Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=tphm20
Philosophical Magazine
ISSN: 1478-6435 (Print) 1478-6443 (Online) Journal homepage: https://www.tandfonline.com/loi/tphm20
Thermal and magnetic characterisation of
(Co
0.402
Fe
0.201
Ni
0.067
B
0.227
Si
0.053
Nb
0.05
)
100–x
Cu
x
bulk
metallic glasses
Kagan Sarlar & Ilker Kucuk
To cite this article: Kagan Sarlar & Ilker Kucuk (2017) Thermal and magnetic characterisation of (Co0.402Fe0.201Ni0.067B0.227Si0.053Nb0.05)100–xCux bulk metallic glasses, Philosophical Magazine, 97:7,
489-496, DOI: 10.1080/14786435.2016.1266101
To link to this article: https://doi.org/10.1080/14786435.2016.1266101
Published online: 13 Dec 2016.
Submit your article to this journal
Article views: 294
View related articles
View Crossmark data
http://dx.doi.org/10.1080/14786435.2016.1266101
Thermal and magnetic characterisation of (Co
0.402Fe
0.201Ni
0.067B
0.227Si
0.053Nb
0.05)
100–xCu
xbulk metallic glasses
Kagan Sarlara,b and Ilker Kucuka
aFaculty of arts and sciences, Physics Department, Uludag University, Bursa, Turkey; bKamil ozdag Faculty of
sciences, Physics Department, Karamanoglu Mehmetbey University, Karaman, Turkey
ABSTRACT
In this work, Co-based (Co0.402Fe0.201Ni0.067B0.227Si0.053Nb0.05)100–xCux bulk glassy alloys (BMG) with 2 mm diameters were formed by suction-casting method and effect of Cu in this system’s thermal stability, glass forming ability and magnetic properties were also investigated. The curves of thermal analysis, obtained using differential scanning calorimetry, show that (Co0.402Fe0.201Ni0.067B0.227Si0.053Nb0.05)100-xCux (x = 0–2) has supercooled liquid region (∆Tx) of about 45 K, and reduced glass transition temperature (Tg/Tl) lies in the range from 0.663 to 0.678. The saturation magnetisation (Js) and coercivity (Hc) for as-cast BMG were in the range of 0.46 T–0.65 T and 13 A/m, respectively.
1. Introduction
The development of Fe- and Co-based BMGs alloys has not been as rapid as the other sys-tems such as Mg-, Pd-, Al-, Cu- and Zr-based BMGs [1]. In 1995, bulk glassy alloys (BMG) with ferromagnetic properties have been synthesised for the first time [2]. It has been found that adding Nb increases stability of supercooled liquid region and enhances the GFA in ferromagnetic (Fe, Co, Ni)-(B, Si) glassy alloys [3]. Before adding Nb, Fe–Co–Ni–B–Si, as it is a pseudoternary system, does not show glass transition phenomenon and it is important to note that, addition of Nb satisfies the three empirical component rules which are pointed out by A. Inoue [4]. Unfortunately, satisfying the three empirical component rules causes reduction in magnetic atoms in the composition and inevitably leads to the deterioration of magnetic properties. However, recent studies show that minor Cu addition has an effect on the magnetic properties of Fe-based BMGs [5,6]. Many researchers try to find out which element and concentration ratio in Fe–Co and Co-based BMGs are suitable for replacing Nb and to optimise the alloy compositions by modifying the B to Si concentration ratio [7]. By copper mould casting method, Co40.2Fe20.1Ni6.7B22.7Si5.3Nb5 alloy could be formed as cylindrical glassy rods with diameter of 4.5 mm and this alloy system has wide supercooled liquid region. This Co-based Co40.2Fe20.1Ni6.7B22.7Si5.3Nb5 ferromagnetic BMG with high GFA and excellent soft magnetic properties is recommended for future applications as new structural and functional materials [8,9].
KEYWORDS
Bulk metallic glasses (BMgs); suction casting; magnetic materials; glass forming ability (gFa)
ARTICLE HISTORY Received 8 January 2016 accepted 22 november 2016
© 2016 informa UK limited, trading as Taylor & Francis group CONTACT ilker Kucuk ikucuk@uludag.edu.tr
490 K. SARLAR AND I. KUCUK
In this paper, the effects of Cu addition on the GFA and magnetic properties of the (Co0.402 Fe0.201Ni0.067B0.227Si0.053Nb0.05)100–xCux (x = 0, 0.5, 0.75, 1, 1.5, 2) BMGs are investigated. The primary motivation of this present study is that, Cu with a positive heat of mixing with the main constituents Co (+6 kJ/mol) and Fe (+13 kJ/mol) [10] in (Co0.402Fe0.201Ni0.067B0.227 Si0.053Nb0.05)100-xCux BMGs, results of having positive heat of mixing and substitution Cu for all over the percentage composition improves soft magnetic properties, and has an influence on the GFA.
