 16mg/LforVCM,64mg/LforTEIC,and32mg/Lfor LZD[14].
Obtainingtheprimarychondrocyteculture
The study included tissues obtained from gonarthrosis patients whose medical and con-servative treatments had failed and who were undergoing total knee arthroplasty surgery [n=6; meanage 65years(59—71);Stage4gonarthrosis]. Patient informed consent was obtained. Osteo-chondral tissues were resected from the distal femurand the proximaltibia, whichareroutinely cut away during total kneearthroplasty. Standard human primary chondrocyte cultures were then performed[15—18].
The cells were counted on a Thoma counting chamber in trypan blue and placed in a 24-well platewith 125×103 cells in eachwell;theywere thenstored inanincubatorfor48h.Atthe endof thistimeperiod,thedrugswereintroducedintothe cellcultures,whichbecameconfluentandstuckto thebottom.
Exposureofthedrugsintothecultures
The control group, named Group I, consisted of pure primary chondrocyte cultures. Groups II, III, and IV consisted of primary chondrocyte cultures
that contained VCM, LZD, and TEIC, respectively. Anadditionalsamplewaskeptasidefromboththe study and control groups for MTT-ELISA and ESEM analyses.
Analyses
Cellsthatdisplayedchondrotoxicactivityandstuck to the inner surface of the flasks were exam-inedunderaninvertedmicroscope, andthemicro images were recorded. Inverted microscopy, MTT-ELISAcellviabilityandtoxicity, andESEManalyses wereperformedonGroupIatboth0and24h.The sameanalyseswereperformedonGroupsII,III,and IVat0and24hafterintroducingtheantibacterial agents.
ESEManalysis. Inthisstage,thesurface topogra-phyand theextracellularmatrixstructures ofthe chondrocyteswereexamined,andthecell-culture contentsofthewellswereexpelledusinggun pipet-tors. A 2.5% glutaraldehyde solution composed of 97.5mL cacodylate buffer and 2.5mL glutaralde-hyde was then added to the wells to cover the samples.Theglutaraldehydesolutionwasremoved fromthe samples usinga pipette gunand kept at roomtemperaturefor 2h.Thesampleswere then washedthreetimeswithcacodylatebuffer. Follow-ingthelastwashing,thesampleswerecoveredwith cacodylatebufferandkeptat4◦Cuntilanalysis. The MTTcell viability, toxicity, and proliferation assays. Viability tests were performed using a commercial MTT kit (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MW=414) and following the manufacturer’s instructions. The kit works as follows: mitochondrial dehydrogenase enzymes cleave to the tetrazolium ring, yield-ing blue formazan crystals, which do not occur in dead cells. A 12mM MTT stock solution was prepared by dissolving 5mg MTT in sterile phos-phate buffer saline. The antibiotic medium was removed from the culture medium and the MTT medium was added to reach a final concentra-tion of 10% (v/v). This mixture was incubated at 37◦Cfor 2h.Atthe endoftheincubationperiod, 500LDMSOwereaddedasasolventandthe mix-ture was incubatedagain at 37◦C for 10min. For eachsample,spectrophotometrywasperformedat 540nm to measure the absorbance. The project coordinatortranscribedletterlabelsdesignatedfor eachsample.Thevitalityofthecontrolgroupwas assumed to be 100% before the transfer of the antibiotics(0h)totheculturemedium.Twenty-four hours after the administration of the antibiotics, theviabilityabsorbance ofthecells wasrecorded in nanometers. Proliferation analyses for 24—36h werethenrecorded.
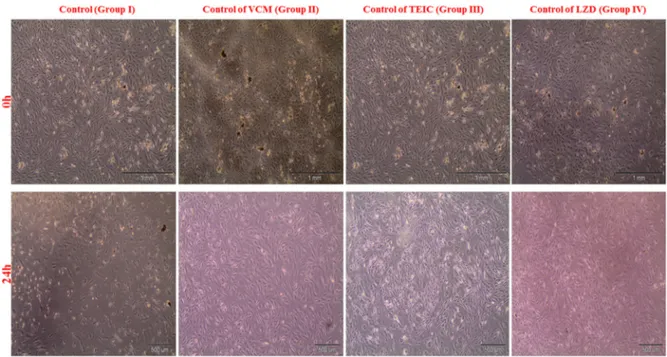
Figure1 Invertmicroscopyimagesofchondrocytesat0and24hinthecontrolgroup.