2. Experimental procedure
The Co-based multi-component alloy ingots with nominal compositions of (Co0.402Fe0.201Ni0.067 B0.227Si0.053Nb0.05)100-xCux (x = 0–2) weighing approximately 2 g were prepared by arc-melting appropriate amounts of the high purity (>%99.9) constituent elements in Zr-gettered argon atmosphere for at least four times. During suction casting method, change in each stage corresponds to arc-melting from 623 K, a stream of molten alloy with temperature between 1923 and 2583 K was suction-cast in a Edmund Buhler MAM-1 arc melting system into a cylindrical copper mould with a length of 30 mm and a diameter of 2 mm. The structures of as-quenched samples were identified by X-ray diffraction (XRD) with CuKα radiation. The glass transition temperature Tg, crystallisation temperature Tx, liquidus temperature
Tl and melting temperature Tm were measured with Seteram SETSYS 16/18 differential scanning calorimetry (DSC) under flowing high purity argon gas with 15–20 mg samples at a ramp rate of 0.67 K/s. Magnetic hysteresis measurements were conducted with an ADE Magnetics EV9 vibrating sample magnetometer (VSM) with maximum magnetic field strength of 1750 kA/m, real-time field control and dynamic gauss range capable of reaching a resolution of 0.08 A/m at low fields. In the coercivity measurement, we align the magnet with the horizontal component of the earth magnetic field, then the VSM power supply can effectively cancel out the horizontal component of the earth magnetic field and because the field control is done by a real-time feedback loop, fluctuations in the earth magnetic field that are within the bandwidth of the field control loop will be compensated for. Alloy den-sities were measured with Archimedean principle by AND HR-250AZ analytical balances’ density determination kit.
3. Results and discussion
3.1. Structural and thermal analysis
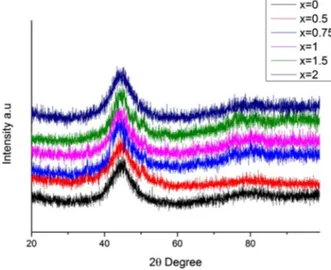
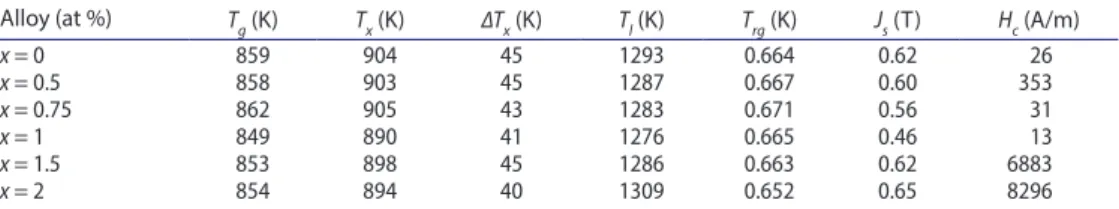
XRD was performed to investigate the structural analysis of the alloy system in an amor-phous state or in a crystalline state. Figure 1 shows XRD patterns of the (Co0.402Fe0.201Ni0.067 B0.227Si0.053Nb0.05)100-xCux (x = 0–2) BMG cylinders with a diameter of 2 mm produced by suction-casting exhibit the typical broad diffraction of an amorphous structure, although small amounts of crystalline phases cannot be excluded due to the noise level of the XRD patterns. The DSC patterns exhibited by the BMGs are also given in Figures 2 and 3. Each DSC curve as shown Figure 2 exhibits glass transition, followed by a supercooled liquid region, ∆Tx of about 45 K. The Tg and Tx (marked by arrows, respectively) determined from the temperature region of 840–910 K are summarised in Table 1. In the presence of Cu, the crystallisation peak is followed by a peak around 1210 K as shown in Figure 3. Tl values were extracted from the DSC curves given in Figure 3. Table 1 also shows us that
upon increasing x from 0 to 0.75, Tl decreases from 1293 to 1276 K, and increases to 1286 K again as x = 1. It means that increasing Cu concentration ratio to x = 0.75 is effective in decreasing Tl of the alloy system. Reduced glass transition temperature Trg = Tg/Tl values are in the range of 0.652–0.671. As a result high Trg value causes a low nucleating rate in the undercooled liquid, and a low critical cooling rate for glass formation.