Statistical analysis. Cell numbers and prolifera-tionresults,alongwithMinitabR15software,were used to analyze the data. Each drug was com-paredwithitsowncontrolgroupusingthevariance analysis, andtheproliferationdatawereanalyzed usingTukey’sMethod(with95.0%confidence inter-vals). The statistical significance level was set at ˛<0.05.
Findings
Analysis
of
inverted
microscopy
results
Images of proliferated healthy chondrocytes (91—99%) in the control group, which did not receiveantibiotics(Fig.1).
The
statistical
evaluation
of
the
effects
of
the
agents
on
chondrocytes
via
MTT
ELISA/MTT-proliferation
assays
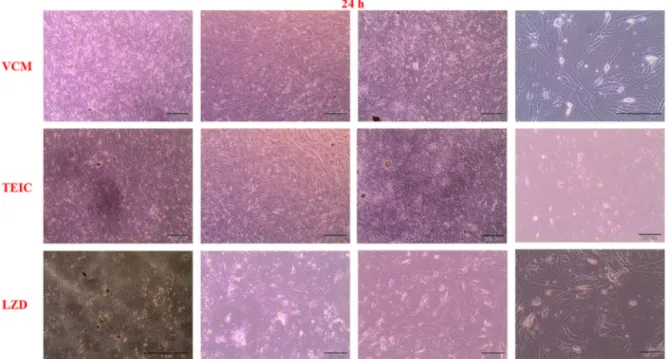
The alive cell counts in all of the groups that received antibiotics were similar with respect to thecontrolgroupsat24h.Allthreeantibiotics con-tinuedtoproliferateinthe36hoftestthatbegan attheendof24thhour(Fig.2).
The 24h viability and 36h proliferation test resultsshowedthatthere wasnostatically differ-ence between the control groups and the groups thatreceivedantibiotics.Allthreeagentswerenot toxictochondrocytes(Table1andFig.3).
An
evaluation
of
the
ESEM
analysis
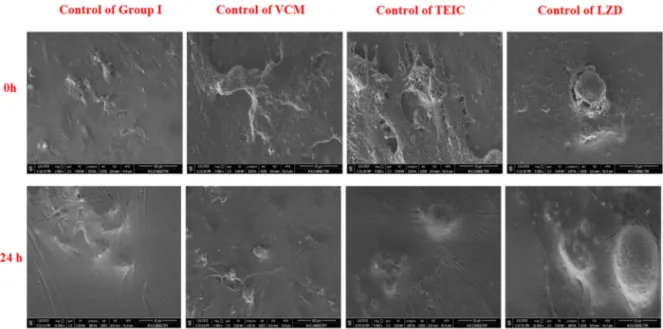
Healthycellsandextracellularstructureswereseen in the morphological evaluations of the chondro-cyte surfaces before antibiotic exposure (Fig. 4). ESEMimagesafter24hofantibiotic exposure sup-porttheresultsoftheMTT-ELISAviability,toxicity, and MTT-proliferationdataanalysesaswell asthe invert images (Fig. 5). Healthy chondrocytes and extracellularmatrices,whichwerethe markersof chondroblasticactivity,andnaturalsurface charac-teristicswerevisiblein theimagesfromallofthe groups.
Table1 CartilagecellsgrowthassessedbytheMTT viability, toxicity, and proliferation assays (540nm) afterexposuretothreedifferentantibiotics.
Groups Mean±SD pa
MTTcellviabilityandtoxicities
ControlofVCM 0.28200±0.01699 VCM 0.2735±0.0594 0.743 ControlofTEIC 0.2240±0.0288 TEIC 0.2362±0.0752 0.719 ControlofLZD 0.2653±0.0482 LZD 0.2705±0.0540 0.865
MTTcellproliferationtest
VCM 107.4±35.5Ab
0.914
TEIC 105.8±28.3Ab
LZD 101.63±2.08Ab
aAnalysisofvariancetest.
bGrouping information usingTukey’s Methodand 95.0%
Figure2 Invertmicroscopyimagesofchondrocytesafter36h(the24thhourproliferationtest)ofexposuretoVCM, TEIC,andLZD.
Discussion
Orthopedic infections, which occasionally jeopar-dize the outcome of the surgery, usually require long-term antibiotic treatments. This molecular study investigated the cytotoxicity of VCM, TEIC, and LZD in human primary chondrocyte cultures. These agents were selected because they can
be administered locally and systemically to treat methicillin-resistant S. aureus(MRSA), one ofthe leadingcausesoforthopedicinfections.