3.2. Soft magnetic properties
Figure 4 shows the magnetisation-magnetic field strength (J–H) loops of amorphous (Co0.402 Fe0.201Ni0.067B0.227Si0.053Nb0.05)100-xCux (x = 0, 0.5, 0.75, 1, 1.5, 2) rods with a diameter of 2 mm. The J values have been calculated using the data of density and magnetisation (M) for
Figure 1. (colour online) XRD patterns of (co0.402Fe0.201ni0.067B0.227si0.053nb0.05)100-xcux (x = 0–2) alloy rods.
Figure 2. (colour online) Dsc heating curves and (co0.402Fe0.201ni0.067B0.227si0.053nb0.05)100-xcux (x = 0–2) alloy rods.
492 K. SARLAR AND I. KUCUK
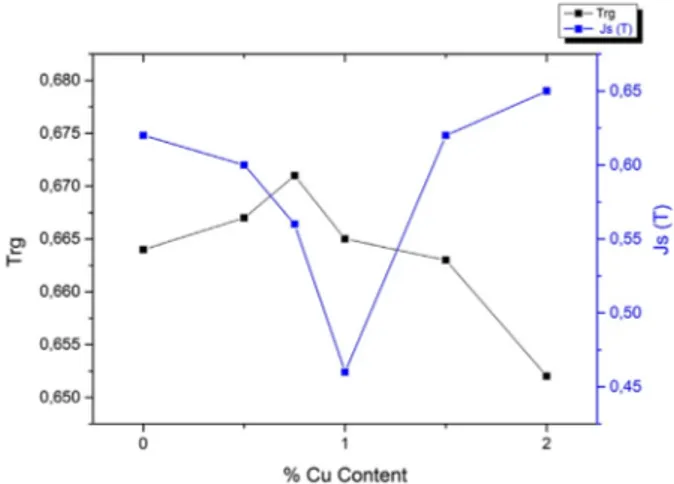
each measurement. The saturation magnetisation (Js) and coercivity (Hc) values determined from hysteresis loops are presented in Table 1. Hc increases suddenly from 13 to 6883 A/m and 13 to 8296 A/m when Cu contents are 1.5 and 2%, respectively. Js value decreases ini-tially from 0.62 to 0.46 T with Cu content up to x = 1, then it increases as a function of Cu content as shown in Figure 5.
Figure 3. (colour online) Dsc cooling patterns of (co0.402Fe0.201ni0.067B0.227si0.053nb0.05)100-xcux (x = 0–2) alloy rods.
Table 1. Thermal and magnetic properties of (co0.402Fe0.201ni0.067B0.227si0.053nb0.05)100-xcux (x = 0, 0.5, 0.75, 1, 1.5, 2).
Alloy (at %) Tg (K) Tx (K) ∆Tx (K) Tl (K) Trg (K) Js (T) Hc (A/m)
x = 0 859 904 45 1293 0.664 0.62 26 x = 0.5 858 903 45 1287 0.667 0.60 353 x = 0.75 862 905 43 1283 0.671 0.56 31 x = 1 849 890 41 1276 0.665 0.46 13 x = 1.5 853 898 45 1286 0.663 0.62 6883 x = 2 854 894 40 1309 0.652 0.65 8296
Js value is determined by the total magnetic moment in the amorphous structure. In our research Cu substitution for all over the percentage composition reduces the total con-centration of Co, Fe and Ni atoms in the alloy system. In this situation Js values of BMGs decreases. On the other hand, Cu has positive heat of mixing with Fe atoms. Heat of mixing (ΔHmix) is only due to the bond energies between adjacent atoms. ΔHmix was calculated by where HS, HA, and HB are the internal energies of the solid solution and of pure A and B atoms, respectively. xA and xB are the mole fractions of the A and B components.
Here we define
where WAA is the interaction energy of an A–A bond, WBB energy of a B–B bond and WAB as the energy of an A–B bond, N is the number of atoms in system and Z is the coordination number. Defining the work of mixing per mole to be
where NA is Avogadro’s number.
We find the molar energy enthalpy of mixing to be
(1) ΔHmix= HS− xAHA− xBHB (2) HA= 1 2NZWAA (3) HB = 1 2NZWBB (4) HS= 1 2NZ(x 2 AWAA+ xB2WBB+2xAxBWAB) (5) WH= 1 2NAZ(2WAB− WAA− WBB) (6) ΔHmix= xAxBWH
494 K. SARLAR AND I. KUCUK
This result shows us that WH has an important role for bonding structure between A and B atoms. We know that Cu have positive heat of mixing with Fe-, Co- and Ni atoms from earlier studies [10]. In our case, if ΔHmix is larger than zero, WH must be larger than zero. Because
WH > 0 there is an energy barrier for forming A–B bonds. In these compositions A–A and B–B bonds are more favourable. The composition will create areas which will be richer in terms of A–A and B–B atom [11] so that increasing the Cu percentage could promote the bonding of Fe–Fe pairs [12]. Proper Cu substitution leads to increased Js values because, according to Heisenberg [13], the atomic magnetic moment is assumed to be dependent on the number of the nearest-neighbour Fe atoms. When minor Cu substitution is increased up to 1%, the amount of magnetic atoms in the compositions decrease. Consequently, alloy system’s Js values decrease with increasing Cu in this case. On the other hand, further addition of Cu in to the composition increases the Js value because of the number of the magnetic atom pairs (Fe–Fe, Fe–Co, Fe–Ni, Co–Co, Co–Ni, Ni–Ni) increases. Therefore,
Js first reaches minimum value at the alloy with 1% Cu and then increases at the alloy con-taining 1.5 and 2% Cu. On the other hand, the Fe–Fe pairs resulted in increasing Hc values with the same percentage of copper in the composition.
Figure 5 shows us that increasing Cu content also increases Trg value up to 0.75% Cu content. But further increasing Cu content decreases Trg value when Js value increases and starts to form Fe–Fe pairs.
3.3. Effect of the Cu content on the electronegativity 3.3.1. Electronegativity and doping contents
Electronegativity difference plays key role on the optimum doping content [14]. So the relationship between doping content of elements is inversely proportional to the electroneg-ativity. When the electronegativity difference between the elements is large, the percentage of doping content of elements is reduced. In other words, electronegativity difference of elements which are close to each other could be further added. Previous studies [6,12,14] have shown that in Fe-based composition copper must be added in small proportions of up to 1%. Because the atoms have negative or positive electronegativity difference with Fe, adding large amount of these atoms causes destabilisation of the liquid phase and new competing crystalline phases are created [14]. So their doping content is low. And another reason is Cu has a positive heat of mixing with Fe also limits the doping content of Cu. Since the electronegativity difference between Fe and Cu is slightly large in comparison to the electronegativity difference between Co and Cu we can add larger amount of Cu to the Co-based alloy system than Fe-based alloy system.
3.3.2. Composition electronegativity and Cu content
The glass forming ability (GFA) strongly depends on the electronegativity of the alloying elements because electronegativity is directly related to the chemical bonding and it is crit-ical for glass formation [14]. Fang et al. [15] suggested simple models for electronegativity difference (∆x).
According to their models the electronegativity of multi-component alloy can be defined as: (8) Δx = √ √ √ √ n ∑ i=1 Ci(xi− ̄x)2
where n is the number of components of the alloy, xi is the Pauling electronegativity of ele-ment i, C
i is the atomic percentage of element i in the alloy; and ̄x is the arithmetical mean
value of electronegativity for a compound which can be calculated as follows:
Pauling electronegativity for all elements can be found in Ref. [15]. Table 2 shows us that upon increasing Cu content suddenly the electronegativity in alloys systems increases. Adding materials to the base composition causes new covalent bonding between alloying metals and metalloids. During solidification, it is necessary to break this covalent bond. Metal-metalloids covalent bond becomes stronger with the increase in the electronegativity according to the Pauling’s theory. So liquid phase stability is enhanced and formation of primary phases is slowed down, leading to large GFA.