EarlierstudiesclaimedthatVCMischondrotoxic
[6].Inadditiontoitssystemicuse,itisalso admin-istered locally to joints [3]. Studies comparing jointandserumlevelsfollowingtheintra-articular and parenteral administration of VCM concluded
Figure4 Morphologicalevaluationsofchondrocytesurfacesinthecontrolgroup(ESEMimagesatthe0and24thhour.
that intra-articular administration is more effec-tive[19]. Aninvivostudyusingamurinebacterial arthritis model reported that VCM and LZD can safelytreatMRSAbasedonthefindingsthat reduc-ing inflammatory cytokines in the group treated with VCM and LZD led to decreased accumulated neutrophils[20].
Previous cytotoxicity studies investigated the chondrotoxicity of not only various antibacterial agents of the glycopeptide group but also other antibiotic agents, such as the quinolone group
[21—23]. These studies examined the antibacte-rial agents used in orthopedic surgeries and the chondrotoxicity of a wide range of pharmaceuti-calagents, suchaslocalanesthetics,opioids,and corticosteroids,onamolecularlevel[18,24,25].
Previous in vitro studies reported that VCM causes less osteoclast activity compared to tobramycin, cefazolin, and gentamicin. Moreover, cell-linecomparisonsshowedthatVCMin125g/mL dosesis toxictoosteoblast- and chondroblast-like cells[1,6,26,27].
Antocietal.investigatedthechondrotoxicityof the glycopeptide group in cell lines and observed VCM to be chondrotoxic. They investigated cyto-toxicityusingalactatedehydrogenase(LDH) assay
[6]. However, the LDH assay was developed as a guide when the direct isolation of cells is not achievedinhigh-densityattachedcells.Therefore, the metabolite level is measured by the amount of LDH released into the cell-culture supernatant and taken as the quantitative value for the loss of live cells. In other words, the measurements are not performed directly in cells but through the reduction ofenzymes released bycells in the medium. The data would have been more robust ifmetabolicactivityandDNAsynthesis,whichare usedin asingle-course culture method, had been usedinsteadofspectrophotometricanalysisofthe reductionfrompyruvatetolactate[28,29].
The most popular cytotoxicity analysis per-formed in single-course cell cultures involves the colorimetric measurement of cell proliferation using MTT tetrazolium salt. The MTT method has beeninusesince1983forcellproliferation, viabil-ity,andtoxicityidentification[30,31].
Thismethodisquitesensitivein the quantifica-tionofcellproliferationandcytotoxicity,especially inchemotherapeuticdrugs,inwhichonlylivecells with metabolicactivityreduce tetrazoliumsalt to colored and insoluble formazan. Therefore, this methodidentifiesonlylivecells.Itnotonly identi-fiescellviabilityandcytotoxicitybutalsoidentifies cellactivation andproliferation.Tetrazoliumsalts havebeenprovenintheliteratureasthemost sen-sitive and most soluble methods for accuratecell vitality, toxicity, and proliferation analyses, and severalnew methods,suchasWST,XTT,MTS,and MTT,havebeendeveloped[28—32].
Toxicitystudiesare generallyperformedon tis-sues from animal articular cartilage. Obviously, human and animal tissues have different sensi-tivities, and thus, results may be different and misleading[24].Theuse ofasingletypeofcellin cell-line systems, the lack of complicated coordi-nationmechanismsinbetweencellsandthemicro frame, and the inhibitionof interactionswith the extracellular matrix structure make in vitro tests problematictoadopttoinvivoconditions,interms ofcompatibility[33]. Thebiggestdisadvantageof celllinesisthattheydonotcarryallofthe genotyp-icalandphenotypicalfeaturesoftheoriginal struc-turebecausetheyaregeneticallymodified[34].
Inthepresentstudy,humanprimarychondrocyte cultureswereusedtoinvestigatethe chondrotoxi-cityofthreeagents.Althoughtheexperimentwas in vitro,cellcultureswere notobtainedfrom ani-mals, anda cellline wasnot used.Therefore,we
believe that the findings are robust. Considering the MBC reference values, VCM (16mg/L), TEIC (64mg/L),andLZD(32mg/L)havenochondrotoxic effects when administered locally. The present study observed that these antibacterial agents, which are frequently used locally in orthopedic infections,arenottoxictocartilage,especiallyin termsofviabilityandproliferation.
Conclusion
Webelieve thatthese antibacterial agents,which showed no chondrotoxicity in either a cellular dimension or on a molecular level, can safely be locally used in orthopedic surgery, especially against MRSA agents. However, this is only an in vitro study. Antibiotics did not show adverse effectsonculturedchondrocytesfromsixpatients in the age range of 59—71 years. We cannot conclude that the antibiotics are safe in vivo in orthopedic surgery. The evidence supports the hypothesis only, and it does not disprove the hypothesis.Inanextensionofthisstudy,theeffect of the antibiotics on cell function should be per-formed in different phases of growth and for variablelengths ofexposure time. Furtherin vivo molecularstudies investigating these agents com-paratively and in a cellular dimensionwith larger samplesarewarranted.
Funding
Nofundingsources.Competing
interests
Nonedeclared.Ethical
approval
Notrequired.References
[1]EdinML,MiclauT,LesterGE,LindseyRW,DahnersLE.Effect ofcefazolinand vancomycinonosteoblastsinvitro. Clin OrthopRelatRes1996;333:245—51.
[2]Shuhendler AJ, Pu K, Cui L, Uetrecht JP, Rao J. Real-timeimagingofoxidativeandnitrosativestressintheliver of live animals for drugtoxicity testing. Nat Biotechnol 2014;32:373—80.
[3]JuddD,BottoniC,KimD,BurkeM,HookerS. Infections following arthroscopic anterior cruciate ligament recon-struction.Arthroscopy2006;22:375—84.
[4]MaderJT,CalhounJ.Bone,joint,andnecrotizingsofttissue infections. In:BaronS, editor. Medicalmicrobiology.4th ed.Galveston,TX:UniversityofTexasMedicalBranchat Galveston;1996.p.1—11.
[5]ShirtliffME,MaderJT.Acutesepticarthritis.ClinMicrobiol Rev2002;15:527—44.
[6]Antoci Jr V, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res 2007;462: 200—6.
[7]El-Husseiny M, Patel S, MacFarlane RJ, Haddad FS. Biodegradableantibioticdeliverysystem.JBoneJointSurg Br2011;93:151—7.
[8]KalilGZ,ErnstEJ,JohnsonSJ,JohannssonB,PolgreenPM, BertolatusJA,etal.Systemicexposuretoaminoglycosides following knee and hip arthroplasty with aminoglyco-side loaded bone cement implants. Ann Pharmacother 2012;46:929—34.
[9]KlemmKW.Antibioticbeadchains.ClinOrthopRelatRes 1993;295:63—76.
[10]McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012;105:37—42.
[11]Penn-Barwell JG, Murray CK, Wenke JC. Local antibiotic deliverybyabioabsorbablegelissuperiortoPMMAbead depotinreducinginfectioninanopen fracturemodel.J OrthopTrauma2014;28:370—5.
[12]AmbroseCG,GogolaGR,ClyburnTA,RaymondAK,PengAS, MikosAG.Antibioticmicrospheres:preliminarytestingfor potentialtreatmentofosteomyelitis.ClinOrthopRelatRes 2003;415:279—85.
[13]Difenbeck M, Muckley T, Hoffmann GO. Prophylaxis and treatmentofimplant-relatedinfectionsbylocalapplication ofantibiotics.Injury2006;2:95—104.
[14]Barcia-Makay M, Seral C, Mingeot-Leclercq MP, Tulkens PM,Van BambekeF.Pharmacodynamicevaluationofthe intracellular activitiesofantibiotics against Staphylococ-cusaureusinamodelofTHP-1macrophages.Antimicrob AgentsChemother2006;50:841—51.
[15]GokceA,YilmazI,BircanR,TonbulM,GokayNS,GokceC. SynergisticeffectofTGF-1andBMP-7onchondrogenesis andextracellularmatrixsynthesis:aninvitrostudy.Open OrthopJ2012;6:406—13.
[16]Gokce A, Yilmaz I, Gokay NS, Can L, Gokce C. Does insülin transferrin and selenous acid preparation effect chondrocyte proliferation? Acta Orthop Traumatol Turc 2014;48:313—9.
[17]Yilmaz I, Gokay NS, Gokce A, Tonbul M, Gokce C. Consecutively controlled release of TGF-Beta1 and BMP-7 for synergistic growth of chondrocyte culture. J Med Sci 2013;33:18—32, http://dx.doi.org/10.5336/ medsci.2011-27807.