4. Conclusions
Effects of Cu on the GFA and soft magnetic properties in a Co-based soft magnetic BMGs system were investigated. Adding appropriate amounts of Cu leads to magnetic atom pairs formation. These pairs increase the atomic magnetic moment of the alloys; thus, it results in Js increasing. The formation of the Fe–Fe pairs also reduces Trg and affects the GFA of the alloy system. And it is found that in Co-based BMGs addition of Cu amount is larger than Fe-based BMGs because Cu and Co atoms have similar electronegativity. And also electronegativity and Cu content of the alloy system have a correlation and have influence on the GFA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partially supported by the Commission of Scientific Research Projects of Uludag University [Project number KUAP(F)-2015/50], [Project number KUAP(F)-2013/25].
References
[1] M. Miller and P. Liaw (eds.), Bulk Metallic Glasses an Overview, Springer Science Business
Media, LLC, New York, 2008.
[2] A. Inoue and J.S. Gook, Effect of additional elements (M) on the thermal stability of supercooled liquid in Fe72-xAl5Ga2P11C6B4Mx glassy alloys, Mater. Trans. JIM 37 (1996), pp. 32–38.
(9) ̄x = n ∑ i=1 Cixi
Table 2. Relationship between cu content and electronegativity.
Cu content (%) Δx 0 0.1004 0.5 1.1437 0.75 1.8608 1 2.6515 1.5 4.4434 2 6.4888
496 K. SARLAR AND I. KUCUK
[3] A. Inoue and B. Shen, New Fe-based bulk glassy alloys with high saturated magnetic flux density of 1.4–1.5T, Mater. Sci. Eng. A 375–377 (2004), pp. 302–306.
[4] A. Inoue, Bulk Amorphous Alloys: Preparation and Fundamental Characteristics. Vol. 4 of Materials Science Foundations, Uetikon-Zurich: Trans Tech Publications. 1998.
[5] A. Rahman, Q. Luo, Y. Lu, and J. Shen, Recurring effects of Cu addition on magnetic properties in Fe-based bulk metallic glasses, J. Non-Crys. Solids 422 (2015), pp. 1–5.
[6] L. Dou, H. Liu, L. Hou, L. Xue, W. Yang, Y. Zhao, C. Chang, and B. Shen, Effects of Cu substitution for Fe on the glass-forming ability and soft magnetic properties for Fe-based bulk metallic glasses, J. Magn. Magn. Mater. 358–359 (2014), pp. 23–26.
[7] I. Kucuk, M. Aykol, O. Uzun, M. Yıldırım, M. Kabaer, N. Duman, F. Yılmaz, K. Ertürk, M.V. Akdeniz, and A.O. Mekhrabov, Effect of (Mo, W) substitution for Nb on glass forming ability and magnetic properties of Fe–Co-based bulk amorphous alloys fabricated by centrifugal casting, J. Alloys Compd. 509 (2011), pp. 2338–2341.
[8] Y. Dong, Q. Man, H. Sun, B. Shen, S. Pang, T. Zhang, A. Makino, and A. Inoue, Glass-forming ability and soft magnetic properties of (Co0.6Fe0.3Ni0.1)67B22+xSi6−xNb5 bulk glassy alloys, J. Alloys
Compd. 509 (2011), pp. S206–S209.
[9] K. Sarlar and I. Kucuk, Glass forming ability and magnetic properties of Co(40.2-x)
Fe(20.1+x)Ni6.7B22.7Si5.3Nb5 (x=0–10), J. Magn. Magn. Mater. 374 (2015), pp. 607–610.
[10] A. Takeuchi and A. Inoue, Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element, Trans Mater. 46 (2005), pp. 2817–2829.
[11] A. Cottrell. An Introduction to Metallurgy, 2nd ed., Institute of Materials, London, 121995. [12] J.E. Gao, H.X. Li, Z.B. Jiao, Y. Wu, Y.H. Chen, T. Yu, and Z.P. Lu, Effects of nanocrystal formation
on the soft magnetic properties of Fe-based bulk metallic glasses, Appl. Phys. Lett. 99 (2011), p. 052504.
[13] W. Heisenberg, Zur Theorie des Ferromagnetismus [On the theory of ferromagnetism], Z. Phys. 49 (1928), pp. 619–636.
[14] Z.B. Jiao, H.X. Li, J.E. Gao, Y. Wu, and Z.P. Lu, Effects of alloying elements on glass formation, mechanical and soft-magnetic properties of Fe-based metallic glasses, Intermetallics 19 (2011), pp. 1502–1508.
[15] S. Fang, X. Xiao, L. Xia, W. Li, and Y. Dong, Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses, J. Non-Cryst. Solids 321 (2003), pp. 120–125.