[18]Yilmaz I, Gokay NS, Bircan R, Saracoglu GV, Dervisoglu S, Gokce A. How different methodologies of harvest-ing and analysing the samples affect the test results in determining joint mediators. Arthritis 2013:631959, http://dx.doi.org/10.1155/2013/631959.
[19]Roy ME, Peppers MP, Whiteside LA, Lazear RM. Van-comycin concentration in synovialfluid: direct injection
into the knee vs. intravenous infusion. J Arthroplasty 2014;29:564—8.
[20]TanakaM,MrozP,DaiT,HuangL,MorimotoY,KinoshitaM, etal.Linezolidandvancomycindecreasethetherapeutic effectofmethylenebluephotodynamictherapyinmouse model of MRSA bacterial arthritis. Photochem Photobiol 2013;89:679—82.
[21]GotoK,ImaokaM,GotoM,KikuchiI,SuzukiT,JindoT,etal. Effect ofbodyweightloadingontothearticularcartilage ontheoccurrenceofquinolone-inducedchondrotoxicityin juvenilerats.ToxicolLett2013;216:124—9.
[22]Goto K, Yabe K, Suzuki T, Takasuna K, Jindo T, Manabe S. Gene expression profilesin the articular cartilage of juvenile ratsreceivingthequinoloneantibacterial agent ofloxacin.Toxicology2008;249:204—13.
[23]StueberT,KarstenJ,StoetzerC,LefterA.Differential cyto-toxicpropertiesofdrugsusedforintra-articularinjection onhumanchondrocytes:anexperimentalin-vitrostudy.Eur JAnaesthesiol2014;31:640—5.
[24]BeyzadeogluT, TorunKG, Ekinci ID,Bekler H, YilmazC. Cytotoxicity oflocal anesthetics to rats’ articular carti-lage:anexperimental study.ActaOrthopTraumatolTurc 2012;46:201—7.
[25]Breu A, Rosenmeier K, Kujat R, Angele P, Zink W. The cytotoxicity of bupivacaine, ropivacaine, and mepiva-caineonhumanchondrocytesandcartilage.AnesthAnalg 2013;117:514—22.
[26]HanssenAD,SpangehlMJ.Practicalapplicationsof antibi-otic loadedbonecementfor treatmentofinfectedjoint replacements.ClinOrthopRelatRes2004;427:79—85. [27]Vertullo CJ, Quick M, Jones A, Grayson JE. A surgical
techniqueusingpresoakedvancomycinhamstringgraftsto decreasetheriskofinfectionafteranteriorcruciate liga-mentreconstruction.Arthroscopy2012;28:337—42. [28]CoryAH,OwenTC,BarltropJA,CoryJG.Useofan
aque-oussolubletetrazolium/formazan13assayforcellgrowth assaysinculture.CancerCommun1991;3:207—12. [29]SylvesterPW. Optimizationofthetetrazoliumdye (MTT)
colorimetricassayforcellulargrowthandviability.Methods MolBiol2011;716:157—68.
[30]BakerJF,BryneDP,WalshPM,MulhallKJ.Human chondro-cyteviabilityaftertreatmentwithlocalanestheticand/or magnesium: results from an in vitro study. Arthroscopy 2011;27,213-207.
[31]Gieni RS, Li Y, HayGlass KT. Comparison of [3H] thymi-dineincorporationwithMTT-andMTS-baredbioassaysfor humanandmurineIL-2andIL-4analysis.Tetrazoliumassays providemarkedlyenhancedsensitivity.JImmunolMethods 1995;17:85—93.
[32]BastianJD,EgliRJ,GanzR,HofstetterW, LeunigM. Dif-ferentialresponseofporcineosteoblastsandchondrocytes incellortissuecultureafter5-aminolevulinicacid-based photodynamictherapy.OsteoarthCartil2009;17:539—46. [33]Wataha JC. Biocompatibility of dental materials. In:
AnusaviceKJ,editor.Phillip’sscienceofdentalmaterials. Missouri:ElsevierScience;2003.p.171—202.
[34]Alberts B, Bray D, Hopkin K. Essential cell biology. In: AlbertsB,BrayD,HopkinK,editors.Manipulatinggenesand cells.2nded.NewYork:GarlandScience,Taylor&Francis Group;2004.p.323—7.
Availableonlineatwww.sciencedirect.com